The Potential Role of Drove Roads as Connecting Corridors for Birds between Natura 2000 Sites
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
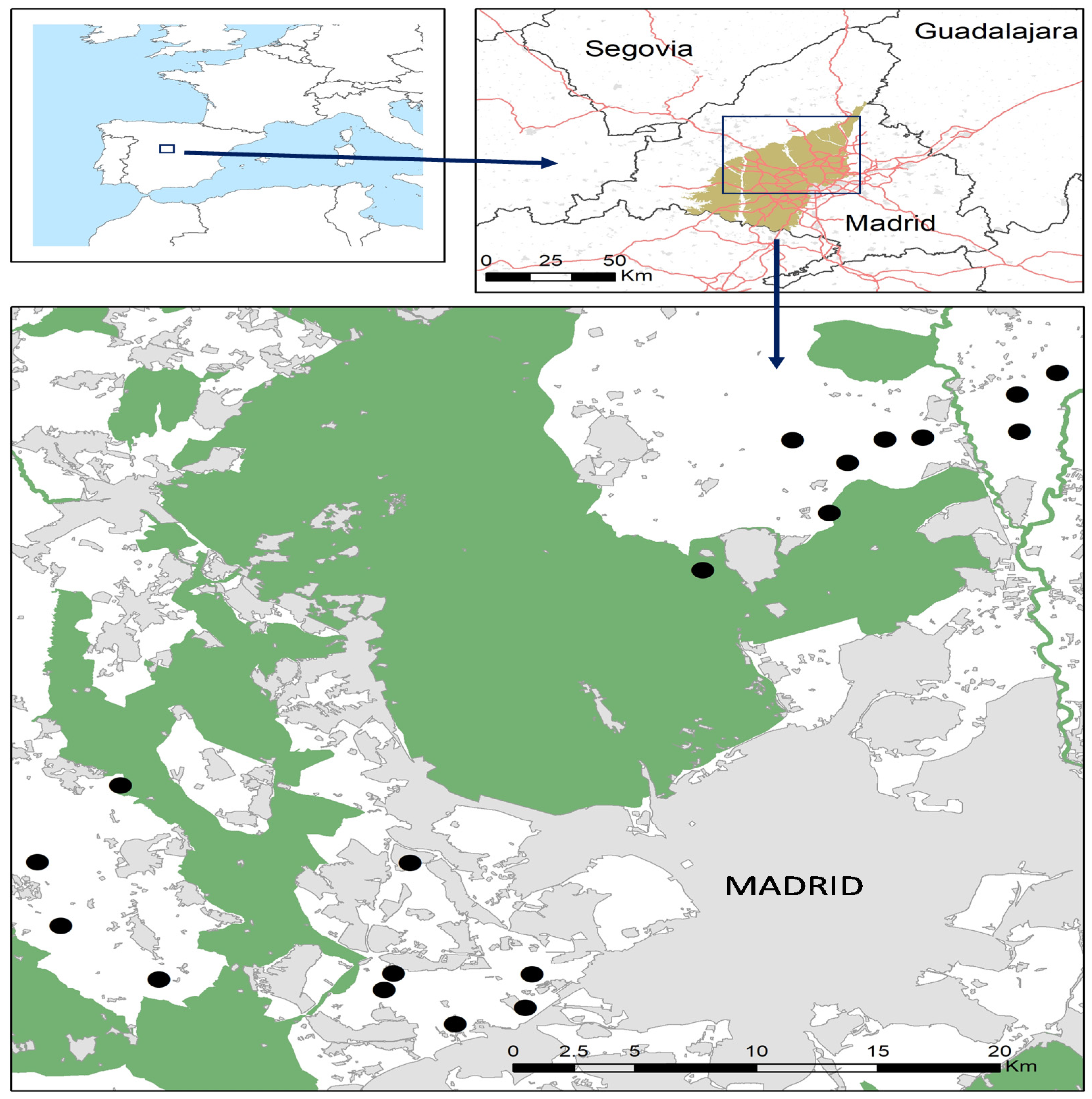
2.1. Study Area and Sampling
2.2. Data Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crooks, K.R.; Sanjayan, M. Conenctivity conservation: Maintaining connections for nature. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–19. [Google Scholar]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Araújo, M.B.; Alagador, D.; Cabeza, M.; Nogués-Bravo, D.; Thuiller, W. Climate change threatens European conservation areas. Ecol. Lett. 2011, 14, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Mingarro, M.; Lobo, J. Connecting protected areas in the Iberian peninsula to facilitate climate change tracking. Environ. Conserv. 2021, 1–10. [Google Scholar] [CrossRef]
- Jongman, R.H.G. Nature conservation planning in Europe: Developing ecological networks. Landsc. Urban Plan. 1995, 32, 169–183. [Google Scholar] [CrossRef]
- Agnoletti, M. Rural landscape, nature conservation and culture: Some notes on research trends and management approaches from a (southern) European perspective. Landsc. Urban Plan. 2014, 126, 66–73. [Google Scholar] [CrossRef]
- Garmendia, E.; Apostolopoulou, E.; Adams, W.M.; Bormpoudakis, D. Biodiversity and Green Infrastructure in Europe: Boundary object or ecological trap? Land Use Policy 2016, 56, 315–319. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Guidance on a Strategic Framework for Further Supporting the Deployment of EU-Level Green and Blue Infrastructure; European Commission: Brussel, Belgium, 2019; p. 101. Available online: https://ec.europa.eu/environment/nature/ecosystems/pdf/SWD_2019_193_F1_STAFF_WORKING_PAPER_EN_V4_P1_1024680.PDF (accessed on 9 September 2021).
- Hermoso, V.; Morán-Ordóñez, A.; Lanzas, M.; Brotons, L. Designing a network of green infrastructure for the EU. Landsc. Urban Plan. 2020, 196, 103732. [Google Scholar] [CrossRef]
- Oneal, A.S.; Rotenberry, J.T. Scale-dependent habitat relations of birds in riparian corridors in an urbanizing landscape. Landsc. Urban Plan. 2009, 92, 264–275. [Google Scholar] [CrossRef]
- de la Fuente, B.; Mateo-Sánchez, M.C.; Rodríguez, G.; Gastón, A.; de Ayala, R.P.; Colomina-Pérez, D.; Melero, M.; Saura, S. Natura 2000 sites, public forests and riparian corridors: The connectivity backbone of forest green infrastructure. Land Use Policy 2018, 75, 429–441. [Google Scholar] [CrossRef]
- European Commission. Building a Green Infrastructure for Europe; Publications Office of the European Union: Luxembourg, 2013; p. 24. [Google Scholar] [CrossRef]
- European Commission. LIFE and Climate Change Mitigation; Publications Office of the European Union: Luxembourg, 2015; p. 92. [Google Scholar] [CrossRef]
- Snäll, T.; Lehtomäki, J.; Arponen, A.; Elith, J.; Moilanen, A. Green infrastructure design based on spatial conservation prioritization and modeling of biodiversity features and ecosystem services. Environ. Manag. 2016, 57, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzimentor, A.; Apostolopoulou, E.; Mazaris, A.D. A review of green infrastructure research in Europe: Challenges and opportunities. Landsc. Urban Plan. 2020, 198, 103775. [Google Scholar] [CrossRef]
- Gippoliti, S.; Battisti, C. More cool than tool: Equivoques, conceptual traps and weaknesses of ecological networks in environmental planning and conservation. Land Use Policy 2017, 68, 686–691. [Google Scholar] [CrossRef]
- Foltête, J.-C. How ecological networks could benefit from landscape graphs: A response to the paper by Spartaco Gippoliti and Corrado Battisti. Land Use Policy 2019, 80, 391–394. [Google Scholar] [CrossRef]
- Bennet, A.F. Linkages in the Landscape: The Role of Corridors and Connectivity in Wildlife Conservation, 2nd ed.; IUCN: Gland, Switzerland, 2003. [Google Scholar]
- Hevia, V.; Azcárate, F.M.; Oteros-Rozas, E.; González, J.A. Exploring the role of transhumance drove roads on the conservation of ant diversity in Mediterranean agroecosystems. Biodivers. Conserv. 2013, 22, 2567–2581. [Google Scholar] [CrossRef]
- Azcárate, F.M.; Robleño, I.; Seoane, J.; Manzano, P.; Peco, B. Drove roads as local biodiversity reservoirs: Effects on landscape pattern and plant communities in a Mediterranean region. Appl. Veg. Sci. 2013, 16, 480–490. [Google Scholar] [CrossRef]
- Bunce, R.; Aranzabal, I.D.; Schmitz, M.; Pineda, F. A Review of the Role of Drove Roads (Cañadas) as Ecological Corridors; Alterra-rapport 1428: Wageningen, The Netherlands, 2006; Available online: http://content.alterra.wur.nl/Webdocs/PDFFiles/Alterrarapporten/AlterraRapport1428.pdf (accessed on 9 September 2021).
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Guidelines for conserving connectivity through ecological networks and corridors. In Best Practice Protected Area Guidelines Series No. 30; IUCN: Gland, Switzerland, 2020; p. 30. [Google Scholar] [CrossRef]
- Fischer, S.; Poschlod, P.; Beinlich, B. Experimental studies on the dispersal of plants and animals on sheep in calcareous grasslands. J. Appl. Ecol. 1996, 33, 1206–1222. [Google Scholar] [CrossRef]
- Baonza, J. Poblaciones de Halimium ocymoides disyuntas de su principal área de distribución madrileña: ¿un caso de zoocoria dirigida por las cañadas? Ecología 2000, 14, 151–157. [Google Scholar]
- Manzano, P.; Malo, J.E. Extreme long-distance seed dispersal via sheep. Front. Ecol. Environ. 2006, 4, 244–248. [Google Scholar] [CrossRef]
- Ley 3/1995. Ley de Vías Pecuarias (Drove Roads Act). 1995. Available online: www.boe.es/buscar/doc.php?id=BOE-A-1995-7241 (accessed on 9 May 2021).
- Sanderson, J.; Da Fonseca, G.A.B.; Galindo-Leal, C.; Alger, K.; Inchausty, V.H.; Morrison, K.; Rylands, A. Escaping the minimalist trap: Design and implementation of large- scale biodiversity corridors. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 620–648. [Google Scholar]
- Clucas, B.; McHugh, K.; Caro, T. Flagship species on covers of US conservation and nature magazines. Biodivers. Conserv. 2008, 17, 1517. [Google Scholar] [CrossRef]
- Veríssimo, D.; Fraser, I.; Groombridge, J.; Bristol, R.; MacMillan, D.C. Birds as tourism flagship species: A case study of tropical islands. Anim. Conserv. 2009, 12, 549–558. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Clavel, J.; Jiguet, F.; Lee, A.; Couvet, D. Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 2008, 17, 252–261. [Google Scholar] [CrossRef]
- Pellissier, V.; Cohen, M.; Boulay, A.; Clergeau, P. Birds are also sensitive to landscape composition and configuration within the city centre. Landsc. Urban Plan. 2012, 104, 181–188. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Y.; Qi, K.; Luo, Z.; Wang, X. Detecting the response of bird communities and biodiversity to habitat loss and fragmentation due to urbanization. Sci. Total. Environ. 2018, 624, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Minor, E.; Urban, D. Forest bird communities across a gradient of urban development. Urban Ecosyst. 2010, 13, 51–71. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Can human disturbance promote nestedness? A case study with breeding birds in urban habitat fragments. Oecologia 2002, 131, 269–278. [Google Scholar] [CrossRef]
- González-Oreja, J.A.; Hernández-Santín, L.; Bonache-Regidor, C.; Buzo-Franco, D. Can human disturbance promote nestedness? Songbirds and noise in urban parks as a case study. Landsc. Urban Plan. 2012, 104, 9–18. [Google Scholar] [CrossRef]
- Laiolo, P. Spatial and Seasonal Patterns of Bird Communities in Italian Agroecosystems. Conserv. Biol. 2004, 1547–1556. [Google Scholar] [CrossRef]
- Valente, J.J.; Betts, M.G. Response to fragmentation by avian communities is mediated by species traits. Divers. Distrib. 2019, 25, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Bregman, T.P.; Şekercioğlu, C.H.; Tobias, J.A. Global patterns and predictors of bird species responses to forest fragmentation: Implications for ecosystem function and conservation. Biol. Conserv. 2014, 169, 372–383. [Google Scholar] [CrossRef]
- Huhta, E.; Mappes, T.; Jokimäki, J. Predation on artificial ground nests in relation to forest fragmentation, agricultural land and habitat structure. Ecography 1996, 19, 85–91. [Google Scholar] [CrossRef]
- Hartley, M.J.; Hunter, M.L., Jr. A meta-analysis of forest cover, edge effects, and artificial nest predation rates. Conserv. Biol. 1998, 12, 465–469. [Google Scholar] [CrossRef]
- Opdam, P. Metapopulation theory and habitat fragmentation: A review of Holarctic breeding bird studies. Landsc. Ecol. 1991, 5, 93–106. [Google Scholar] [CrossRef]
- Schlesinger, M.D.; Manley, P.N.; Holyoak, M. Distinguishing stressors acting on land bird communities in an urbanizing environment. Ecology 2008, 89, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Minor, E.S.; Park, C.-R.; Lee, D. Effects of habitat structure, human disturbance, and habitat connectivity on urban forest bird communities. Urban Ecosyst. 2015, 18, 857–870. [Google Scholar] [CrossRef]
- Gilbert-Norton, L.; Wilson, R.; Stevens, J.R.; Beard, K.H. A Meta-Analytic Review of Corridor Effectiveness. Conserv. Biol. 2010, 24, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, D.F.; Miller, C.; Possingham, H.P.; Fuller, R.A. The influence of patch area and connectivity on avian communities in urban revegetation. Biol. Conserv. 2011, 144, 722–729. [Google Scholar] [CrossRef]
- Brotons, L.; Herrando, S. Factors affecting bird communities in fragments of secondary pine forests in the north-western Mediterranean basin. Acta Oecol. 2001, 22, 21–31. [Google Scholar] [CrossRef]
- Mönkkönen, M.; Rajasärkkä, A.; Lampila, P. Isolation, patch size and matrix effects on bird assemblages in forest reserves. Biodivers. Conserv. 2014, 23, 3287–3300. [Google Scholar] [CrossRef]
- Chalfoun, A.D.; Thompson, F.R.; Ratnaswamy, M.J. Nest predators and fragmentation: A review and meta-analysis. Conserv. Biol. 2002, 16, 306–318. [Google Scholar] [CrossRef]
- Tellería, J.L. Manual Para el Censo de Los Vertebrados Terrestres; Editorial Raíces: Madrid, Spain, 1986. [Google Scholar]
- Lee, M.B.; Carroll, J.P. Relative importance of local and landscape variables on site occupancy by avian species in a pine forest, urban, and agriculture matrix. For. Ecol. Manag. 2014, 320, 161–170. [Google Scholar] [CrossRef]
- PNOA (National Plan for Aerial Orthophotography) IGN. 2020. Available online: https://pnoa.ign.es/ (accessed on 9 September 2021).
- IDEM (Infrastructure of Spatial Data of Madrid region). Land use and vegetation map of Madrid Region. Regional Government of Madrid. 2018. Available online: https://idem.madrid.org/visor/ (accessed on 4 December 2020).
- ESRI. ArcGIS 10.7.1: Geographical Information System; ESRI: West Redlands, CA, USA, 2019. [Google Scholar]
- JRC 2021 NED-Nestedness for dummies. Joint Research Centre. Available online: https://ecosoft.alwaysdata.net/ (accessed on 9 September 2021).
- Ulrich, W.; Almeida-Neto, M.; Gotelli, N.J. A consumer’s guide to nestedness analysis. Oikos 2009, 118, 3–17. [Google Scholar] [CrossRef]
- Mac Nally, R.; Duncan, R.P.; Thomson, J.R.; Yen, J.D.L. Model selection using information criteria, but is the “best” model any good? J. Appl. Ecol. 2018, 55, 1441–1444. [Google Scholar] [CrossRef]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 8.0; StatSoft Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Wilson, D.S. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology 1992, 73, 1984–2000. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, United Kingdom, 1999. [Google Scholar]
- Mouquet, N.; Loreau, M. Community patterns in source–sink metacommunities. Am. Nat. 2003, 162, 544–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Lussier, S.M.; Enser, R.W.; Dasilva, S.N.; Charpentier, M. Effects of habitat disturbance from residential development on breeding bird communities in riparian corridors. Environ. Manag. 2006, 38, 504–521. [Google Scholar] [CrossRef]
- Santos, T.; Tellería, J.L.; Carbonell, R. Bird conservation in fragmented Mediterranean forests of Spain: Effects of geographical location, habitat and landscape degradation. Biol. Conserv. 2002, 105, 113–125. [Google Scholar] [CrossRef]
- Doherty, P.F.; Grubb, T.C. Survivorship of permanent-resident birds in a fragmented forested landscape. Ecology 2002, 83, 844–857. [Google Scholar] [CrossRef]
- IUCN 2021. The IUCN Red List of Threatened Species; Version 2021-1; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2021; ISSN 2307-8235; Available online: https://www.iucnredlist.org (accessed on 9 September 2021)ISSN 2307-8235.
- BirdLife International. Aquila adalberti; The IUCN Red List of Threatened Species 2019: E.T22696042A152593918; BirdLife International: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Herrera, A. Análisis del Carácter de las Vías Pecuarias Como Refugio del Conejo (Oryctolagus cuniculus, L. 1758) en el Centro Peninsular. Master’s Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2021. [Google Scholar]
- Chamberlain, D.E.; Toms, M.P.; McHarg, R.C.; Banks, A.N. House sparrow (Passer domesticus) habitat use in urbanized landscapes. J. Ornithol. 2007, 148, 453–462. [Google Scholar] [CrossRef]
- De Laet, J.; Summers-Smith, J.D. The status of the urban house sparrow Passer domesticus in north-western Europe: A review. J. Ornithol. 2007, 148 (Suppl. 2), S275–S278. [Google Scholar] [CrossRef]
- Shaw, L.M.; Chamberalin, D.; Evans, M. The House Sparrow Passer domesticus in urban areas: Reviewing a possible link between post-decline distribution and human socieoeconomic status. J. Ornithol. 2008, 149, 293–299. [Google Scholar] [CrossRef]
- Robillard, A.; Garant, D.; Be’lisle, M. The Swallow and the Sparrow: How agricultural intensification affects abundance, nest site selection and competitive interactions. Landsc. Ecol. 2013, 28, 201–215. [Google Scholar] [CrossRef]
- Rottenborn, S.C. Predicting the impacts of urbanization on riparian bird communities. Biol. Conserv. 1999, 88, 289–299. [Google Scholar] [CrossRef]
- Benítez-López, A.; Alkemade, R.; Verweij, P.A. The impacts of roads and other infrastructure on mammal and bird populations: A meta-analysis. Biol. Conserv. 2010, 143, 1307–1316. [Google Scholar] [CrossRef] [Green Version]
- Halfwerk, W.; Holleman, L.J.M.; Lessells, C.M.; Slabbekoorn, H. Negative impact of traffic noise on avian reproductive success. J. Appl. Ecol. 2011, 48, 210–219. [Google Scholar] [CrossRef]
- Buxton, R.T.; McKenna, M.F.; Mennitt, D.; Fristrup, K.; Crooks, K.; Angeloni, L.; Wittemyer, G. Noise pollution is pervasive in U.S. protected areas. Science 2017, 356, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Summers, P.D.; Cunnington, G.M.; Fahrig, L. Are the negative effects of roads on breeding birds caused by traffic noise? J. Appl. Ecol. 2011, 48, 1527–1534. [Google Scholar] [CrossRef]
- Francis, C.D.; Ortega, C.P.; Cruz, A. Noise pollution changes avian communities and species interactions. Curr. Biol. 2009, 19, 1415–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Limón, J.; De Lucío Fernández, J.V. Changes in use and landscape preferences on the agricultural-livestock landscapes of the central Iberian Peninsula (Madrid, Spain). Landsc. Urban Plan. 1999, 44, 165–175. [Google Scholar] [CrossRef]
- Neuschulz, L.; Brown, M.; Farwig, N. Frequent bird movements across a highly fragmented landscape: The role of species traits and forest matrix. Anim. Conserv. 2013, 16, 170–179. [Google Scholar] [CrossRef]
- Taylor, J.J.; Lepczyk, C.A.; Brown, D.G. Patch and matrix level influences on forest birds at the rural-urban interface. Landsc. Ecol. 2016, 31, 1005–1020. [Google Scholar] [CrossRef]
- Traba, J.; de la Morena, E.L.G.; Morales, M.B.; Suárez, F. Determining high value areas for steppe birds in Spain: Hot spots, complementarity and the efficiency of protected areas. Biodivers. Conserv. 2007, 16, 3255–3275. [Google Scholar] [CrossRef]
- Villard, M.A.; Trzcinski, M.K.; Merriam, G. Fragmentation effects on forest birds: Relative influence of woodland cover and configuration on landscape occupancy. Conserv. Biol. 1999, 13, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Dakos, V.; Bascompte, J. Critical slowing down as early warning for the onset of collapse in mutualistic communities. Proc. Natl. Acad. Sci. USA 2014, 11, 17546–17551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verboom, J.; Schotman, A.; Opdam, P.; Metz, J.A.J. European Nuthatch Metapopulations in a Fragmented Agricultural Landscape. Oikos 1991, 61, 149–156. [Google Scholar] [CrossRef]
- Van der Sluis, T.; Schmidt, A.M. E-BIND Handbook (Part B): Scientific Support for Successful Implementation of the Natura 2000 network; Wageningen Environmental Research/Ecologic Institute/Milieu Ltd.: Wageningen, The Netherlands, 2021. [Google Scholar]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Manzano, P.; Casas, R. Past, present and future of Trashumancia in Spain: Nomadism in a developed country. Pract. Action Publ. 2010. [Google Scholar] [CrossRef]
- Miller, S.G.; Knight, R.L.; Miller, C.K. Influence of recreational trails on breeding bird communities. Ecol. Appl. 1998, 8, 162–169. [Google Scholar] [CrossRef]
- Rochelle, S.; Pickering, J.C.; Castley, G. A review of the impacts of nature based recreation on birds. J. Environ. Manag. 2011, 92, 2287–2294. [Google Scholar] [CrossRef]

| Variables | Mean ± SD [Max.–Min.] |
|---|---|
| Ground cover at the drove road (50 m buffer) scale | |
| Bare ground (%) | 7.52 ± 4.55 [0–16.0] |
| Urban (%) | 1.00 ± 1.97 [0–6.4] |
| Crops (%) | 26.50 ± 31.47 [0–86.9] |
| Grassland (%) | 40.41 ± 31.35 [0–89.3] |
| Low open scrub (%) | 1.64 ± 2.71 [0–6.9] |
| Retama sphaerocarpa scrub (%) | 16.79 ± 24.84 [0–79.0] |
| Riparian vegetation (%) | 1.24 ± 2.99 [0–12.0] |
| Holm oak forest (%) | 5.14 ± 9.73 [0–34.8] |
| Ground cover at the landscape (500 m buffer) scale | |
| Urban and bare ground (%) | 8.44 ± 17.39 [0–69.5] |
| Crops (%) | 34.22 ± 33.58 [0–86.3] |
| Grassland (%) | 10.76 ± 15.68 [0–53.3] |
| Low open scrub (%) | 0.94 ± 2.16 [0–8.1] |
| Retama sphaerocarpa scrub (%) | 20.35 ± 20.43 [0–62.0] |
| Riparian vegetation (%) | 1.99 ± 2.25 [0–7.2] |
| Holm oak forest (%) | 23.29 ± 33.36 [0–96.6] |
| Location of the transect | |
| Distance (m) to the nearest Natura 2000 site | 2254.2 ± 2044.0 [0–7098] |
| Distance (m) to the closest urban area or high capacity roads | 734.3 ± 570.4 [35.3–2052] |
| Distance (m) to the nearest forest patch larger than 5 Ha | 486.7 ± 602.9 [0–2391] |
| Species | Presence in Transects | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passer domesticus | |||||||||||||||||||
| Pica pica | |||||||||||||||||||
| Galerida cristata | |||||||||||||||||||
| Sturnus unicolor | |||||||||||||||||||
| Serinus serinus | |||||||||||||||||||
| Columba palumbus | |||||||||||||||||||
| Carduelis carduelis | |||||||||||||||||||
| Curruca melanocephala | |||||||||||||||||||
| Cyanistes caeruleus | |||||||||||||||||||
| Turdus merula | |||||||||||||||||||
| Miliaria calandra | |||||||||||||||||||
| Saxicola rubicola | |||||||||||||||||||
| Lullula arborea | |||||||||||||||||||
| Fringilla coelebs | |||||||||||||||||||
| Sylvia atricapilla | |||||||||||||||||||
| Alectoris rufa | |||||||||||||||||||
| Carduelis cannabina | |||||||||||||||||||
| Luscinia megarhynchos | |||||||||||||||||||
| Parus major | |||||||||||||||||||
| Cyanopica cyanus | |||||||||||||||||||
| Lanius senator | |||||||||||||||||||
| Bubulcus ibis | |||||||||||||||||||
| Corvus corone | |||||||||||||||||||
| Periparus ater | |||||||||||||||||||
| Myiopsitta monachus | |||||||||||||||||||
| Upupa epops | |||||||||||||||||||
| Hippolais polyglotta | |||||||||||||||||||
| Motacilla alba | |||||||||||||||||||
| Clamator glandarius | |||||||||||||||||||
| Curruca cantillans | |||||||||||||||||||
| Chloris chloris | |||||||||||||||||||
| Ficedula hypoleuca | |||||||||||||||||||
| Picus viridis | |||||||||||||||||||
| Cettia cetti | |||||||||||||||||||
| Streptopelia decaocto | |||||||||||||||||||
| Merops apiaster | |||||||||||||||||||
| Species richness | 14 | 13 | 13 | 12 | 11 | 11 | 10 | 9 | 9 | 8 | 7 | 7 | 6 | 6 | 5 | 5 | 5 | 4 | 3 |
| Variables | Estimate ± SD | Wald Statistic | p |
|---|---|---|---|
| Intercept | 2.13965 ± 0.11220 | 363.63 | <0.0001 |
| Distance to Natura 2000 site | −0.00012 ± 0.00004 | 8.20 | 0.0042 |
| Forest cover (500 m) | 0.00560 ± 0.00224 | 6.23 | 0.0126 |
| Holm oak forest (50 m) | 0.01254 ± 0.00683 | 3.37 | 0.0665 |
| Variables | Estimate ± SD | Wald Statistic | p |
|---|---|---|---|
| Intercept | 1.33285 ± 0.09783 | 185.62 | <0.0001 |
| Distance to urban areas | 0.00026 ± 0.00007 | 13.00 | 0.0003 |
| Forest cover (500 m) | 0.00200 ± 0.00123 | 2.62 | 0.1057 |
| Bare ground (50 m) | −0.01580 ± 0.00883 | 3.20 | 0.0738 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malo, J.E.; Mata, C. The Potential Role of Drove Roads as Connecting Corridors for Birds between Natura 2000 Sites. Birds 2021, 2, 314-328. https://doi.org/10.3390/birds2030023
Malo JE, Mata C. The Potential Role of Drove Roads as Connecting Corridors for Birds between Natura 2000 Sites. Birds. 2021; 2(3):314-328. https://doi.org/10.3390/birds2030023
Chicago/Turabian StyleMalo, Juan E., and Cristina Mata. 2021. "The Potential Role of Drove Roads as Connecting Corridors for Birds between Natura 2000 Sites" Birds 2, no. 3: 314-328. https://doi.org/10.3390/birds2030023
APA StyleMalo, J. E., & Mata, C. (2021). The Potential Role of Drove Roads as Connecting Corridors for Birds between Natura 2000 Sites. Birds, 2(3), 314-328. https://doi.org/10.3390/birds2030023






