Effect of the Croton rhamnifolioides Essential Oil and the Inclusion Complex (OEFC/β-CD) in Antinociceptive Animal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Substances
2.2. Animals
2.3. Assays
2.4. Effect of the OEFC and COEFC on the Central Nervous System
2.4.1. Open Field
2.4.2. Rota-Rod
2.5. Antinociceptive Effect of the OEFC and COEFC
2.5.1. Acetic Acid-Induced Abdominal Contortions
2.5.2. Formalin Test (2.5%)
2.5.3. Hot Plate
2.6. Pain Signaling Pathways Involved in the OEFC and COEFC Antinociceptive Response (Opioid, Cholinergic, α1 and α2 Adrenergic, Serotonergic, Nitric Oxide, Adenosinergic, Dopaminergic, Glutamatergic, and Vanilloid)
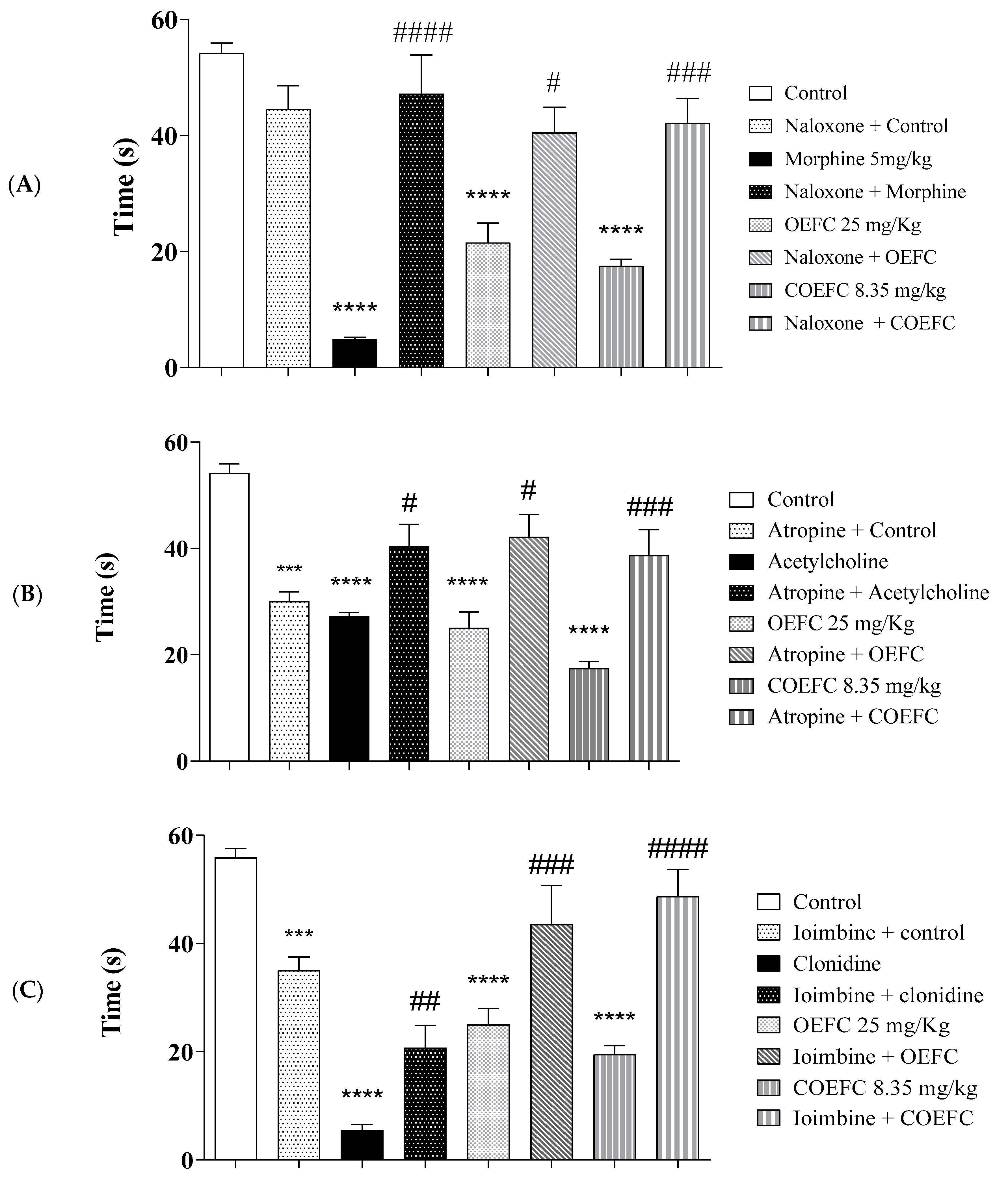
2.6.1. Opioid System Participation
2.6.2. Cholinergic System Participation
2.6.3. α-1. Adrenergic Receptor Participation
2.6.4. α-2. Adrenergic Receptor Participation
2.6.5. L-arginine/Nitric Oxide/cGMP Pathway Participation
2.6.6. Participation of the Vanilloid System
2.6.7. Participation of Serotonergic Pathways
2.6.8. Participation of the Dopaminergic System
2.6.9. Participation of the Adenosinergic System
2.6.10. Participation of the Glutamatergic System
2.7. Data Expression and Statistical Analysis
3. Results
3.1. Effect of the OEFC and COEFC on the Central Nervous System (CNS)
3.1.1. Open Field
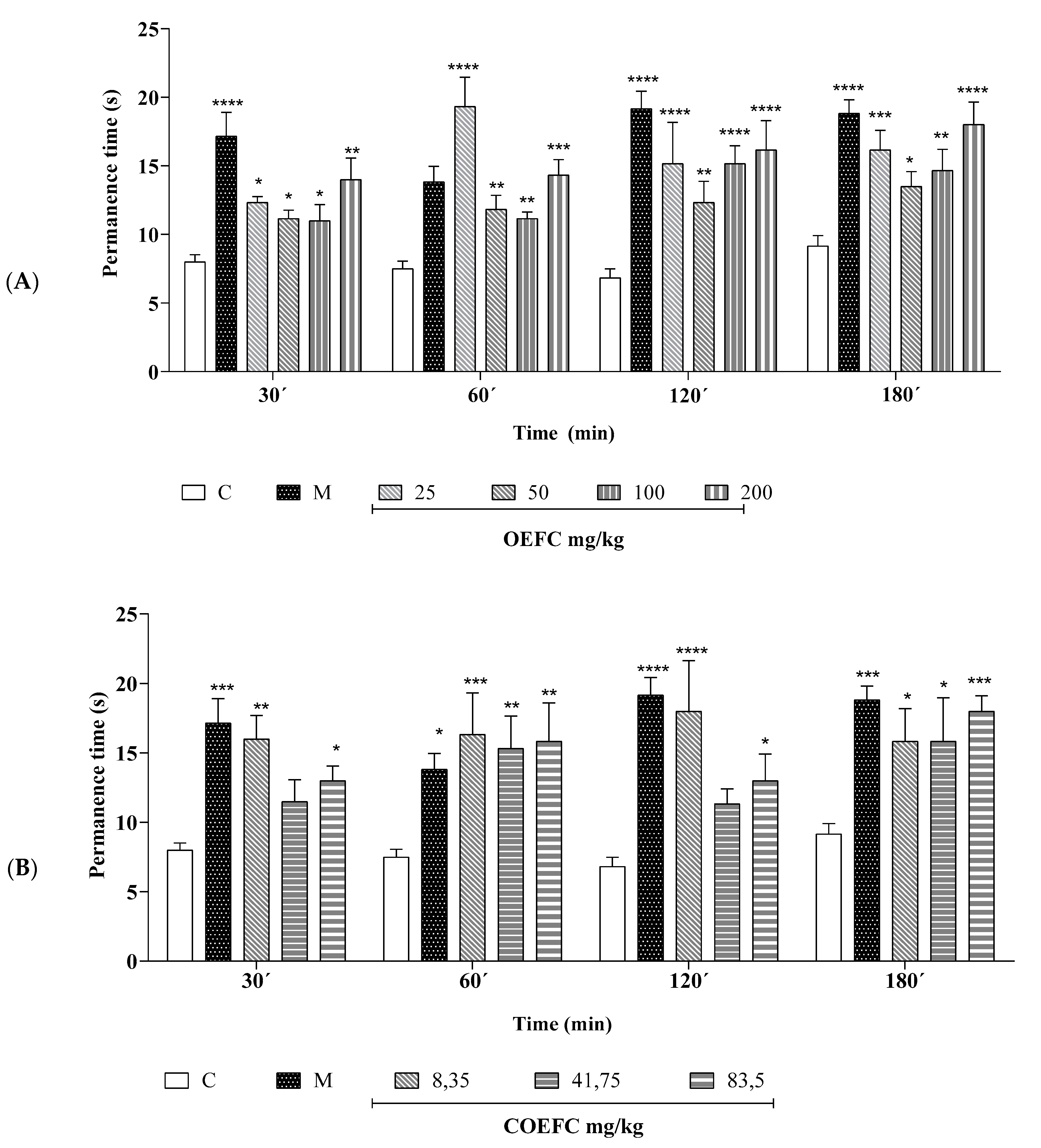
3.1.2. Rota-Rod
3.2. Antinociceptive Effect of the OEFC and COEFC
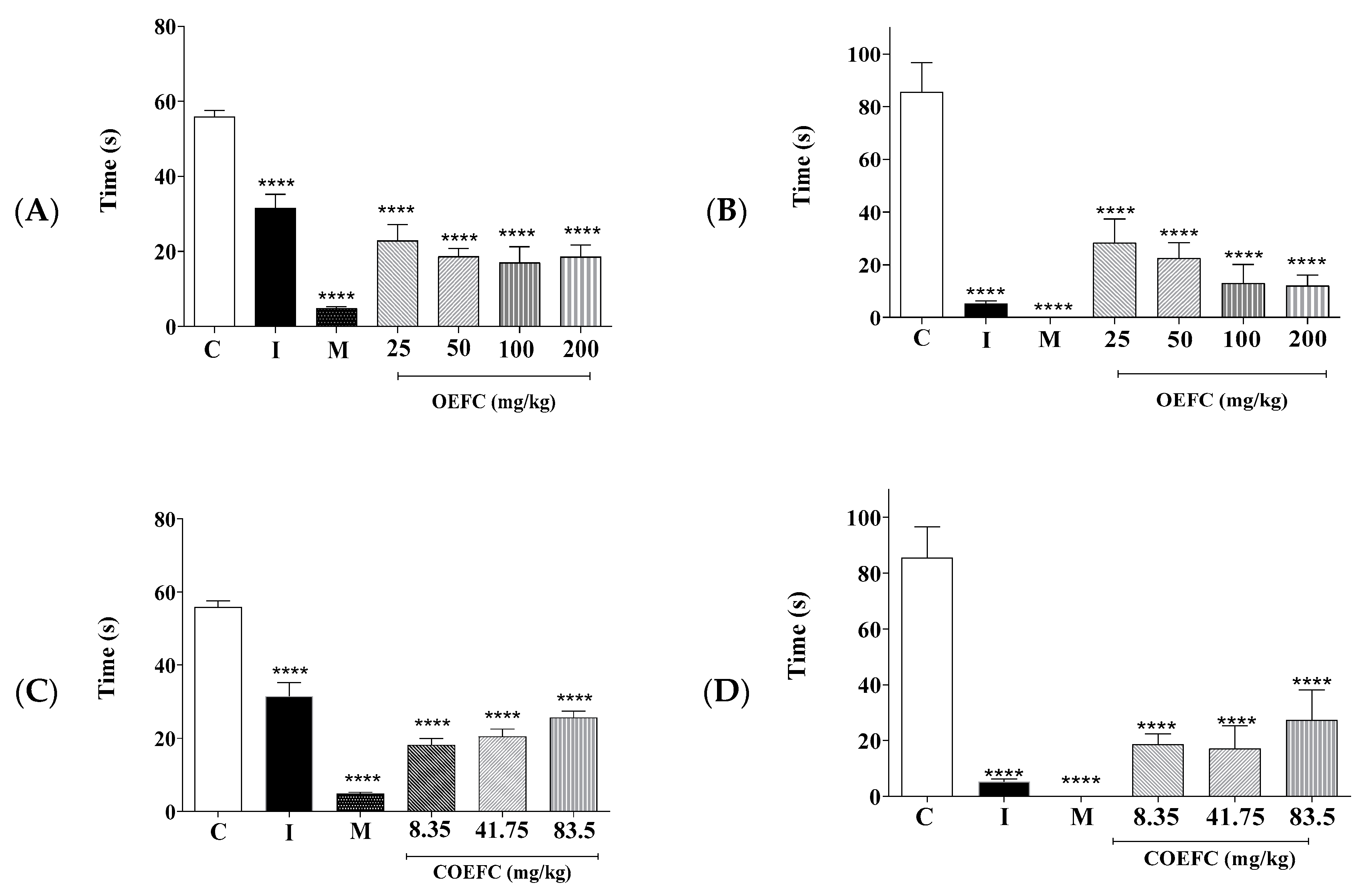
3.2.1. Acetic Acid-Induced Abdominal Contortions
3.2.2. Formalin Test (2.5%)
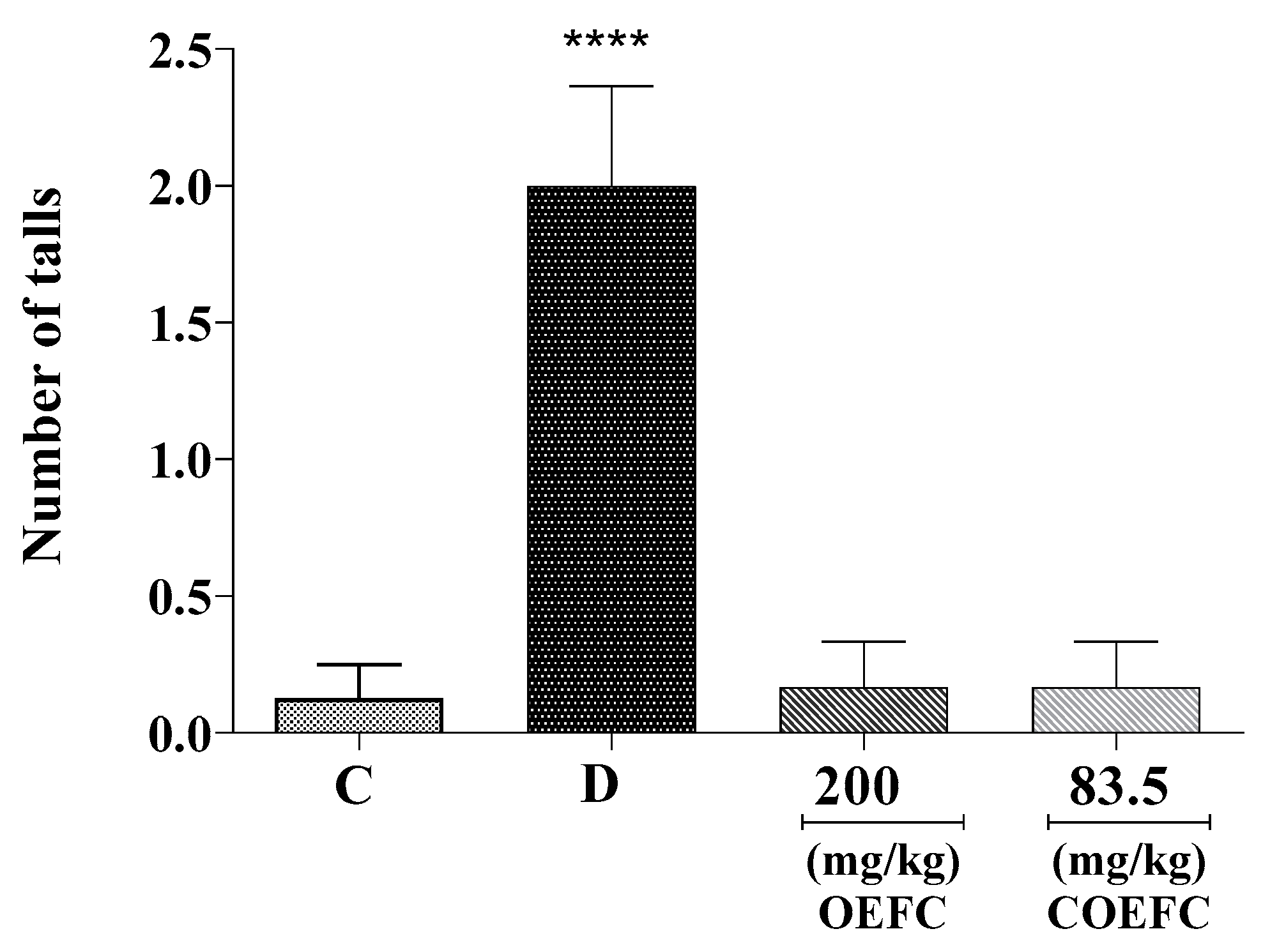
3.2.3. Hot Plate
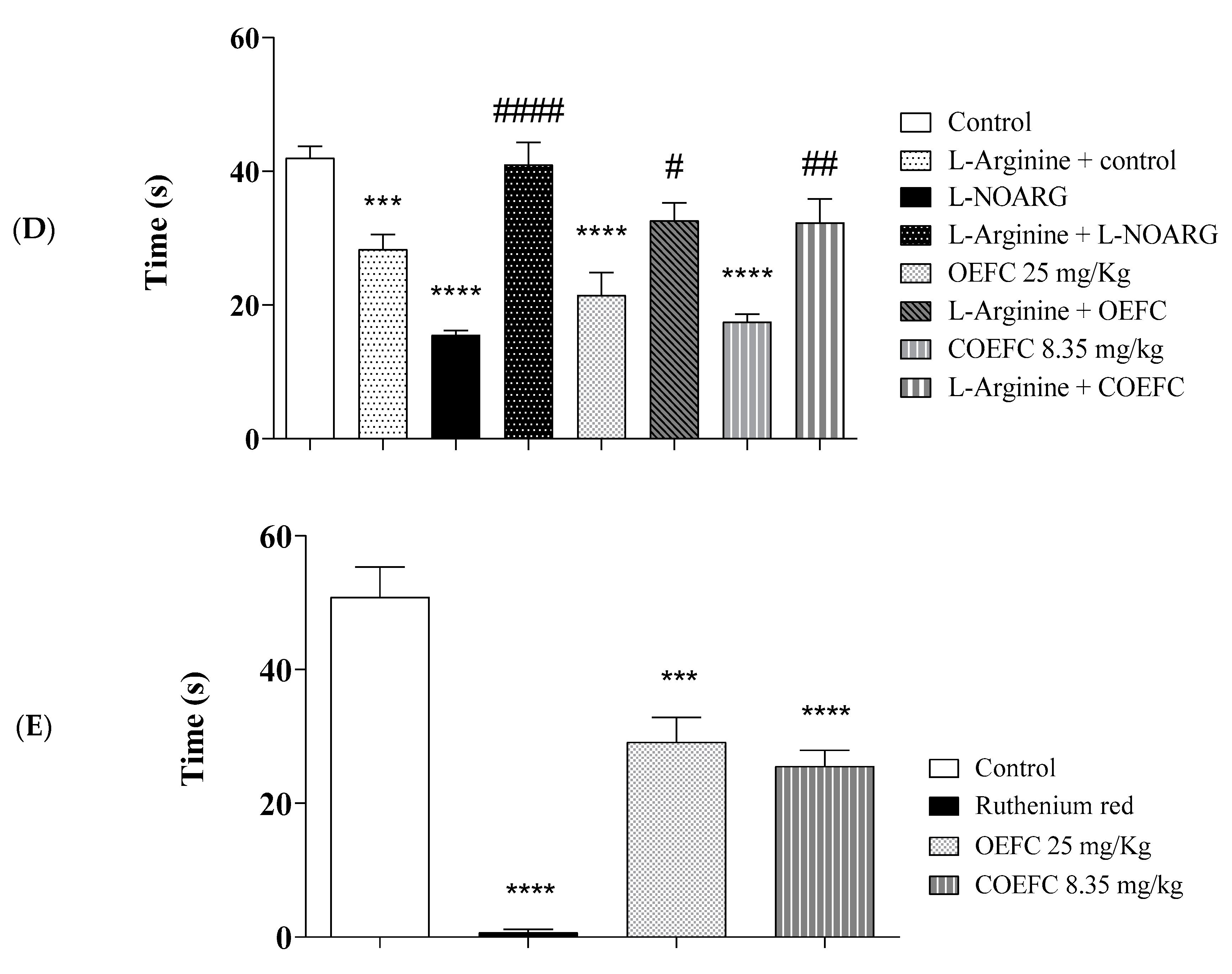
3.3. Pain Signaling Pathways Involved in the OEFC and COEFC Antinociceptive Response (Opioid, Cholinergic, α1 and α2 Adrenergic, Serotonergic, Nitric Oxide, Adenosinergic, Dopaminergic, Glutamatergic and Vanilloid)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, D.A.; Macêdo, D.G.; Oliveira, L.G.S.; Saraiva, M.E.; Oliveira, S.F.; Souza, M.M.A.; Menezes, I.R. Therapeutic potential and use of medicinal plants in an area of the Caatinga in the state of Ceará, northeastern Brazil. Rev. Bras. Pl. Med. 2014, 16, 912–930. [Google Scholar] [CrossRef]
- Randau, K.P.; Florêncio, D.C.; Ferreira, C.P.; Xavier, H.S. Pharmacognostic study of Croton rhamnifolius HBK and Croton rhamnifolioides Pax & Hoffm.(Euphorbiaceae). Rev. Bras. Farmacogn. 2004, 14, 89–96. [Google Scholar]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; de Oliveira, M.R.C.; Tintino, C.D.M.; e Castro, F.F.; Alcântara, I.S.; Fernandes, M.N.M.; de Albuquerque, T.R.; da Silva, M.S.A. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1, 8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.K.N.; Dutra, K.A.; Lira, C.S.; Lima, B.N.; Napoleão, T.H.; Paiva, P.M.G.; Maranhão, C.A.; Brandão, S.S.F.; Navarro, D.M.A.F. Effects of Croton rhamnifolioides essential oil on Aedes aegypti oviposition, larval toxicity and trypsin Activity. Molecules 2014, 19, 16573–16587. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.S.; Martins, A.O.B.P.B.; de Alencar Silva, A.; de Oliveira, M.R.C.; Ribeiro-Filho, J.; de Albuquerque, T.R.; Coutinho, H.D.M.; da Silva Almeida, J.R.G.; Quintans, L.J.; de Menezes, I.R.A. Gastroprotective effect and mechanism of action of Croton rhamnifolioides essential oil in mice. Biomed. Pharmacother. 2017, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, U.N.; de Lima, S.G.; Moura, L.C.B.; de Almeida, L.T.G.; Oliveira, T.M.; de Freitas, R.M.; Silva, R.M.; Rocha, M.S. Preparação e caracterização do complexo de inclusão do óleo essencial de Croton zehntneri com β-Ciclodextrina. Quim. Nova 2014, 37, 50–55. [Google Scholar] [CrossRef]
- CUNHA-FILHO, M.S.S.; Sá-BARRETO, L.C.L. Utilização de ciclodextrinas na formação de complexos de inclusão de interesse farmacêutico. Rev. Ciências Farm. Básica Apl. 2009, 28, 1–9. [Google Scholar]
- de Lima Guedes, F.; Alves, G.M.C.; dos Santos, F.L.A.; de Lima, L.F.; Rolim, L.A.; Neto, P.J.R. Ciclodextrinas: Como adjuvante tecnológico para melhorar a biodisponibilidade de fármacos. Rev. Bras. Farm 2008, 89, 3. [Google Scholar]
- Sithole, M.N.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Development of a Novel Polymeric Nanocomposite Complex for Drugs with Low Bioavailability. AAPS PharmSciTech. 2018, 19, 303–314. [Google Scholar] [CrossRef]
- Brito, M.; JÚNIOR, C.S.N.; SANTOS, H.F. Análise estrutural de ciclodextrinas: Um estudo comparativo entre métodos teóricos clássicos e quânticos. Química. Nov. 2004, 6, 882–888. [Google Scholar] [CrossRef]
- Lapa, A.J.; Souccar, C.; Lima-Landman, M.T.R.; de Castro, M.S.A.; Lima, T.C.M. Métodos de avaliação da atividade farmacológica de plantas medicinais. Soc. Bras. Plantas Med. 2003, 64, 66. [Google Scholar]
- WOOLFE, G.; MacDonald, A.D. The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Peura, D.A.; Goldkind, L. Balancing the gastrointestinal benefits and risks of nonselective NSAIDs. Arthritis Res. Ther. 2005, 7, S7. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, C.R.; Scully, S.S. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005, 78, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.P.; Serafini, M.R.; Quintans-Júnior, L.J.; Silva, G.F.; Oliveira, J.F.; Carvalho, F.M.S.; Souza, J.C.C.; Matos, J.R.; Alves, P.B.; Matos, I.L. Inclusion complex of (−)-linalool and β-cyclodextrin. J. Therm. Anal. Calorim. 2014, 115, 2429–2437. [Google Scholar] [CrossRef]
- Martins, A.O.B.P.B.; Wanderley, A.G.; Alcântara, I.S.; Rodrigues, L.B.; Cesário, F.R.A.; Oliveira, M.R.C.; Castro, F.F.; Albuquerque, T.R.; Silva, M.S.A.; Ribeiro-Filho, J.; et al. Anti-Inflammatory and Physicochemical Characterization of the Croton Rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology 2020, 9, 114. [Google Scholar]
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Bilsky, E.J.; Negus, S.S. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: Effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J. Pain 2006, 7, 408–416. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.-G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Jacob, J.J.C.; Ramabadran, K. Enhancement of a nociceptive reaction by opioid antagonists in mice. Br. J. Pharmacol. 1978, 64, 91–98. [Google Scholar] [CrossRef]
- Schechtmann, G.; Song, Z.; Ultenius, C.; Meyerson, B.A.; Linderoth, B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain 2008, 139, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.S.; Gadotti, V.M.; Oliveira, G.L.; Tibola, D.; Paszcuk, A.F.; Neto, A.; Spindola, H.M.; Souza, M.M.; Rodrigues, A.L.S.; Calixto, J.B. Mechanisms involved in the antinociception caused by agmatine in mice. Neuropharmacology 2005, 48, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, A.; Tegeder, I.; Geisslinger, G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 2009, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Mantovani, M.; Kaster, M.P.; Pertile, R.; Calixto, J.B.; Rodrigues, A.L.S.; Santos, A.R.S. Mechanisms involved in the antinociception caused by melatonin in mice. J. Pineal. Res. 2006, 41, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Nayebi, A.M.; Garjani, A. Effects of central and peripheral depletion of serotonergic system on carrageenan-induced paw oedema. Int. Immunopharmacol. 2005, 5, 1723–1730. [Google Scholar] [CrossRef]
- Finan, P.H.; Smith, M.T. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med. Rev. 2013, 17, 173–183. [Google Scholar] [CrossRef]
- Ferré, S.; Diamond, I.; Goldberg, S.R.; Yao, L.; Hourani, S.M.O.; Huang, Z.L.; Urade, Y.; Kitchen, I. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry: Implications for drug addiction, sleep and pain. Prog. Neurobiol. 2007, 83, 332–347. [Google Scholar] [CrossRef]
- Zeraati, F.; Araghchian, M.; Farjoo, M.H. Ascorbic Acid interaction with analgesic effect of morphine and tramadol in mice. Anesthesiol. Pain Med. 2014, 4, 5. [Google Scholar] [CrossRef]
- Talarek, S.; Orzelska, J.; Listos, J.; Fidecka, S. Effects of sildenafil treatment on the development of tolerance to diazepam-induced motor impairment and sedation in mice. Pharmacol. Rep. 2010, 62, 627–634. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Barreto, R.S.S.; Menezes, P.P.; Almeida, J.R.G.S.; Viana, A.F.S.C.; Oliveira, R.; Oliveira, A.P.; Gelain, D.P.; Lucca Júnior, W.; Araújo, A.A.S. β-Cyclodextrin-complexed (−)-linalool produces antinociceptive effect superior to that of (−)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.P.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef]
- Santos, F.A.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phyther. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Theodosopoulos, S.; Galanopoulou, P. Antinociceptive properties of 1, 8-cineole and β-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 2007, 73, 1247–1254. [Google Scholar] [CrossRef]
- Cordeiro, K.W.; Felipe, J.L.; Malange, K.F.; do Prado, P.R.; de Oliveira Figueiredo, P.; Garcez, F.R.; de Cássia Freitas, K.; Garcez, W.S.; Toffoli-Kadri, M.C. Anti-inflammatory and antinociceptive activities of Croton urucurana Baillon bark. J. Ethnopharmacol. 2016, 183, 128–135. [Google Scholar] [CrossRef]
- del Carmen, R.-O.J.; Willam, H.M.J.; del Carmen, G.M.A.; Nataly, J.-G.; Stefany, C.O.S.; Anahi, C.A.; Domingo, P.T.J.; Leonardo, G.P.; de la Mora Miguel, P. Antinociceptive effect of aqueous extracts from the bark of Croton guatemalensis Lotsy in mice. Res. Pharm. Sci. 2016, 11, 15. [Google Scholar] [PubMed]
- de Nogueira, L.M.; da Silva, M.R.; dos Santos, S.M.; de Albuquerque, J.F.C.; Ferraz, I.C.; de Albuquerque, T.T.; de Mota, C.R.F.C.; Araújo, R.M.; de Viana, G.S.B.; Martins, R.D. Antinociceptive effect of the essential oil obtained from the leaves of Croton cordiifolius Baill.(Euphorbiaceae) in mice. Evid. Based Complement Altern. Med. 2015, 2015, 620865. [Google Scholar] [CrossRef]
- Gavériaux-Ruff, C. Opiate-induced analgesia: Contributions from mu, delta and kappa opioid receptors mouse mutants. Curr. Pharm. Des. 2013, 19, 7373–7381. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Basbaum, A.I.; Way, W.L. Opioid Analgesics and Antagonists; Katzung, B.G., Masters, S.B., Trevor, A.J., Eds.; McGraw-Hill Educationpublisher: San Francisco, CA, USA, 2012. [Google Scholar]
- Tasker, R.A.R.; Connell, B.J.; Yole, M.J. Systemic injections of alpha-1 adrenergic agonists produce antinociception in the formalin test. Pain 1992, 49, 383–391. [Google Scholar] [CrossRef]
- Otsuka, N.; Kiuchi, Y.; Yokogawa, F.; Masuda, Y.; Oguchi, K.; Hosoyamada, A. Antinociceptive efficacy of antidepressants: Assessment of five antidepressants and four monoamine receptors in rats. J. Anesth. 2001, 15, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, W.A.; Lemônica, L. Mecanismos celulares e moleculares da dor inflamatória. Modulação periférica e avanços terapêuticos. Rev. Bras. Anestesiol. 1998, 48, 137–158. [Google Scholar]
- Shih, C.-C.; Hwang, H.-R.; Chang, C.-I.; Su, H.-M.; Chen, P.-C.; Kuo, H.-M.; Li, P.-J.; Wang, H.-M.D.; Tsui, K.-H.; Lin, Y.-C. Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica. Int. J. Mol. Sci. 2017, 18, 2437. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; Schloss, P. Differential regulation of serotonin transporter cell surface expression. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 259–268. [Google Scholar] [CrossRef]
- Millan, M.J.; Seguin, L.; Honoré, P.; Girardon, S.; Bervoets, K. Pro-and antinociceptive actions of serotonin (5-HT) 1A agonists and antagonists in rodents: Relationship to algesiometric paradigm. Behav. Brain Res. 1995, 73, 69–77. [Google Scholar] [CrossRef]
- Bobinski, F.; Ferreira, T.A.A.; Córdova, M.M.; Dombrowski, P.A.; da Cunha, C.; do Espírito Santo, C.C.; Poli, A.; Pires, R.G.W.; Martins-Silva, C.; Sluka, K.A. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain following sciatic nerve injury in mice. Pain 2015, 156, 2595. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef]
- Kellendonk, C.; Simpson, E.H.; Polan, H.J.; Malleret, G.; Vronskaya, S.; Winiger, V.; Moore, H.; Kandel, E.R. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006, 49, 603–615. [Google Scholar] [CrossRef]
- Lin, M.T.; Wu, J.J.; Chandra, A.; Tsay, B.L. Activation of striatal dopamine receptors induces pain inhibition in rats. J. Neural Transm. 1981, 51, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Benitez, J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin. Pharmacokinet. 2000, 39, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Padgett, W.L.; Daly, J.W. Caffeine analogs: Effects on ryanodine-sensitive calcium-release channels and GABA A receptors. Cell. Mol. Neurobiol. 2003, 23, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada, M.; Hatai, C.; Shimizu, M.; Tominaga, M. 1, 8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain 2012, 8, 86. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain® 2013, 154, 2169–2177. [Google Scholar] [CrossRef]
- de Oliveira Júnior, R.G.; Ferraz, C.A.A.; Silva, J.C.; de Oliveira, A.P.; Diniz, T.C.; Silva, M.G.; Quintans Júnior, L.J.; de Souza, A.V.V.; dos Santos, U.S.; Turatti, I.C.C. Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules 2017, 22, 900. [Google Scholar] [CrossRef]
- de Ramos, J.M.O.; dos Santos, C.A.; Santana, D.G.; Antoniolli, A.R.; de Santos, D.A.; Alves, P.B.; Thomazzi, S.M. Impact of Croton argyrophyllus essential oil on behavioural models of nociception. Flavour Fragr. J. 2017, 32, 40–45. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira Brito Pereira Bezerra Martins, A.; Correia de Oliveira, M.R.; Alcântara, I.S.; Rodrigues, L.B.; Cesário, F.R.A.S.; da Silva, M.S.A.; Castro, F.F.e.; Nascimento, E.P.d.; Albuquerque, T.R.d.; Quintans Júnior, L.J.; et al. Effect of the Croton rhamnifolioides Essential Oil and the Inclusion Complex (OEFC/β-CD) in Antinociceptive Animal Models. Macromol 2021, 1, 94-111. https://doi.org/10.3390/macromol1020008
Oliveira Brito Pereira Bezerra Martins A, Correia de Oliveira MR, Alcântara IS, Rodrigues LB, Cesário FRAS, da Silva MSA, Castro FFe, Nascimento EPd, Albuquerque TRd, Quintans Júnior LJ, et al. Effect of the Croton rhamnifolioides Essential Oil and the Inclusion Complex (OEFC/β-CD) in Antinociceptive Animal Models. Macromol. 2021; 1(2):94-111. https://doi.org/10.3390/macromol1020008
Chicago/Turabian StyleOliveira Brito Pereira Bezerra Martins, Anita, Maria Rayane Correia de Oliveira, Isabel Sousa Alcântara, Lindaiane Bezerra Rodrigues, Francisco Rafael Alves Santana Cesário, Maria Sanadia Alexandre da Silva, Fyama Ferreira e Castro, Emmily Petícia do Nascimento, Thaís Rodrigues de Albuquerque, Lucindo José Quintans Júnior, and et al. 2021. "Effect of the Croton rhamnifolioides Essential Oil and the Inclusion Complex (OEFC/β-CD) in Antinociceptive Animal Models" Macromol 1, no. 2: 94-111. https://doi.org/10.3390/macromol1020008
APA StyleOliveira Brito Pereira Bezerra Martins, A., Correia de Oliveira, M. R., Alcântara, I. S., Rodrigues, L. B., Cesário, F. R. A. S., da Silva, M. S. A., Castro, F. F. e., Nascimento, E. P. d., Albuquerque, T. R. d., Quintans Júnior, L. J., Araújo, A. A. d. S., Coutinho, H. D. M., Menezes, I. R. A. d., & Wanderley, A. G. (2021). Effect of the Croton rhamnifolioides Essential Oil and the Inclusion Complex (OEFC/β-CD) in Antinociceptive Animal Models. Macromol, 1(2), 94-111. https://doi.org/10.3390/macromol1020008






