Influence of Habitat and Food Resource Availability on Common Raven Nest Site Selection and Reproductive Success in Mediterranean Forests
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
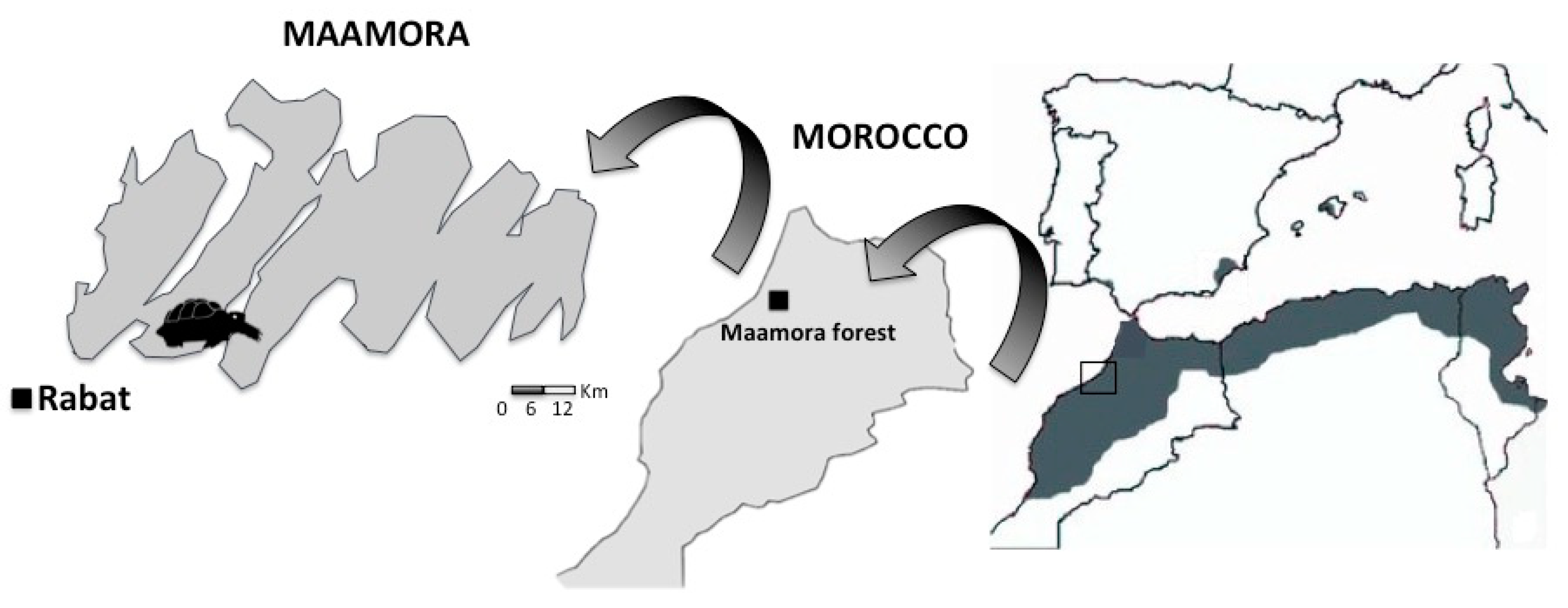
2.1. Study Area
2.2. On the Study Species
2.3. Sampling Common Ravens and Tortoises
2.4. Environmental Characteristics and Anthropogenic Influence
2.5. Modeling Nest Site Selection and Reproductive Success
3. Results
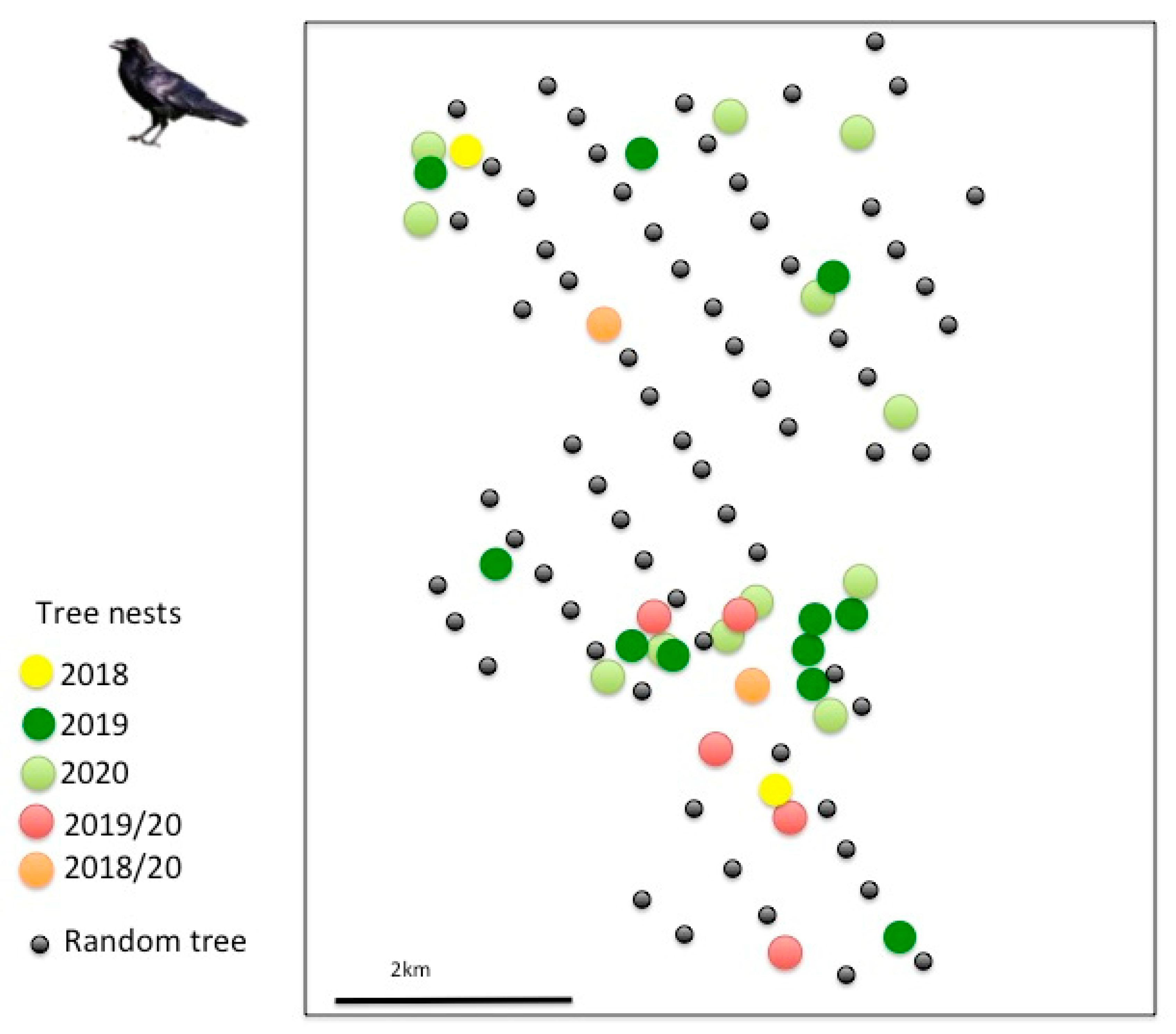
3.1. Tree Selection for Nesting
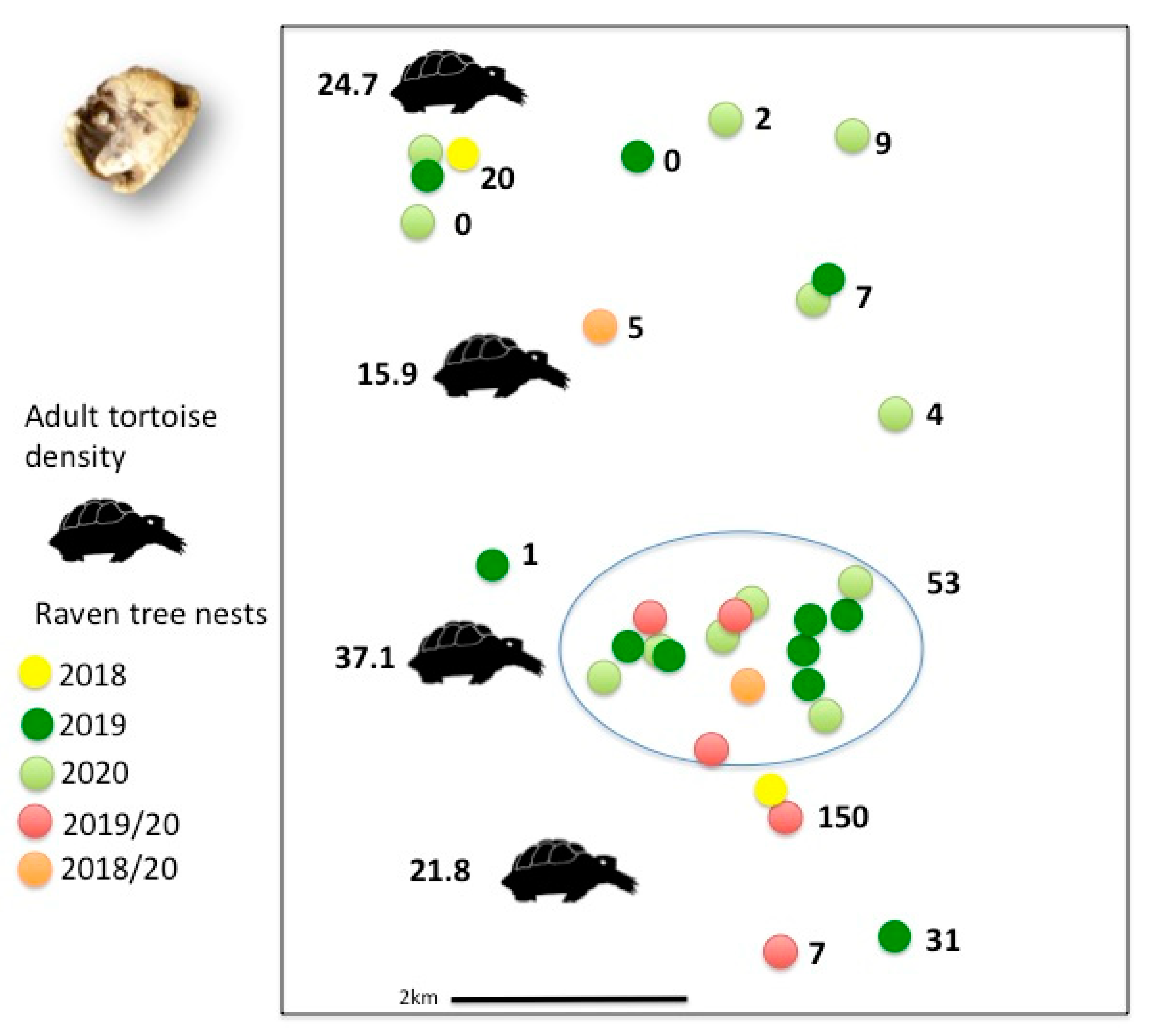
3.2. Reproductive Success and Predation of Young Tortoises
4. Discussion
4.1. Common Raven Nest Site Selection
4.2. Common Raven Reproductive Success and Predation on Young Tortoises
4.3. Implication for Conservation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, T.E. Avian life history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 1995, 65, 101–127. [Google Scholar] [CrossRef]
- Jara, R.F.; Crego, R.D.; Samuel, M.D.; Rozzi, R.; Jiménez, J.E. Nest-site selection and breeding success of passerines in the world´s southernmost forests. PeerJ 2020, 8, e9892. [Google Scholar] [CrossRef]
- Ewins, P.J. Osprey (Pandium haliaetus) populations in forest areas of North America: Changes, their causes and management recommendations. J. Raptor Res. 1997, 31, 138–150. [Google Scholar]
- Petty, S.J.; Avery, M.I. Forest Bird Communities; Occasional Papers 26; Forestry Commission: Edinburgh, Scotland, 1990. [Google Scholar]
- Barrientos, R.; Arroyo, B. Nesting habitat selection of Mediterranean raptors in managed pinewoods: Searching for Common patterns to derive conservation recommendations. Bird Conserv. Int. 2014, 24, 138–151. [Google Scholar] [CrossRef]
- Burke, A.D. Mature Forest Breeding Bird Use of Early Successional Habitat. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2013. [Google Scholar]
- Ratcliffe, D. The Raven; T. & A.D. Poyser: London, UK, 1997. [Google Scholar]
- Dunk, J.R.; Smith, R.N.; Cain, S.L. Nest-site selection and reproductive success in common ravens. Auk 1997, 114, 116–120. [Google Scholar] [CrossRef]
- Holyoak, D. A comparative study of the food of some British corvidae. Bird Study 1968, 15, 147–153. [Google Scholar] [CrossRef]
- Andrén, H. Corvid density and nest predation in relation to forest fragmentation: A landscape perspective. Ecology 1992, 73, 794–804. [Google Scholar] [CrossRef]
- Loretto, M.-C.; Reimann, S.; Schuster, R.; Graulich, D.M.; Bugnyar, T. Shared space, individually used: Spatial behavior of non-breeding ravens (Corvus corax) close to a permanent anthropogenic food source. J. Ornithol. 2016, 157, 439–450. [Google Scholar] [CrossRef]
- Beck, K.B.; Loretto, M.-C.; Bugnyar, T. Effects of site fidelity, group size and age on food-caching behaviour of common ravens, Corvus corax. Anim. Behav. 2020, 164, 51–64. [Google Scholar] [CrossRef]
- Baltensperger, A.P.; Mullet, T.C.; Schmid, M.S. Seasonal observations and machine-learning-based spatial model predictions for the common raven (Corvus corax) in the urban, sub-arctic environment of Fairbanks, Alaska. Polar Biol. 2013, 36, 1587–1599. [Google Scholar] [CrossRef]
- Davis, P.E.; Davis, J.E. The breeding biology of a Raven population in central Wales. Nat. Wales 1986, 3, 44–54. [Google Scholar]
- Rosner, S.; Selva, N.; Mueller, T.; Pugacewicz, E. Raven Corvus corax ecology in a primeval temperate forest. In Ptaki krukowate Polski (Corvids of Poland); Jerzak, L., Kavanagh, B.P., Tryjanowski, P., Eds.; Bogucky Wyd. Nauk: Poznan, Poland, 2005. [Google Scholar]
- Engel, K.A.; Young, L.S. Spatial and temporal patterns in the diet of common ravens in southwester Idaho. Condor 1989, 91, 372–378. [Google Scholar] [CrossRef]
- Amat, J.A.; Obeso, J.R. Alimentación del Cuervo (Corvus corax) en un ambiente marismeño. Ardeola 1989, 36, 219–224. [Google Scholar]
- Sara, M.; Busalacchi, D. Diet and feeding habits of nesting and non-nesting ravens (Corvus corax) on a Mediterranean island (Vulcano, Eolian archipelago). Ethol. Conserv. Evol. 2003, 15, 119–131. [Google Scholar] [CrossRef]
- Nogales, M. High density and distribution patterns of a raven Corvus corax population on an oceanic island (El Hierro, Canary islands). J. Avian Biol. 1994, 25, 80–84. [Google Scholar] [CrossRef][Green Version]
- Knight, R.L.; Camp, R.J. Common ravens and number and type of linear rights-of-way. Biol. Conserv. 1995, 74, 65–67. [Google Scholar] [CrossRef]
- Boarman, W.I.; Heindrich, B. Common raven Corvus corax. Birds N. Am. 1999, 476, 1–31. [Google Scholar]
- Boarman, W.I.; Camp, R.J.; Hagan, M.; Deal, W. Raven Abundance at Anthropogenic Resources in the Western Mojave Desert, California; Report to Edwards Air Force Base; Edwards Air Force Base: Edwards, CA, USA, 1995. [Google Scholar]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Tellería, J.L. Passerine bird communities of Iberian dehesas: A review. Anim. Biod. Cons. 2001, 24, 67–78. [Google Scholar]
- Rosalino, L.M.; Rosario, J.; Santos-Reis, M. The role of habitat patches on mammalian diversity in cork oak agroforestry systems. Acta Oecol. 2009, 35, 507–512. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Aguiar, C.A.S.; Niemela, J.; Sousa, J.P.; Serrano, A.R.M. Cork oak woodlands as key habitats for biodiversity conservation in Mediterranean landscapes: A case study using rove and ground beetles (Coleoptera: Staphylinidae, Carabidae). Biod. Conser. 2009, 18, 605–619. [Google Scholar] [CrossRef]
- Segura, A. How does vegetation structure influence woodpeckers and secondary cavity nesting birds in African cork oak forest? Acta Oecol. 2017, 83, 22–28. [Google Scholar] [CrossRef]
- Lahssini, S.; Lahlaoi, H.; Alaoui, H.M.; Hlal, E.; Bagaram, M.; Ponette, Q. Predicting cork oak suitability in Maamora forest using random forest algorithm. J. Geog. Inf. Syst. 2015, 7, 202–210. [Google Scholar]
- Segura, A.; Jimenez, J.; Acevedo, P. Predation of young tortoises by ravens: The effect of habitat structure on tortoise detectability and abundance. Sci. Rep. 2020, 10, 1874. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Acevedo, P. The importance of protected and unprotected areas for the Mediterranean Spur-thighed tortoise demography in northwest Morocco. Amphib.-Reptil. 2019, 40, 369–371. [Google Scholar] [CrossRef]
- Aafi, A. Etude de la Diversité Floristique de L´Écosystème de Chene-Liège de la Foret de la Maamora. Ph.D. Thesis, Institut Agron et Vétér Hassan II, Rabat, Morocco, 2007. [Google Scholar]
- Giménez, A.; Esteve-Selma, M.A.; Pérez, I.; Anadón, J.D.; Martínez, M.A.; Martínez-Fernández, J. La Tortuga mora en la region de Murcia. In Conservación de Una Especie Amenazada; Diego Marin Librero Editor SL: Murcia, Spain, 2005. [Google Scholar]
- Rosner, S.; Selva, N. Use of the bait-marking method to estimate the territory size of scavenging birds a case study on ravens Corvus corax. Wildl. Biol. 2005, 11, 183–191. [Google Scholar] [CrossRef]
- Haffer, J.; Kirchner, H. Corvus corax—Kolkrabe. In Handbuch der Vögel Mitteleuropas; Von Blotzheim, U.G., Bauer, K., Bezzel, E., Eds.; AULA: Wiesbaden, Germany, 1993; pp. 1947–2022. [Google Scholar]
- Dall, S.R.X.; Wright, J. Rich pickings near large communal roosts favor ‘gang’ foraging by juvenile common ravens, Corvus corax. PLoS ONE 2009, 4, e4530. [Google Scholar] [CrossRef]
- Heinrich, B.; Kaye, D.; Knight, T.; Schaumburg, K. Dispersal and association among common ravens. Condor 1994, 96, 545–551. [Google Scholar] [CrossRef]
- Stiehl, R.B. Brood chronology of the common raven. Wilson Bull. 1985, 97, 78–87. [Google Scholar]
- Kristan, W.B., III; Boarman, W.I.; Crayon, J. Diet composition of Common ravens across the urban-wildland interface of the West Mojave Desert. Wildl. Soc. Bull. 2004, 32, 244–253. [Google Scholar] [CrossRef]
- Birdlife International. Corvus corax (Amended Version Published in 2016) the IUCN Red List of Threatened Species 2017: e. T22706068A113271893; Birdlife International: Cambridge, UK, 2017. [Google Scholar]
- Liebezeit, J.R.; George, T.L. A Summary of Predation by Corvids on Threatened and Endangered Species in California and Management Recommendations to Reduce Corvid Predation; Calif. Dept. Fish and Game, Species Conservation and Recovery Program, Report 2002-02; California Department of Fish and Wildlife: Sacramento, CA, USA, 2002. [Google Scholar]
- Kristan, W.B.; Boarman, W.I. Spatial pattern of risk of common raven predation on desert tortoises. Ecology 2003, 84, 2432–2443. [Google Scholar] [CrossRef]
- Sherman, M.W. Activity Patterns and Foraging Ecology of Nesting Common Ravens in the Mojave Desert, California. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 1993. [Google Scholar]
- James, F.C.; Shugart, J.R. A quantitative method of habitat description. Audubon Field Notes 1970, 24, 727–736. [Google Scholar]
- ESRI. ArcGIS Desktop, Versión 10; Sistemas Ambientales Instituto de investigación: Redlands, CA, USA, 2011. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 25 May 2021).
- Steenhof, K.; Kochert, M.N.; Rope, J.A. Nesting by raptors and common ravens on electrical transmission line towers. J. Wildl. Manag. 1993, 57, 271–281. [Google Scholar] [CrossRef]
- Xiong, A. Spatial Analysis of Common Raven Monitoring and Management Data for Desert Tortoise Critical Habitat Units in California. Master’s Thesis, University of Nevada, Reno, NV, USA, 2020. [Google Scholar]
- Khamcha, D.; Powell, L.A.; Gale, G.A. Effects of roadside edge on nest predators and nest survival of Asian tropical forest birds. Glob. Ecol. Conserv. 2018, 16, e00450. [Google Scholar] [CrossRef]
- Guerzou, A.; Guerzou, M.; Derdoukh, W.; Karim Souttou, K.; Doumandji, S. Corvus corax Diet composition in different agricultural lands in Algeria. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 41–57. [Google Scholar] [CrossRef]
- Harju, S.M.; Olson, C.V.; Hess, J.E.; Bedrosian, B. Common raven movement and space use: Influence of anthropogenic subsidies within greater sage-grouse nesting habitat. Ecosphere 2018, 9, e02348. [Google Scholar] [CrossRef]
- Capstick, L.A.; Sage, R.; Madden, J.R. Predation of artificial nests in UK farmland by magpies (Pica pica): Interacting environmental, temporal, and social factors influence a nest’s risk. Eur. J. Wildl. Res. 2019, 65, 50. [Google Scholar] [CrossRef]
- Sonerud, G.; Fjeld, P. Long-term memory in egg predators: An experiment with a hooded crow. Ornis Scand. 1987, 18, 323–325. [Google Scholar] [CrossRef]
- Birkhead, T.R. The Magpies: The Ecology and Behavior of Black-Billed and Yellow-Billed Magpies; T. & A.D. Poyser: London, UK, 1991. [Google Scholar]
- Zinkivskay, A.; Nazir, F.; Smulders, T.V. What-where-when memory in magpies (Pica pica). Anim. Cogn. 2009, 12, 119–125. [Google Scholar] [CrossRef]
- Kövér, L.; Toth, N.; Lengyel, S.; Juhasz, L. Corvid control in urban environments: A comparison of trap types. North-West. J. Zool. 2018, 14, 85–90. [Google Scholar]
- Brussee, B.E.; Coates, P.S. Reproductive success of common ravens influences nest predation rates of their prey: Implications for egg-oiling techniques. Avian Cons. Ecol. 2018, 13, 17. [Google Scholar] [CrossRef]
| 2018 | 2019 | 2020 | Random Tree | |
|---|---|---|---|---|
| Vegetation | ||||
| H (m) | 12 ± 1 | 12.4 ± 1.7 | 12.4 ± 1.8 | 9 ± 1.7 |
| DBH (cm) | 96.3 ± 27.5 | 79.9 ± 27.2 | 70.9 ± 19.9 | 54 ± 26 |
| Mature tree density | 3.8 ± 1.5 | 3.8 ± 1.7 | 3.8 ± 1.3 | 2.7 ± 1.7 |
| Scrub cover (%) | 23.7 ± 10.3 | 30.5 ± 19.1 | 26.9 ± 20.6 | 41.8 ± 30.8 |
| Bare ground cover (%) | 13.7 ± 9.4 | 10.3 ± 8.7 | 8.6 ± 6.6 | 3.9 ± 5.6 |
| Scrub richness | 3.5 ± 1.3 | 2.6 ± 1.4 | 2.6 ± 1.4 | 2.2 ± 1.5 |
| Distance to road (m) | 48 ± 36.7 | 78.4 ± +60.1 | 76.1 ± 59.5 | 184.7 ± 177.2 |
| Nest conspecific distance (m) Ravens | 340 ± 225 | 325 ± 200 | ||
| Nests | 4 | 19 | 18 | |
| Breeding pairs | 4 | 19 | 16 | |
| Fledglings | 3.5 ± 0.6 (n = 14) | 2.6 ± 0.8 (n = 50) | 2 ± 1.1 (n = 36) | |
| Predated tortoises | 102 | 125 | 119 | 0 |
| Predictors | Estimate | Std Error | Z Value | p Value |
|---|---|---|---|---|
| Intercept | −704.81 | 42.246 | −16.68 | <0.05 |
| Height | 45.91 | 2.952 | 15.55 | <0.05 |
| Bare ground cover | 470.45 | 28.922 | 16.27 | <0.05 |
| Tortoise density | 66.04 | 4.648 | 14.21 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura, A.; Acevedo, P. Influence of Habitat and Food Resource Availability on Common Raven Nest Site Selection and Reproductive Success in Mediterranean Forests. Birds 2021, 2, 302-313. https://doi.org/10.3390/birds2030022
Segura A, Acevedo P. Influence of Habitat and Food Resource Availability on Common Raven Nest Site Selection and Reproductive Success in Mediterranean Forests. Birds. 2021; 2(3):302-313. https://doi.org/10.3390/birds2030022
Chicago/Turabian StyleSegura, Amalia, and Pelayo Acevedo. 2021. "Influence of Habitat and Food Resource Availability on Common Raven Nest Site Selection and Reproductive Success in Mediterranean Forests" Birds 2, no. 3: 302-313. https://doi.org/10.3390/birds2030022
APA StyleSegura, A., & Acevedo, P. (2021). Influence of Habitat and Food Resource Availability on Common Raven Nest Site Selection and Reproductive Success in Mediterranean Forests. Birds, 2(3), 302-313. https://doi.org/10.3390/birds2030022






