Simple Summary
Grey-faced Petrels breed on islands around the upper North Island of New Zealand and raise one chick per year from September to December. We regularly (weekly or fortnightly) monitored the growth rates of chicks from 2011 to 2015 at the colony on Te Hāwere-a-Maki (Goat Island). Monthly variation in tropical Pacific Ocean winds bringing warmer surface air temperatures was found to negatively affect the growth rates of chicks, ultimately causing important annual differences in chick growth rates and colony productivity. This pattern is likely to be consistent across other Grey-faced Petrel colonies on islands in the Hauraki Gulf. Measurements of chicks that were not included in our study at the end of each breeding season showed no negative effects of our regular monitoring on the growth rates of chicks in our study. Due to the combined impacts of predation and climate, the Grey-faced Petrel colony on Te Hāwere-a-Maki remains at a constant size.
Abstract
Grey-faced Petrels (Pterodroma gouldi) are a colonial burrowing seabird predominantly nesting on offshore islands of the upper North Island of New Zealand. We studied their annual breeding biology and the impact of Southern Oscillation Index climatic effects by measuring colony productivity and chick growth rates from 2011 to 2015 on Te Hāwere-a-Maki as unfavorable warmer La Niña conditions changed to favorable cooler El Niño conditions. Across all five years, annual chick hatching consistently occurred within a one-week period at the end of August but fledging variably occurred over a three-week period following Christmas. Because ship rats are pest controlled on Te Hāwere-a-Maki, we found only a slight reduction in breeding success with nearby predator-free islands. However, chick growth and fledging rates were significantly higher under El Niño conditions occurring towards the end of our study, rather than La Niña conditions at the start of our study. Our regular handling of chicks for monitoring had no discernible impact compared to a set of control chicks. The combined impacts of annual variation in predation and climate mean the Grey-faced Petrel colony on Te Hāwere-a-Maki maintains a constant population size of around 100 burrows.
1. Introduction
For many species, survival and reproduction are affected by climatic variation over the breeding period [1]. Climatic variation includes short-term localized weather events, particularly extreme events such as heat waves, storms and flooding, and long-term regional events, such as seasonal and annual variations. The impacts of long-term climate variation are particularly profound for slow-breeding k-selected species with few and altricial offspring [2]. Variations in climate, and consequent indirect effects on prey, can have broad impacts on species [3]. The outcome of such impacts can be the difference between ‘good’ and ‘bad’ breeding years, leading to either population growth or decline, respectively [4], and are likely to be exacerbated by predicted climate change [5].
Pelagic seabirds are highly mobile and for many species their foraging range can be widespread over large marine areas [6]. Oceanic oscillations (naturally occurring atmospheric cycles affecting sea surface temperature, precipitation and wind direction) can impact seabirds through multiple pathways including prevailing wind direction and changes in prey availability due to sea surface temperature [7], but this impact can be variable depending on species [8,9]. Nevertheless, seabird species must breed on land, often in dense colonies. Colony breeding success is therefore impacted by both short-term local events at their terrestrial breeding sites, such as predation and weather, and long-term regional events at marine foraging grounds, such as oceanic oscillations [10]. The impact of climate variation is further exacerbated during reproduction, when seabirds are anchored to breeding colonies and must tend not just to their own physical condition but also that of their offspring and are thus most sensitive to changes in resource availability [11]. Such changes can occur gradually over decades due to oceanic oscillations [7].
The Grey-faced Petrel (Pterodroma gouldi) is a medium-sized winter breeding Procellarid classified as ‘least concern’ with an estimated 250,000 pairs breeding in the upper North Island of New Zealand [12,13], although robust population size estimates and trends are difficult to obtain [14]. Introduced mammalian predators are the primary threat to the Grey-faced Petrel [15] and most colonies are found on predator-free offshore islands. However, weather is known to impact colony attendance [16] and climate variation, particularly the El Niño Southern Oscillation (ENSO: El Niño or La Niña events), is also suspected to impact Grey-faced Petrel colonies. In El Niño years, sea temperatures decrease, and more southerly winds occur in northern New Zealand. In contrast, La Niña brings more north-easterly winds and warmer seas towards northern New Zealand [17]
We investigated the annual breeding success of Grey-faced Petrels for five years over a gradient of shifting ocean-atmosphere conditions from warming La Niña (2011) to cooling El Niño (2015). We hypothesized that inter-annual variation in breeding success could in part be explained by variations in at-sea conditions. Such variations have already been shown to impact adult foraging strategies [18]. We thus predicted a strong gradient in breeding success across the five years of our study correlating with the shifting ENSO conditions over the period of our study.
2. Materials and Methods
2.1. Study Species
Grey-faced Petrels are gadfly petrels recently split from Great-winged Petrels (Pterodroma macroptera) [19]. They are long-lived—up to 40 years—and commence breeding at around five years of age [20]. Adults return to colonies at the start of March each year prospecting burrows until the end of May, and after a pre-breeding exodus in June return to lay a single egg at the start of July, which is alternately incubated by both parents and hatches around the end of August. The chick is left alone and the parents return from alternating short and long trips until the chick fledges around the end of December [21].
2.2. Study Site
We monitored the breeding biology of Grey-faced Petrels from 2011 to 2015 on Te Hāwere-a-Maki, a 9.3 ha island lying 50 metres offshore from the University of Auckland Leigh marine laboratory in the Cape Rodney-Okakari Point (Goat Island) Marine Reserve (Figure 1). The climate in Auckland is classified as warm temperate and maritime with monthly temperatures averaging from 11.5 to 20.0 °C and annual rainfall just over 1000 mm. The island is covered in native broadleaf and podocarp forest and along the coastal margins there is a single, widespread Grey-faced Petrel colony that supports a breeding population of approximately 100 pairs. Most burrows can be found along the southern landward coast. Reinfestation of introduced mammalian pests is common because of the island’s proximity to the coast. Ship rats (Rattus rattus) annually swim across the 50 m wide channel to reach Te Hāwere-a-maki [22] and during the course of our study were managed ongoingly around the colonies with snap-traps [23]. Further south on the east coast lying further offshore are the larger islands of Motuora (79.8 ha) and Tiritiri Matangi (187.6 ha), which we monitored only in 2011 to investigate consistency in chick growth rates and survival across islands. Introduced rats have historically been eradicated from Tiritiri Matangi (1993) and have never been present on Motuora [24]. Both islands were farmed but have been restored to native broadleaf forest over recent decades. All three islands are managed by the New Zealand Department of Conservation as reserves with public access.
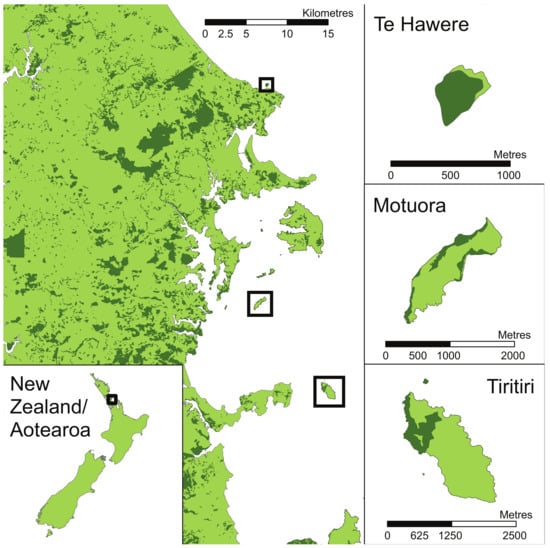
Figure 1.
North Auckland, New Zealand, with study islands inset.
2.3. Field Methods
On Te Hāwere-a-Maki, we annually identified 22 suitable burrows for monitoring when adults were incubating eggs in July, selecting burrows based on the presence of an incubating adult and our ability to regularly handle and monitor chicks (where necessary and possible through a study entrance located by the burrow chamber). Although pairs tend to be monogamous and re-use the same burrow each year, we did not necessarily monitor the same burrows each year. On Motuora and Tiritiri Matangi, we monitored 24 and 15 burrows, respectively. All adults and chicks handled were marked with a uniquely numbered steel ring to enable individual re-identification. The colony was visited either weekly or fortnightly (Table 1), weather dependent, to assess burrow status (egg, chick, abandoned) and take morphological measurements of chicks. Around egg hatching and chick fledging, weekly visits were increased to twice a week to obtain more accurate estimates of these dates. We took five morphological measurements of chicks at each inspection: body mass (grams) using a 1000-g Pesola scale, maximum flattened chord wing length (mm) using a steel butt-stopped rule, and false tarsus length (mm), bill culmen length (mm) and bill depth at nostrils (mm) using a pair of calipers (see [25]), although bill measurements were not subsequently used in analysis. From 2011 to 2013, we also measured unmonitored chicks on Te Hāwere-a-Maki once only at the end of the breeding season as a control to determine whether our regular handling of chicks may have negatively affected their growth rates. Successful fledging was assumed when chicks were last recorded in adult plumage and good health just prior to expected departure, and on a subsequent inspection no further sign was found. We calculated the percentage of eggs hatched and percentage of chicks fledged (from eggs laid).
2.4. Statistical Methods
We tested for any differences in hatching and fledging proportions across islands and years using analysis of variance. We tested for any effect of our handling on chick body masses with an analysis of variance comparing study and control chicks on Te Hāwere-a-Maki with year as a covariate. We then conducted three growth rate investigations: (i) whether growth curves differed across islands in 2011 (‘island effect’); (ii) whether growth curves differed annually from 2011 to 2015 on Te Hāwere-a-Maki (‘annual effect’); and (iii) whether growth curves differed with the monthly Southern Oscillation Index from 2011 to 2015 on Te Hāwere-a-Maki (‘climate effect’). Although growth curves would fit a more biologically appropriate model to growth rates, they do not easily allow the incorporation of complex fixed and random effects [26]. We thus chose to analyze chick growth rates for each morphological measure using more simplistic linear mixed models but including age as an interaction term with each covariate. Mixed models allowed us to incorporate fixed effects for chick age and survivorship (fledged or not), and either island, year or climate, with a random effect for chicks to account for repeated measures. Investigating the interaction of each covariate with age allows us to determine where our growth curves start at identical values at hatching but diverge over time. Chick age was measured as days since hatching. When the hatching date was not known, we estimated it based on morphometrics at the time of first measurement. To meet assumptions of normality, body mass was squared, wing length log transformed and tarsus length cubed. We assessed ENSO conditions using Southern Oscillation Index (SOI) data obtained from the Climatic Research Unit of the University of East Anglia [27]. All analyses were conducted in R 4.1.0 [28] with α = 0.05 for significance and we used package nlme for mixed models [29].
3. Results
We monitored 149 burrows where eggs were laid and incubated (Table 1). The average hatching proportion across burrows was 0.80 and did not differ significantly between islands or years, and fledging proportion across burrows (excluding breeding failure due to stoat (Mustela erminea) invasion and predation of all chicks on Te Hāwere-a-Maki in 2014) was 0.47, not differing significantly between islands but differing significantly by year. On Te Hāwere-a-Maki, only 60% of hatched chicks fledged (excluding stoat invaded Te Hāwere-a-Maki in 2014).

Table 1.
Hatching and fledging proportions and average dates (estimated hatching date and last measurement prior to fledging) for burrows monitored from 2011 to 2015. SOI = Average Southern Oscillation Index over the chick-rearing period from August to January (positive = La Niña, negative = El Niño). NA = not available.
Table 1.
Hatching and fledging proportions and average dates (estimated hatching date and last measurement prior to fledging) for burrows monitored from 2011 to 2015. SOI = Average Southern Oscillation Index over the chick-rearing period from August to January (positive = La Niña, negative = El Niño). NA = not available.
| Year | Island | Visits | Burrows | Hatched | Date | Fledged | Date | SOI |
|---|---|---|---|---|---|---|---|---|
| 2011 | Motuora | fortnightly | 24 | 0.88 | NA | 0.54 | NA | +1.15 |
| 2011 | Tiritiri | weekly | 15 | 0.93 | 30 August | 0.47 | 13 January | +1.15 |
| 2011 | Te Hāwere | weekly | 22 | 0.86 | 26 August | 0.36 | 5 January | +1.15 |
| 2012 | Te Hāwere | fortnightly | 22 | 0.77 | 25 August | 0.41 | 31 December | −0.15 |
| 2013 | Te Hāwere | weekly | 22 | 0.68 | 28 August | 0.45 | 7 January | +0.29 |
| 2014 | Te Hāwere | weekly | 22 | 0.82 | 25 August | 0 1 | ˗ | −0.91 |
| 2015 | Te Hāwere | fortnightly | 22 | 0.73 | 30 August | 0.59 | 22 December | −1.58 |
1 stoat invasion led to catastrophic breeding failure.
We obtained a total of 1000 morphological records for 126 (91%) of the 138 chicks which were included in our study (including control chicks). In both 2012 and 2014 on Te Hāwere-a-Maki, six chicks were depredated by ship rats before any measurements could be taken. Some measurements were missing for 1% of our records when the chick had just hatched or the measurement was taken incorrectly or overlooked. At the end of each breeding season, we found no significant effect of handling on the final body mass of the study chicks (n = 25) when compared to a sample of control chicks (n = 18), which were measured at the same time but had only been handled once.
The Southern Oscillation Index varied significantly between years over the course of our study (Figure 2), with a shift from warmer La Niña conditions in 2011 to cooler El Niño conditions in 2015. In our island effect model, we found no significant differences in growth rates of chick body mass, wing length and tarsus length among islands. In our annual effect model, we found significant differences in growth rates of chick body mass (Figure 3a), wing length (Figure 4a) and tarsus length (Figure 5a) among years. In our climate effect model, we found no significant differences in growth rates of chick body mass (Figure 3b), but we did find significant differences in wing length (Figure 4b) and tarsus length (Figure 5b), associated with the monthly Southern Oscillation Index. At 125 days since hatching (corresponding to around New Year’s Day), fledging chicks are predicted on average to have 7.7 mm longer wings (287.5 vs. 279.8) and 0.3 mm longer tarsi (53.0 vs. 52.7) in El Niño years (SOI = −1.0) compared to La Niña years (SOI = +1.0).
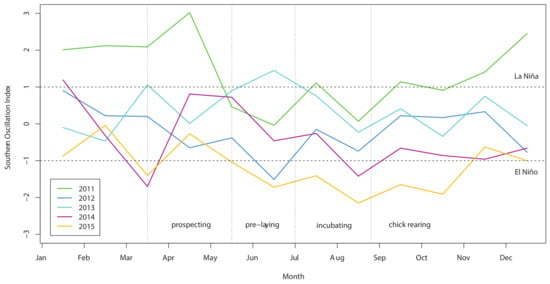
Figure 2.
Monthly Southern Oscillation Index from 2011 to 2015. Horizontal bars indicate thresholds for declaring La Niña and El Niño events. Vertical bars indicate breeding stages for Grey-faced Petrel.
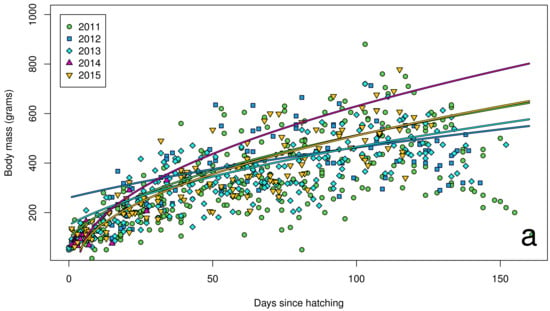
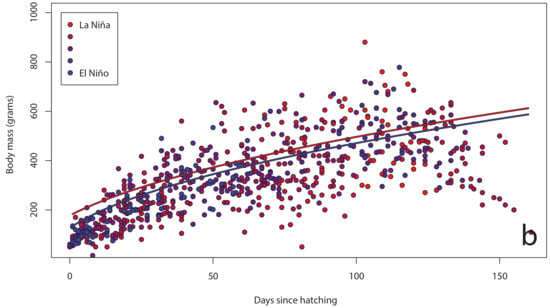
Figure 3.
Body mass growth curves for chicks (n = 72) on Te Hāwere-a-Maki from 2011 to 2015: (a) annually; (b) monthly Southern Oscillation Index. Lines of best fit indicate years and SOI = –1 or +1.
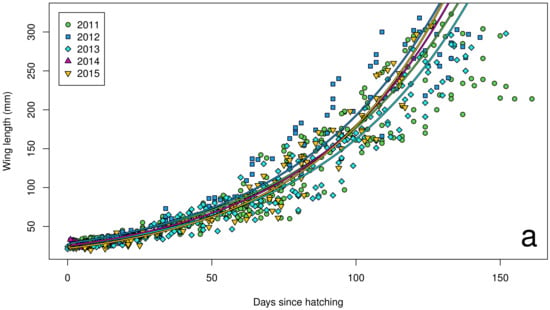
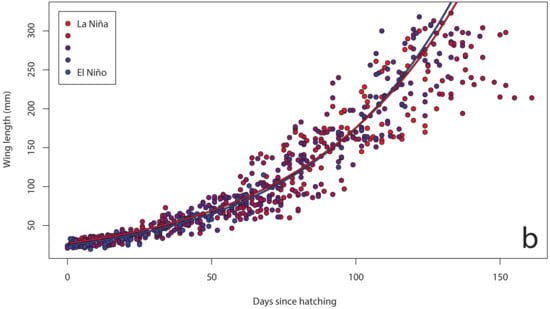
Figure 4.
Wing length growth curves for chicks (n = 72) on Te Hāwere-a-Maki from 2011 to 2015: (a) annually; (b) monthly Southern Oscillation Index. Lines of best fit indicate years and SOI = –1 or +1.
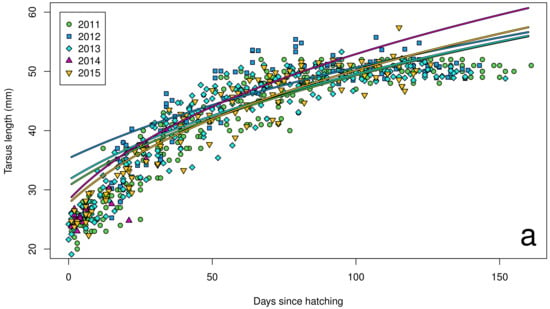
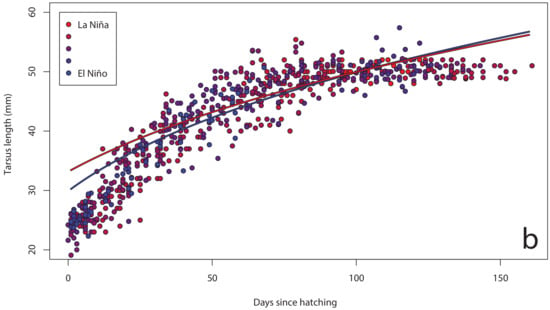
Figure 5.
Tarsus length growth curves for chicks (n = 72) on Te Hāwere-a-Maki from 2011 to 2015: (a) annually; (b) monthly Southern Oscillation Index. Lines of best fit indicate years and SOI = –1 or +1.
4. Discussion
We found significant variation in Grey-faced Petrel chick growth rates and colony reproductive success across five years, which could be predicted by the intensity of the Southern Oscillation Index. This result confirmed our prediction and has been found consistently for other seabird species during other ENSO events in the Pacific Ocean (e.g., [8,9]). Grey-faced Petrels fared better in El Niño years, when sea temperatures decrease and more southerly winds occur in northern New Zealand. This climatic impact was consistent across nearby colonies based on one year of monitoring, when we found no difference in chick growth rates across islands. These results contain nuance, however. No impact of monthly SOI was found on chick body masses, only on wing and tarsus length. Chick wings and tarsi grow consistently across a season and provide a conservative measure of chick condition over an extended period of nourishment. In contrast, chick body masses fluctuate significantly based on time since last provisioning, and thus are a less reliable measure of long-term chick condition, until they grossly differ from growth curves [25].
Importantly, we established a significant effect of monthly variation in SOI on chick growth rates in addition to the broad inter-annual trends that others have already confirmed in petrels due to ENSO events [30]. Annual averages of SOI provide a means to classify an entire season as either weak or strong La Niña or El Niño events, but within such years, significant monthly variation in the intensity of the SOI can still occur, including whether the event takes place earlier or later in the season [17]. For example, in 2011 La Niña conditions intensified over the chick rearing period, while in 2015 El Niño conditions diminished over the chick rearing period (Figure 2). This nuanced effect was further evidenced in our annual models, where complex interactions between the year and days since hatching indicated that the timing within a year of any annual impact differed between years.
Hatching dates for Grey-faced Petrels were generally consistent across our five-year study, with eggs laid around the same one-week period at the end of August. Any variation in hatching date can be attributed to sample variation. In contrast, fledging dates varied markedly across our five-year study, with chicks fledging over a three-week period commencing around Christmas. This variation in fledging date can be directly attributed to the variation in the Southern Oscillation Index, as has been found elsewhere [30]. However, it is important to note that while our hatching dates are directly estimated, our fledging dates are partially contrived, being dependent upon the dates of our last inspections subsequent to a bird fledging. They are hence slight underestimates of actual fledging dates. In 2011, we estimated this bias to be about five days.
Seabirds are vulnerable to multiple threats [31]. On Te Hāwere-a-Maki, introduced rats are pest controlled [23] and so breeding success was only slightly below that on nearby predator-free islands. However, a catastrophic invasion event such as by stoats can still lead to colony reproductive failure in a given year, even though stoats are unlikely to permanently reside on small seabird islands [32]. The effects of annual variations in climate in addition to predators can thus be the difference between positive and negative growth for a colony. This has also been demonstrated on southern islands of New Zealand where the Sooty Shearwater (Ardenna grisea) breeds, although for this species the climatic effect is opposite in direction with improved breeding success in La Niña years [33,34]. The Grey-faced Petrel colony on Te Hāwere-a-Maki has remained constant in size around approximately 100 burrows for at least two decades (JCR unpubl. data), which suggests that the combined impacts of ongoing rat predation and poor breeding La Niña years maintain the population at a constant size. The benefits of intensified pest control over the past decade [35] may take further time to be reflected in the colony size given the slow population growth rate of petrels [36], but predicted climate change may also create additional impacts [37], although it is not strong around north-eastern New Zealand [38].
Despite our efforts, our study had a number of limitations. Our study was not able to monitor post-fledging survival at-sea and chicks that fledge in poor conditions may experience elevated mortality, further exacerbating the impacts of poor breeding seasons [39]. Ultimately, the most appropriate measure of reproductive success is recruitment back into a population. Although our sample sizes were small, they represent a substantial fraction of the colony (e.g., nearly a quarter of burrows on Te Hāwere-a-Maki) and thus are representative of the colony. Although we did not account for parental identity across our burrows, doing so would only have increased precision by further reducing random variation in breeding success. It was reassuring in our study from the use of control burrows that the repeated regular handling of chicks, which occasionally elicited the standard seabird defense mechanism of regurgitation, did not affect their long-term body condition. Establishing this was critical in our study and we urge other seabird scientists to routinely assess the impacts of their studies on their target species [40]. The ENSO is likely to affect other seabird species, although the direction cannot always be predicted [8], and so future research should investigate the causal mechanism by which the Southern Oscillation Index impacted Grey-faced Petrel chick growth rates. We recommend ongoing management of introduced mammalian predators on Te Hāwere-a-Maki to mitigate the impacts of negative climatic effects on breeding success.
Author Contributions
Conceptualization, J.C.R.; Data Collection, J.C.R., J.R.W., K.B., S.D., R.D., M.R.F., M.J.R.; Analysis, J.C.R.; Writing, J.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Auckland Early Career Research Excellence Award to J.C.R.; European Commission Marie Curie International Outgoing Fellowship to K.B.; Forest and Bird J.S. Watson Trust grant to K.B.
Institutional Review Board Statement
The study was approved by the Animals Ethics Committee of the University of Auckland (R898 and R1373) and the New Zealand Department of Conservation (Wildlife Act Authorities AK-31697, 34780-RES, 38573-FAU and permit to band birds 2010/021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in FigShare at 10.17608/k6.auckland.17088584 and 10.17608/k6.auckland.17088608.
Acknowledgments
Our thanks are extended to the landowners and kaitiaki who granted us access to islands; Ngāti Manuhiri, Supporters of Tiritiri Matangi and the Motuora Restoration Society. Thanks to the field assistants who helped collect data, and Leigh Marine laboratory staff for supporting access to islands. Thanks to Katherine Russell for the map, and two anonymous reviewers and the editor for feedback on an earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grosbois, V.; Gimenez, O.; Gaillard, J.M.; Pradel, R.; Barbraud, C.; Clobert, J.; Weimerskirch, H. Assessing the impact of climate variation on survival in vertebrate populations. Biol. Rev. 2008, 83, 357–399. [Google Scholar] [CrossRef]
- Bronson, F.H. Climate change and seasonal reproduction in mammals. Phil. Trans. Roy. Soc. B 2009, 364, 3331–3340. [Google Scholar] [CrossRef] [Green Version]
- White, T.C.R. The role of food, weather and climate in limiting the abundance of animals. Biol. Rev. 2008, 83, 227–248. [Google Scholar] [CrossRef]
- Frederiksen, M.; Harris, M.P.; Daunt, F.; Rothery, P.; Wanless, S. Scale-dependent climate signals drive breeding phenology of three seabird species. Glob. Chang. Biol. 2004, 10, 1214–1221. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beal, M.; Dias, M.P.; Phillips, R.A.; Oppel, S.; Hazin, C.; Pearmain, E.J.; Catry, P. Global political responsibility for the conservation of albatrosses and large petrels. Sci. Adv. 2021, 27, eabd7225. [Google Scholar] [CrossRef]
- Watanuki, Y.; Yamamoto, M.; Okado, J.; Ito, M.; Sydeman, W. Seabird reproductive responses to changing climate and prey communities are mediated by prey packaging. Mar. Ecol. Prog. Ser. 2022, 683, 179–194. [Google Scholar] [CrossRef]
- Anderson, D.J. Differential responses of boobies and other seabirds in the Galápagos to the 1986–1987 El Nino-Southern Oscillation event. Mar. Ecol. Prog. Ser. 1989, 52, 209–216. [Google Scholar] [CrossRef]
- Schreiber, R.W.; Schreiber, E.A. Central Pacific seabirds and the El Niño southern oscillation: 1982 to 1983 perspectives. Science 1984, 225, 713–716. [Google Scholar] [CrossRef]
- Gjerdrum, C.; Vallée, A.M.; St Clair, C.C.; Bertram, D.F.; Ryder, J.L.; Blackburn, G.S. Tufted puffin reproduction reveals ocean climate variability. Proc. Natl. Acad. Sci. USA 2003, 100, 9377–9382. [Google Scholar] [CrossRef] [Green Version]
- Sauve, D.; Charmantier, A.; Hatch, S.A.; Friesen, V.L. Environmental conditions variably affect growth across the breeding season in a subarctic seabird. Oecologia 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.S.; Taylor, G.A.; Earl, R. Distribution, population status and trends of grey-faced petrel (Pterodroma macroptera gouldi) in the northern North Island, New Zealand. Notornis 2015, 62, 143–161. [Google Scholar]
- Miskelly, C.M.; Gilad, D.; Taylor, G.A.; Tennyson, A.J.; Waugh, S.M. A review of the distribution and size of gadfly petrel (Pterodroma spp.) colonies throughout New Zealand. Tuhinga 2019, 30, 93–173. [Google Scholar]
- Russell, J.C.; Welch, J.R.; Dromzée, S.; Bourgeois, K.; Thoresen, J.; Earl, R.; McNutt, K. Developing a National Framework for Monitoring the Grey-Faced Petrel (Pterodroma gouldi) as an Indicator Species; DOC Research and Development Series; Department of Conservation: Wellington, New Zealand, 2017; Volume 350, pp. 1–19.
- Stolpmann, L.M.; Landers, T.J.; Russell, J.C. Camera trapping of Grey-faced Petrel (Pterodroma gouldi) breeding burrows reveals interactions with introduced mammals throughout the breeding season. Emu Austral Ornith. 2019, 119, 391–396. [Google Scholar] [CrossRef]
- Ross, E.L.; Brunton, D. Seasonal trends and nightly variation in colony attendance of grey-faced petrels (Pterodroma macroptera gouldi). Notornis 2002, 49, 153–157. [Google Scholar]
- Mullan, A.B. On the linearity and stability of Southern Oscillation-climate relationships for New Zealand. Int. J. Climat. 1995, 15, 1365–1386. [Google Scholar] [CrossRef]
- Bourgeois, K.; Welch, J.R.; Dromzée, S.; Taylor, G.A.; Russell, J.C. Flexible foraging strategies in a highly pelagic seabird revealed by seasonal isotopic niche variation. Mar. Biol. 2022, 169, 28. [Google Scholar] [CrossRef]
- Wood, J.R.; Lawrence, H.A.; Scofield, R.P.; Taylor, G.A.; Lyver, P.O.; Gleeson, D.M. Morphological, behavioural, and genetic evidence supports reinstatement of full species status for the grey-faced petrel, Pterodroma macroptera gouldi (Procellariiformes: Procellariidae). Zool. J. Linn. Soc. 2017, 179, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.J.; Clifford, H.; Fletcher, D.; Cuming, P.; Lyver, P.O.B. Survival and age-at-first-return estimates for grey-faced petrels (Pterodroma macroptera gouldi) breeding on Mauao and Motuotau Island in the Bay of Plenty, New Zealand. Notornis 2011, 58, 71–80. [Google Scholar]
- Imber, M.J. Breeding biology of the grey-faced petrel Pterodroma macroptera gouldi. Ibis 1976, 118, 51–64. [Google Scholar] [CrossRef]
- Russell, J.C.; Mackay, J.W.; Abdelkrim, J. Insular pest control within a metapopulation context. Biol. Cons. 2009, 142, 1404–1410. [Google Scholar] [CrossRef]
- Pichlmueller, F.; Russell, J.C. Survivors or reinvaders? Intraspecific priority effect masks reinvasion potential. Biol. Cons. 2018, 227, 213–218. [Google Scholar] [CrossRef]
- Clout, M.N.; Russell, J.C. The eradication of mammals from New Zealand islands. In Assessment and Control of Biological Invasion Risks; Koike, F., Clout, M.N., Kawamichi, M., De Poorter, M., Iwatsuki, K., Eds.; IUCN: Gland, Switzerland, 2006; pp. 127–141. [Google Scholar]
- Bourgeois, K.; Dromzée, S.; Welch, J.R.; Russell, J.C. Sex and geographic variation in grey-faced petrel (Pterodroma gouldi) morphometrics. Waterbirds 2017, 40, 144–153. [Google Scholar] [CrossRef]
- Hart, D.R.; Chute, A.S. Estimating von bertalanffy growth parameters from growth increment data using a linear mixed-effects model, with an application to the sea scallop Placopecten magellanicus. ICES J. Mar. Sci. 2009, 66, 2165–2175. [Google Scholar] [CrossRef]
- Ropelewski, C.F.; Jones, P.D. An extension of the Tahiti–Darwin southern oscillation index. Mon. Weather Rev. 1987, 115, 2161–2165. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 7 February 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-152. 2021. Available online: https://CRAN.R-project.org/package=nlme (accessed on 7 February 2022).
- Cruz, J.B.; Cruz, F. Effect of El Niño-Southern Oscillation conditions on nestling growth rate in the Dark-rumped Petrel. Condor 1990, 92, 160–165. [Google Scholar] [CrossRef]
- Rodríguez, A.; Arcos, J.M.; Bretagnolle, V.; Dias, M.P.; Holmes, N.D.; Louzao, M.; Chiaradia, A. Future directions in conservation research on petrels and shearwaters. Front. Mar. Sci. 2019, 6, 94. [Google Scholar] [CrossRef]
- Miskelly, C.M.; Tennyson, A.J.; Stahl, J.C.; Smart, A.F.; Edmonds, H.K.; McMurtrie, P.G. Breeding petrels of Dusky Sound, Fiordland–survivors from a century of stoat invasions. Notornis 2017, 64, 136–153. [Google Scholar]
- McKechnie, S.; Fletcher, D.; Newman, J.; Bragg, C.; Dillingham, P.W.; Clucas, R.; Moller, H. Separating the effects of climate, bycatch, predation and harvesting on tītī (Ardenna grisea) population dynamics in New Zealand: A model-based assessment. PLoS ONE 2020, 15, e0243794. [Google Scholar] [CrossRef]
- Fletcher, D.; Newman, J.; McKechnie, S.; Bragg, C.; Dillingham, P.; Clucas, R.; Moller, H. Projected impacts of climate change, bycatch, harvesting, and predation on the Aotearoa New Zealand tītī Ardenna grisea population. Mar. Ecol. Prog. Ser. 2021, 670, 223–238. [Google Scholar] [CrossRef]
- Gronwald, M.; Russell, J.C. Behaviour of invasive ship rats, Rattus rattus, around Goodnature A24 self-resetting traps. Manag. Biol. Inv. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Russell, J.C.; Lecomte, V.; Dumont, Y.; Le Corre, M. Intraguild predation and mesopredator release effect on long-lived prey. Ecol. Mod. 2009, 220, 1098–1104. [Google Scholar] [CrossRef]
- Brothers, N.; Bone, C. The response of burrow-nesting petrels and other vulnerable bird species to vertebrate pest management and climate change on sub-Antarctic Macquarie Island. Pap. Proc. Roy. Soc. Tasmania 2008, 142, 123–148. [Google Scholar] [CrossRef] [Green Version]
- Shears, N.T.; Bowen, M.M. Half a century of coastal temperature records reveal complex warming trends in western boundary currents. Sci. Rep. 2017, 7, 14527. [Google Scholar] [CrossRef]
- Votier, S.C.; Birkhead, T.R.; Oro, D.; Trinder, M.; Grantham, M.J.; Clark, J.A.; Hatchwell, B.J. Recruitment and survival of immature seabirds in relation to oil spills and climate variability. J. Anim. Ecol. 2008, 77, 974–983. [Google Scholar] [CrossRef]
- Carey, M.J. The effects of investigator disturbance on procellariiform seabirds: A review. N. Z. J. Zool. 2009, 36, 367–377. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).