Relative Water Economy Is a Useful Index of Aridity Tolerance for Australian Poephiline Finches
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
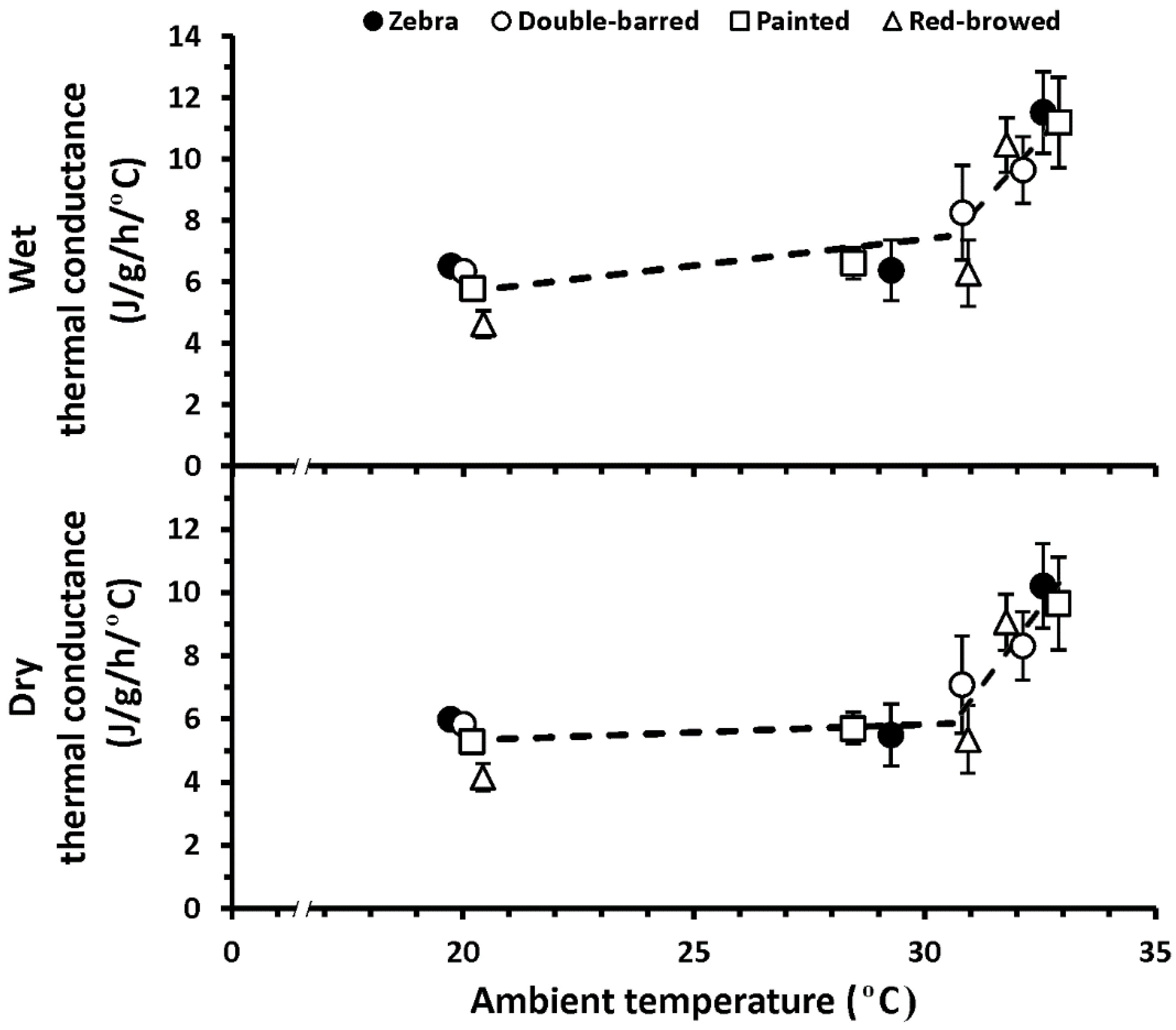
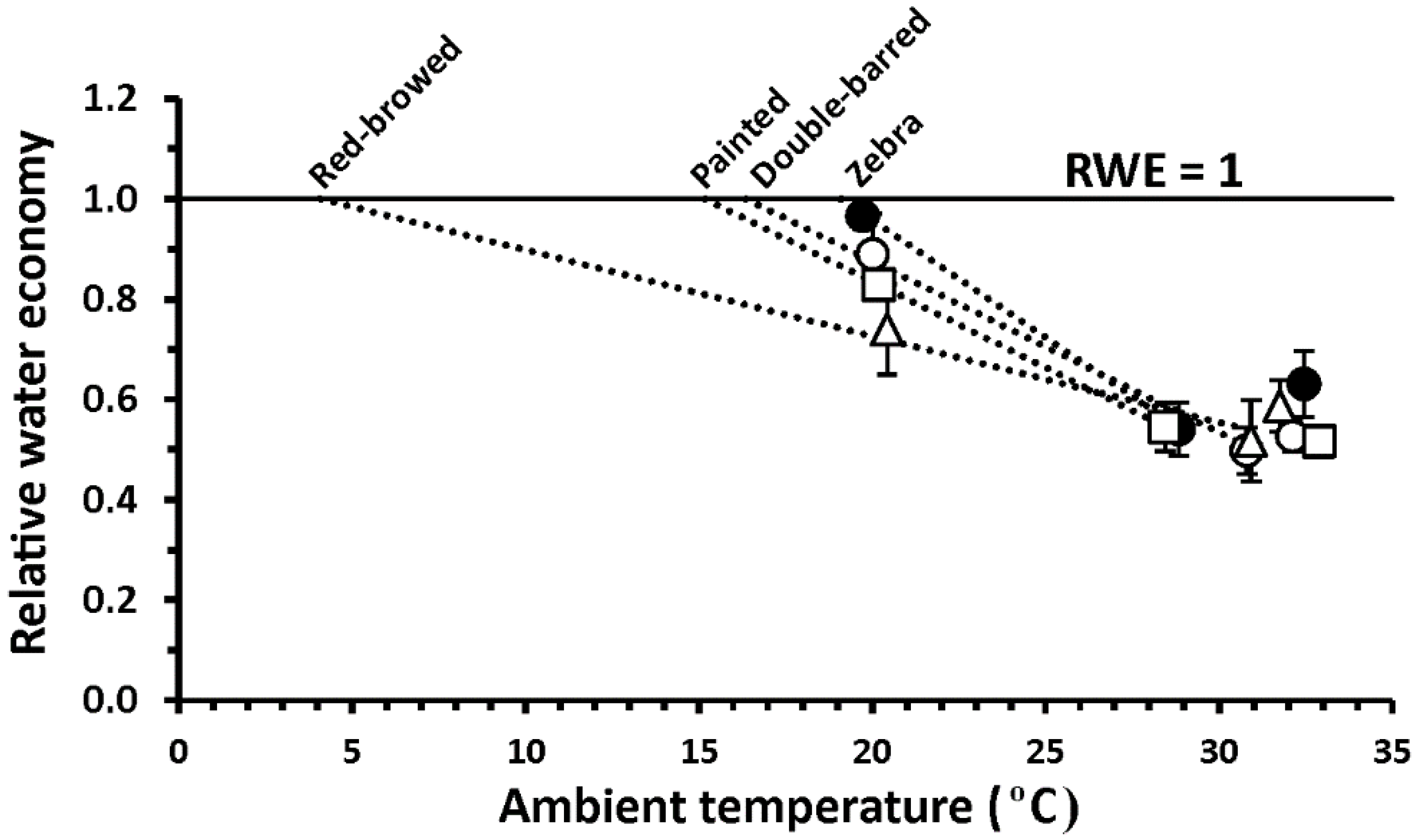
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Immelmann, K. Ecólogy and behaviour of African and Australian grass finches—A comparison. Ostrich 1966, 37, 371–379. [Google Scholar] [CrossRef]
- Immelmann, K. Australian Finches; Angus and Robertson: Sydney, Australia, 1982. [Google Scholar]
- Arnaiz-Villena, A.; Ruiz-del-Valle, V.; Gomez-Prieto, P.; Reguera, R.; Parga-Lozano, C.; Serrano-Vela, I. Estrildinae finches (Aves, Passeriformes) from Africa, South Asia and Australia: A molecular phylogeographic study. Open Ornithol. J. 2009, 2, 29–36. [Google Scholar] [CrossRef]
- Del Hoyo, J.; Elliot, A.; Christie, D. Handbook of Birds of the World, Volume 15: Weavers to New World Warblers; Lynx Edicions: Barcelona, Spain, 2010. [Google Scholar]
- Olsson, U.; Alström, P. A comprehensive phylogeny and taxonomic evaluation of the waxbills (Aves: Estrildidae). Mol. Phylogenet. Evol. 2020, 146, 106757. [Google Scholar] [CrossRef] [PubMed]
- Cade, T.J.; Tobin, C.A.; Gold, A. Water economy and metabolism of two estrildine finches. Physiol. Zool. 1965, 38, 9–33. [Google Scholar] [CrossRef]
- Barrett, G.; Silcocks, A.; Barry, S.; Cunningham, R.; Poulter, R. The New Atlas of Australian Birds; Royal Australasian Ornithologists Union: Victoria, Australia, 2003. [Google Scholar]
- Bartholomew, G.A.; Cade, T.J. The water economy of land birds. Auk 1963, 80, 504–539. [Google Scholar] [CrossRef]
- Bartholomew, G.A. The water economy of seed eating birds which survive without drinking. In Proceedings of the 15th International Ornithological Congress, The Hague, The Netherlands, 30 August–5 September 1970; Voous, K.H., Ed.; E. J. Brill: Leiden, The Netherlands, 1972; pp. 237–254. [Google Scholar]
- Griffith, S.C.; Buchanan, K.L. The Zebra Finch: The ultimate Australian supermodel. Emu 2010, 110, v–xii. [Google Scholar] [CrossRef]
- Griffith, S.C.; Ton, R.; Hurley, L.L.; McDiarmid, C.S.; Pacheco-Fuentes, H. The ecology of the Zebra Finch makes it a great laboratory model but an outlier amongst passerine birds. Birds 2021, 2, 60–76. [Google Scholar] [CrossRef]
- Calder, W.A.; King, J.R. Evaporative cooling in the zebra finch. Experientia 1963, 19, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Calder, W.A. Gaseous metabolism and water relations of the zebra finch Taeniopygia castanotis. Physiol. Zool. 1964, 37, 400–413. [Google Scholar] [CrossRef]
- Skadhauge, E.; Bradshaw, S.D. Saline drinking and cloacal excretion of salt and water in the zebra finch. Am. J. Physiol. 1974, 227, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Withers, P.C.; Hurley, L.L.; Griffith, S.C. The field metabolic rate, water turnover, and feeding and drinking behavior of a small avian desert granivore during a summer heatwave. Front. Physiol. 2019, 10, 1405. [Google Scholar] [CrossRef]
- Cooper, C.E.; Hurley, L.L.; Griffith, S.C. Effect of acute exposure to high ambient temperature on the thermal, metabolic and hygric physiology of a small desert bird. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 244, 110684. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.J.; Peter, J.M.; Cowling, S.J. Handbook of Australian, New Zealand and Antarctic Birds. Volume 7. Boatbill to Starlings; Oxford University Press: Melbourne, Australia, 2006. [Google Scholar]
- Menkhorst, P.; Rogers, D.; Clarke, R.; Davies, J.; Marsack, P.; Franklin, K. The Australian Bird Guide, Revised Edition; CSIRO Publishing: Clayton, Victoria, Australia, 2019. [Google Scholar]
- Oksche, A.; Farner, D.S.; Serventy, D.L.; Wolff, F.; Nicholls, C. The hypothalamo-hypophysial neurosecretory system of the Zebra Finch, Taeniopygia castanotis. Zeit. Zellforsch. Mikro. Anat. 1963, 58, 846–914. [Google Scholar] [CrossRef]
- Lee, P.; Schmidt-Nielsen, K. Respiratory and cutaneous evaporation in the zebra finch—Effect on water balance. Am. J. Physiol. 1971, 220, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.J.F. Nomadism as a response to desert conditions in Australia. J. Arid Environ. 1984, 7, 183–195. [Google Scholar] [CrossRef]
- Burbidge, A.A.; Fuller, P.J. Gibson desert birds: Responses to drought and plenty. Emu-Aust. Ornith. 2007, 107, 126–134. [Google Scholar] [CrossRef]
- Jordan, R.; James, A.I.; Moore, D.; Franklin, D.C. Boom and bust (or not?) among birds in an Australian semi-desert. J. Arid Environ. 2017, 139, 58–66. [Google Scholar] [CrossRef]
- MacMillen, R.E. Water economy of granivorous birds—A predictive model. Condor 1990, 92, 379–392. [Google Scholar] [CrossRef]
- Williams, J.B. A phylogenetic perspective of evaporative water loss in birds. Auk 1996, 113, 457–472. [Google Scholar] [CrossRef]
- MacMillen, R.E.; Hinds, D.S. Water economy of granivorous birds: California House Finches. Condor 1998, 100, 493–503. [Google Scholar] [CrossRef]
- Tieleman, B.I.; Williams, J.B.; Bloomer, P. Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc. R. Soc. B Biol. Sci. 2003, 270, 207–214. [Google Scholar] [CrossRef]
- Williams, J.B.; Tieleman, B.I. Physiological adaptation in desert birds. Bioscience 2005, 55, 416–425. [Google Scholar] [CrossRef]
- White, C.R.; Blackburn, T.M.; Martin, G.R.; Butler, P.J. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B Biol. Sci. 2007, 274, 287–293. [Google Scholar] [CrossRef]
- McKechnie, A.E.; Gerson, A.R.; Wolf, B.O. Thermoregulation in desert birds: Scaling and phylogenetic variation in heat tolerance and evaporative cooling. J. Exp. Biol. 2021, 224 (Suppl. S1), jeb229211. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, G.A.; Dawson, W.R. Respiratory water loss in some birds of the south western United States. Physiol. Zool. 1953, 26, 162–166. [Google Scholar] [CrossRef]
- Jetz, W.; Freckleton, R.P.; McKechnie, A.E. Environment, migratory tendency, phylogeny and basal metabolic rate in birds. PLoS ONE 2008, 3, e3261. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Beissinger, S.R. Environmental determinants of total evaporative water loss in birds at multiple temperatures. Auk 2020, 137, ukz069. [Google Scholar] [CrossRef]
- Maclean, G.L. Ecophysiology of Desert Birds; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Bradshaw, D. Vertebrate Ecophysiology: An Introduction to its Principles and Applications; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Williams, J.B.; Tieleman, B.I. Ecological and evolutionary physiology of desert birds: A progress report. Integr. Comp. Biol. 2002, 42, 68–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacMillen, R.E.; Hinds, D.S. Water regulatory efficiency in heteromyid rodents. A model and its application. Ecology 1983, 64, 152–164. [Google Scholar] [CrossRef]
- Cortés, A.; Rosenmann, M.; Bozinovic, F. Water economy in rodents: Evaporative water loss and metabolic water production. Rev. Chil. Hist. Nat. 2000, 73, 311–321. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, B.; Schmidt-Nielsen, K. A complete account of the water metabolism in kangaroo rats and an experimental verification. J. Cell. Comp. Physiol. 1951, 38, 165–181. [Google Scholar] [CrossRef]
- Cooper, C.E.; Withers, P.C.; Turner, J.M. Physiological implications of climate change for a critically endangered Australian marsupial. Aust. J. Zool. 2020, 68, 200–211. [Google Scholar] [CrossRef]
- Dawson, W.R. Evaporative losses of water by birds. Comp. Biochem. Physiol. A Physiol. 1982, 71, 495–509. [Google Scholar] [CrossRef]
- MacMillen, R.E.; Baudinette, R.V. Water economy of granivorous birds: Australian parrots. Func. Ecol. 1993, 7, 704–712. [Google Scholar] [CrossRef]
- Cooper, C.E.; Withers, P.C. Comparative physiology of Australian quolls (Dasyurus; Marsupialia). J. Comp. Physiol. B 2010, 180, 857–868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Withers, P.C. Design, calibration and calculation for flow-through respirometry systems. Aust. J. Zool. 2001, 49, 445–461. [Google Scholar] [CrossRef]
- Withers, P.C. Comparative Animal Physiology; Saunders College Publishing: Philadelphia, PA, USA, 1992. [Google Scholar]
- Henderson-Sellers, B. A new formula for latent heat of vaporization of water as a function of temperature. Quart. J. R. Met. Soc. 1984, 110, 1186–1190. [Google Scholar] [CrossRef]
- Douglas, T.K.; Cooper, C.E.; Withers, P.C. Avian torpor or alternative thermoregulatory strategies for overwintering? J. Exp. Biol. 2017, 220, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Statist. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.5.1. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 11 November 2021).
- Bicudo, J.E.P.W.; Buttemer, W.A.; Chappell, M.A.; Pearson, J.T.; Bech, C. Ecological and Environmental Physiology of Birds; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Marschall, U.; Prinzinger, R. Vergleichende ökophysiologie von fünf prachtfinkenarten (Estrildidae). J. Ornithol. 1991, 132, 319–323. [Google Scholar] [CrossRef]
- Bech, C.; Rønning, B.; Moe, M. Individual variation in the basal metabolism of Zebra finches Taeniopygia guttata: No effect of food quality during early development. Int. Congr. Ser. 2004, 1275, 306–312. [Google Scholar] [CrossRef]
- Rønning, B.; Moe, B.; Bech, C. Long-term repeatability makes basal metabolic rate a likely heritable trait in the zebra finch Taeniopygia guttata. J. Exp. Biol. 2005, 208, 4663–4669. [Google Scholar] [CrossRef]
- Rønning, B.; Jensen, H.; Moe, B.; Bech, C. Basal metabolic rate: Heritability and genetic correlations with morphological traits in the zebra finch. J. Evol. Biol. 2007, 20, 1815–1822. [Google Scholar] [CrossRef]
- Bernstein, M.H. Cutaneous water loss in small birds. Condor 1971, 73, 468–469. [Google Scholar] [CrossRef]
- Weathers, W.W.; Weathers, D.L.; Van Riper, C. Basal metabolism of the Apapane: Comparison of freshly caught birds with long-term captives. Auk 1983, 100, 977–978. [Google Scholar] [CrossRef]
- Warkentin, I.G.; West, N.H. Impact of long-term captivity on basal metabolism in birds. Comp. Biochem. Physiol. A 1990, 96, 379–381. [Google Scholar] [CrossRef]
- Cooper, C.E.; Withers, P.C. Effect of sampling regime on estimation of basal metabolic rate and standard evaporative water loss using flow-through respirometry. Physiol. Biochem. Zool. 2010, 83, 385–393. [Google Scholar] [CrossRef]
- Page, A.J.; Cooper, C.E.; Withers, P.C. Effects of experiment start time and duration on measurement of standard physiological variables. J. Comp. Physiol. B 2011, 181, 657–665. [Google Scholar] [CrossRef]
- Burton, C.T.; Weathers, W.W. Energetics and thermoregulation of the Gouldian Finch (Erythrura gouldiae). Emu 2003, 103, 1–10. [Google Scholar] [CrossRef]
- Aschoff, J.; Pohl, H. Rhythmic variations in energy metabolism. Fed. Proc. 1970, 29, 1541–1552. [Google Scholar]
- Willoughby, E.J. Evaporative water loss of a small xerophilous finch, Lonchura malabarica. Comp. Biochem. Physiol. 1969, 28, 655–664. [Google Scholar] [CrossRef]
- Lovegrove, B.G.; Smith, G.A. Is ‘nocturnal hypothermia’ a valid physiological concept in small birds?: A study on bronze mannikins Spermestes cucullatus. Ibis 2003, 145, 547–557. [Google Scholar] [CrossRef]
- Weathers, W.W. Temperature regulation in the dusky munia, Lonchura fuscens (Cassin) (Estrilidae). Aust. J. Physiol. 1977, 25, 193–199. [Google Scholar]
- Kendeigh, S.C.; Dol’nik, V.R.; Gavrilov, V.M. Avian energetics. In Granivorous Birds in Ecosystems; Pinowski, J., Kendeigh, S.C., Eds.; Cambridge University Press: Cambridge, UK, 1977; pp. 127–204. [Google Scholar]
- Qiao, Q.-G.; Liang, H.-J.; Bai, M.-L.; Zheng, W.-H.; Liu, J.-S. Interspecific variation of thermoregulation between migratory and resident passerines in Wenzhou. Zool. Res. 2016, 37, 167–175. [Google Scholar]
- Lasiewski, R.C.; Hubbard, S.H.; Moberly, W.R. Energetic relationships of a very small passerine bird. Condor 1964, 66, 212–220. [Google Scholar] [CrossRef]
- Stephens, C.M.; Siegel, R.B.; Weathers, W.W. Thermal conductance and basal metabolism of the Orange-cheeked Waxbill (Estrilda melpoda). Ostrich 2001, 72, 114–126. [Google Scholar] [CrossRef]
- Gavrilov, V.M.; Dol’nik, V.R. Basal metabolic rate, thermoregulation and existence energy in birds: World data. In Acta XVIII Congress Internationalis Ornithologici; Ilyichov, V.D., Gavrilov, V.M., Eds.; Nauka: Moscow, Russia, 1985; pp. 421–466. [Google Scholar]
- McKechnie, A.E.; Lovegrove, B.G. Facultative hypothermic responses in an Afrotropical arid-zone passerine, the red-headed finch (Amadina erythrocephala). J. Comp. Physiol. B 2003, 173, 339–346. [Google Scholar] [CrossRef]
- Cooper, C.E.; Withers, P.C. Effects of measurement duration on the determination of basal metabolic rate and evaporative water loss of small marsupials: How long is long enough? Physiol. Biochem. Zool. 2009, 82, 438–446. [Google Scholar] [CrossRef]
- Connolly, M.K.; Cooper, C.E. How do measurement duration and timing interact to influence estimation of basal physiological variables of a nocturnal rodent? Comp. Biochem. Physiol. A 2014, 178, 24–29. [Google Scholar] [CrossRef][Green Version]
- McKechnie, A.E.; Wolf, B.O. The allometry of avian basal metabolic rate: Good predictions need good data. Physiol. Biochem. Zool. 2004, 77, 502–521. [Google Scholar] [CrossRef]
- Bayly, I.A. Review of how indigenous people managed for water in desert regions of Australia. J. R. Soc. West. Aust. 1999, 82, 17–25. [Google Scholar]
- Pacheco-Fuentes, H.; Cooper, C.E.; Withers, P.C.; Griffith, S.C. Re-evaluating model assumptions suggests that Australian birds are more tolerant of heat and aridity than predicted: A response to Conradie et al. (2020). Cons. Physiol. 2022, 10, coac010. [Google Scholar] [CrossRef]
- Davies, S.J.J.F. Results of 40 hours’ continuous watch at five waterpoints in an Australian desert. Emu-Austral Ornithol. 1972, 72, 8–12. [Google Scholar] [CrossRef]
- Fisher, C.D.; Lindgren, E.; Dawson, W.R. Drinking patterns and behavior of Australian desert birds in relation to their ecology and abundance. Condor 1972, 74, 111–136. [Google Scholar] [CrossRef]
- Evans, S.M.; Collins, J.A.; Evans, R.; Miller, S. Patterns of drinking behaviour of some Australian estrildine finches. Ibis 1985, 127, 348–354. [Google Scholar] [CrossRef]
- Bowler, J.M. Aridity in Australia: Age, origins and expression in aeolian landforms and sediments. Earth-Sci. Rev. 1976, 12, 279–310. [Google Scholar] [CrossRef]
- Williams, J.B.; Tieleman, B.I. Physiological ecology and behavior of desert birds. In Current Ornithology; Nolan, V., Thompson, C.F., Eds.; Kluwer Academics/Plenum Publishers: New York, NY, USA, 2001; Volume 16, pp. 299–353. [Google Scholar]
- Dawson, W.R.; Bartholomew, G.A. Temperature regulation and water economy of desert birds. In Desert Biology; Brown, G.W., Ed.; Academic Press: New York, NY, USA, 1968; Volume 1, pp. 357–394. [Google Scholar]
- Cooper, C.E.; Withers, P.C.; Munns, S.L.; Geiser, F.; Buttemer, W.A. Geographical variation in the standard physiology of brushtail possums (Trichosurus): Implications for conservation translocations. Cons. Physiol. 2018, 6, coy042. [Google Scholar] [CrossRef]
- Conradie, S.R.; Woodborne, S.M.; Wolf, B.O.; Pessato, A.; Mariette, M.M.; McKechnie, A.E. Avian mortality risk during heat waves will increase greatly in arid Australia during the 21st century. Cons. Physiol. 2020, 8, coaa048. [Google Scholar] [CrossRef]
- Cooper, C.E.; Hurley, L.L.; Deviche, P.; Griffith, S.C. Physiological responses of wild zebra finches (Taeniopygia guttata) to heatwaves. J. Exp. Biol. 2020, 223, jeb225524. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Withers, P.C.; Cooper, C.E.; Larcombe, A.N. Relative Water Economy Is a Useful Index of Aridity Tolerance for Australian Poephiline Finches. Birds 2022, 3, 172-183. https://doi.org/10.3390/birds3020012
Withers PC, Cooper CE, Larcombe AN. Relative Water Economy Is a Useful Index of Aridity Tolerance for Australian Poephiline Finches. Birds. 2022; 3(2):172-183. https://doi.org/10.3390/birds3020012
Chicago/Turabian StyleWithers, Philip C., Christine E. Cooper, and Alexander N. Larcombe. 2022. "Relative Water Economy Is a Useful Index of Aridity Tolerance for Australian Poephiline Finches" Birds 3, no. 2: 172-183. https://doi.org/10.3390/birds3020012
APA StyleWithers, P. C., Cooper, C. E., & Larcombe, A. N. (2022). Relative Water Economy Is a Useful Index of Aridity Tolerance for Australian Poephiline Finches. Birds, 3(2), 172-183. https://doi.org/10.3390/birds3020012





