Simple Summary
COVID-19 (caused by the SARS-CoV-2 virus) can be a significant and life-threatening condition. One reason for the seriousness of COVID-19 is an extreme inflammatory response by the body. Historically, low-dose radiation has been used to treat pneumonia and has the potential to reduce an inflammatory state. Therefore, our team investigated the effects of low-dose radiotherapy in two patients admitted to hospital with severe COVID-19. The results of this were positive, and we discuss other research on this topic.
Abstract
Low-dose radiotherapy had historically been used to treat both bacterial and viral pneumonias. In the present day, this is not in use due to the development of antibiotics and other supportive measures as well as a concern regarding late radiation toxicities. COVID-19 presented us with a novel respiratory illness without a strong evidence-based best practice; it was thought, therefore, that there may be a role for low-dose radiotherapy in the absence or failure of a standard treatment. The rationale for this was based around the ability of low-dose radiation to reduce an inflammatory state. We treated two individuals suffering from severe COVID-19 with low-dose whole lung radiotherapy, in the setting of a phase I trial. Both patients improved clinically, biochemically, and radiologically within a matter of days. We discuss why the meta-analyses may not have shown this advantage.
1. Introduction
As of August 2024, infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has accounted for more than 7 million deaths globally [1]. Infection by SARS-CoV-2 results in the clinical syndrome of COVID-19, which widely varies in severity from asymptomatic to severe pneumonia to life-threatening acute respiratory distress syndrome (ARDS) [2]. It is thought that the release of pro-inflammatory cytokines contributes to ARDS [3], with severely ill COVID-19 patients developing an acute systemic inflammatory response consistent with Cytokine Release Syndrome (CRS). This is characterised by an increase in several pro-inflammatory cytokines, including IL-1β, IL-6, interferon gamma, and TNF-α [3]. This inflammation of the lungs impairs gas exchange and may lead to hypoxia requiring ventilatory support. Neutralising these key inflammatory factors in CRS has been shown to be of potential value in reducing mortality from COVID-19 infection [4].
Mortazavi et al. summarised how low-dose radiotherapy (LDRT) may help in this setting [5]. The authors discussed that LDRT triggers anti-inflammatory and anti-thrombosis effects, with immunomodulation, leading to alveolar acceleration and mucous absorption.
From the first half of the 20th century, retrospective reports on over 800 patients with pneumonia suggest that LDRT may reduce mortality and often provide symptom relief within 24–48 h [6]. In Germany, over 50,000 people are treated annually with radiotherapy for non-cancerous conditions, for example, arthritis [6]. LDRT induces M2 macrophage phenotype activation, an increase in CD3, CD4, and CD8 T-cells, as well as an increase in IL-10 and TGF-β, while resulting in a decrease in IL-6 and TNF-α [7]. Overall, LDRT appears to negate the effects of CRS through immunomodulation [8], and, thus, theoretically reduce mortality risk from COVID-19-induced ARDS.
The COVID-19 pandemic presented a unique requirement for rapid evaluation of novel treatments. Below, we report on the clinical course and outcomes of two patients enrolled to a feasibility study (Clinicaltrails.gov ID NCT04572412, IRAS 285167) into the use of LDRT in COVID-19.
2. Methods
This case report follows two patients recruited to a single arm feasibility study which evaluated whether individuals with severe COVID-19 lung infection could tolerate treatment with up to two doses of 0.5 Gy whole lung radiotherapy. The participants were identified and recruited from inpatient wards. Both had confirmed COVID-19 infection with supplemental oxygen therapy but no other respiratory support. This paper reports on the clinical course and outcomes of both.
The mortality and morbidity associated with severe COVID-19 infection in patients eligible for this study were judged to far outweigh the very low risk of acute toxicities (mild nausea and lymphopenia) and the small risk of long-term radiation-induced malignancies. No significant acute toxicity was expected for the proposed dose and volumes. To prevent patients experiencing nausea due to irradiation of large volumes including the gastric mucosa, patients were premedicated with ondansetron.
Study participants were identified and recruited from inpatient wards. As part of the inclusion criteria (this is not an extensive list), patients were required to be above the age of 50 years and have laboratory-confirmed COVID-19 infection requiring supplemental 28–40% oxygen therapy but no other respiratory support. Our inclusion criteria specified treatment should be given within 5 days of admission for patients needing supplemental oxygen. Patients were excluded if amongst other factors, they were known to have a secondary infection (with raised pro-calcitonin), radio-sensitising conditions, conditions pre-disposing them to increased risks of radiation toxicities, or P/F ration ≤ 100 mmHg.
A single fraction of radiotherapy was delivered to the thorax with the aim to cover the entire bilateral lung volume with a prescription dose (0.5 Gy) as close to the lung edge as possible. The prescription was 0.5 Gy mid plane dose (MPD). This was usually calculated at the geometrical field centre (central axis), accounting for the patient separation at chest level, with equal weight from the anterior and posterior beams. The minimum dose had to be over 0.4 Gy. Lung bases and parts of the lungs visually involved by COVID-19 received at least 0.45 Gy. The prescription point could be the MPD off the Central Axis to achieve above constraints. IMRT was not permitted. Treatment was provided via a standard Linear Accelerator with an output rate at approximately 600 MU/minute using large, flattened fields. Parallel pair beams at energies ≤ 10 MV (6 MV usual) were utilised. Proton beams, orthovoltage photons, or electrons were not permitted. The specified dose for patients receiving a planning CT scan was dose to medium as per the computerised CT plan, with inhomogeneity corrections for tissue composition and density differences.
2.1. Patient 1
Patient 1 was a 68-year-old gentleman with confirmed COVID-19 suffering from shortness of breath, fever, and a productive cough. His WHO COVID-19 score [9] was 5 and he was receiving dexamethasone, remdesivir, and oxygen at study recruitment. Comorbidities included type 2 diabetes mellitus, hypertension, benign prostate hyperplasia, hypercholesterolaemia, cervical spondylosis, lumbar spine degeneration, and childhood asthma (now well controlled). Current medications included tamsulosin, solifenacin, losartan, amlodipine, metformin, lansoprazole, atorvastatin, and tadalafil.
The patient received a single dose of 0.5 Gy radiotherapy (with the minimum dose being 0.466 Gy) to the entire lung tissue. This was delivered using a standard dose rate linear accelerator with 6 mega voltage (MV) photons as CT planned parallel pair AP/PA open fields. Treatment was delivered five days after admission. There was no significant acute toxicity; the patient was premedicated with ondansetron.
2.2. Patient 2
Patient 2 was a 67-year-old gentleman with confirmed COVID-19 suffering from cough, dyspnoea, and pharyngitis. His WHO COVID-19 score [9] was 5 and he was receiving dexamethasone and oxygen as treatment at study recruitment. Comorbidities included bilateral knee osteoarthritis, obesity, unstable bladder, previous deep vein thrombosis, and heterozygous factor V Leiden mutation. Current medications included amitriptyline, diclofenac, dihydrocodeine, lansoprazole, naproxen, oxybutynin, and propranolol. CT pulmonary angiogram excluded pulmonary thromboembolism.
The patient received a single dose of 0.5 Gy radiotherapy (with the minimum dose being 0.494 Gy) to the entire lung tissue, utilising the same technique as patient 1. Treatment was delivered 2 days after admission. There were no significant acute toxicities; the patient was premedicated with ondansetron.
3. Results
3.1. Patient 1
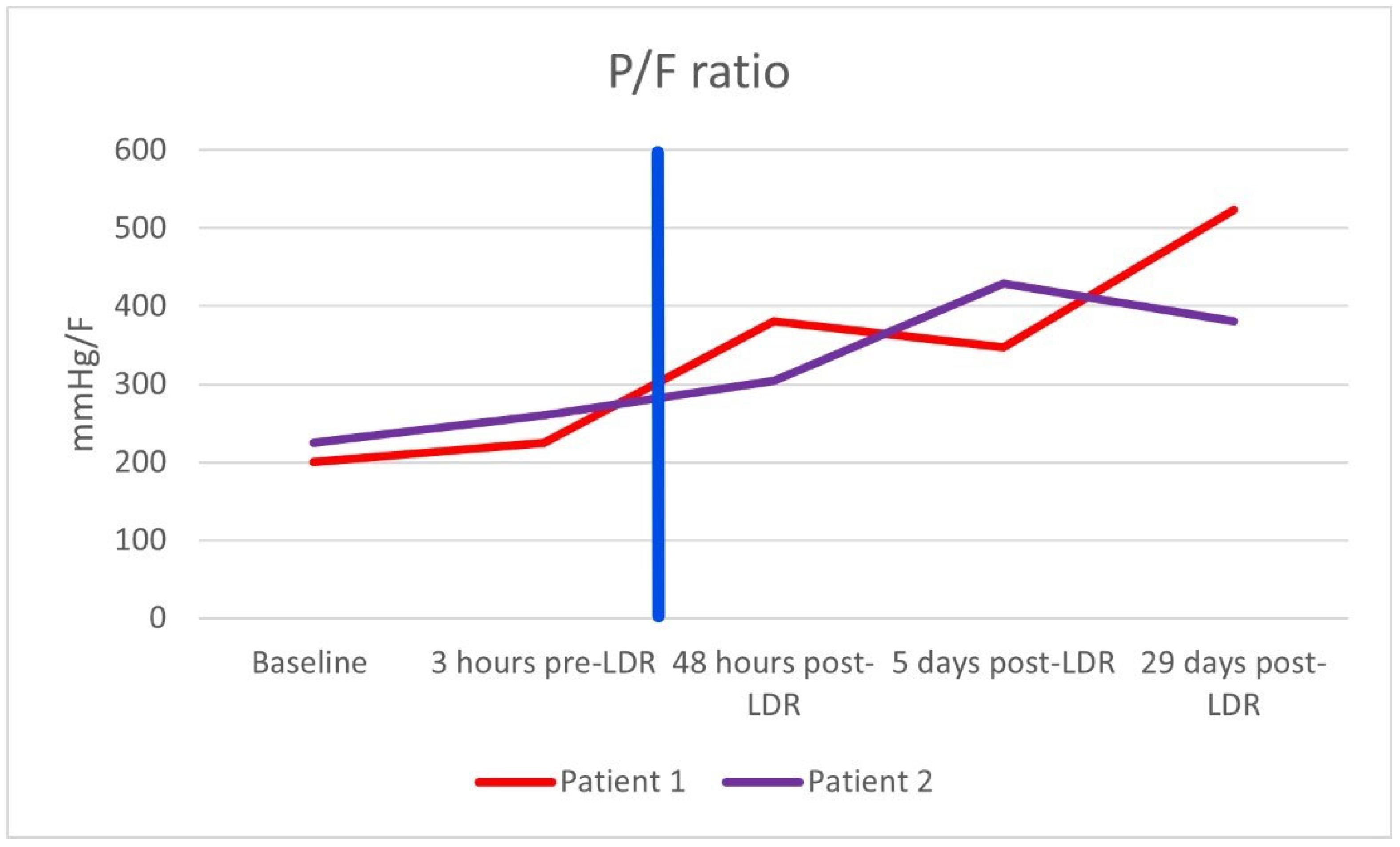
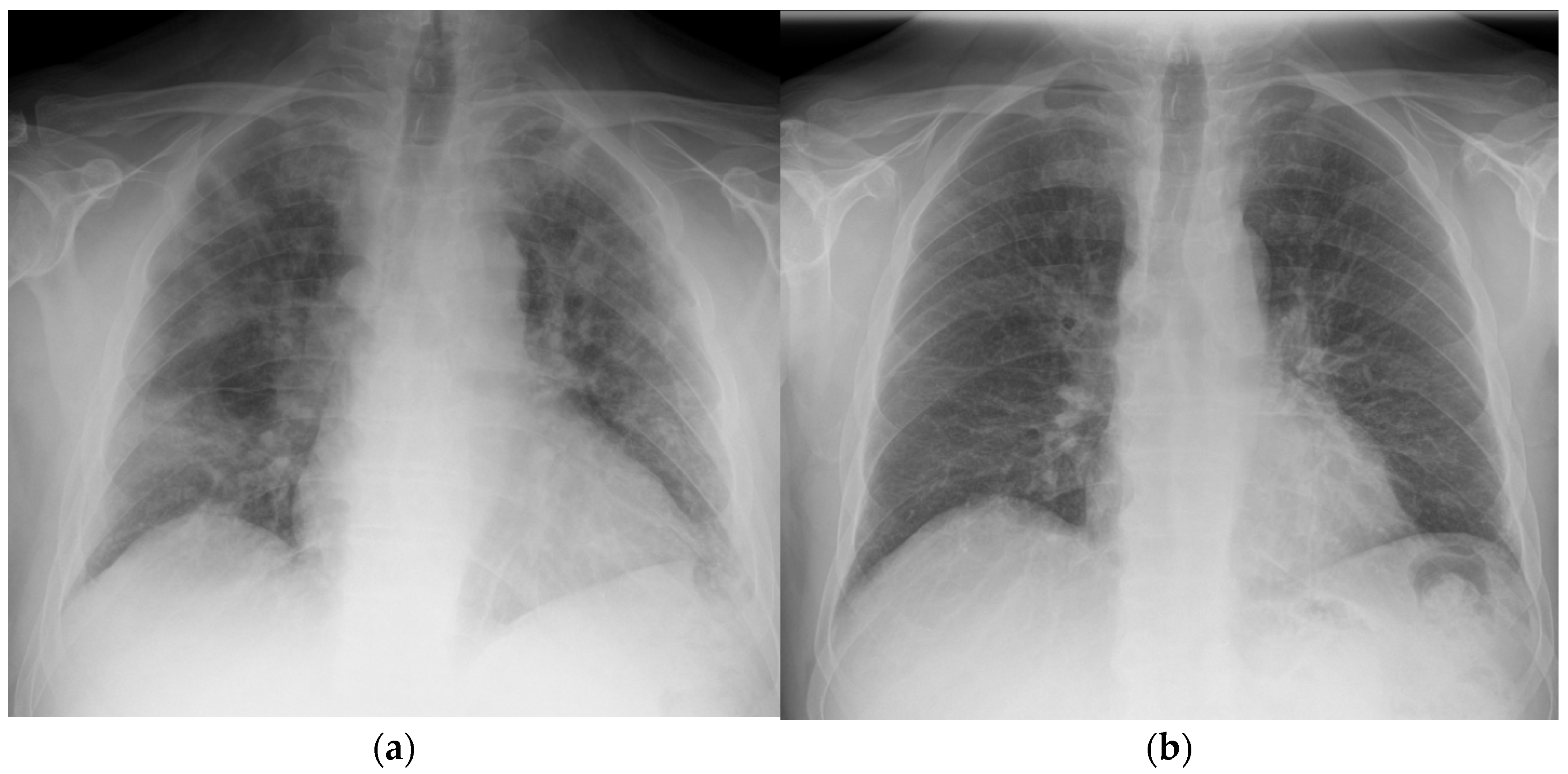
After the single dose of LDRT, improvement was observed clinically, biochemically, and radiologically. Prior to LDRT, the patient required 40% oxygen to maintain oxygen saturations of 95%. Forty-eight hours following LDRT, the patient was able to maintain oxygen saturations of 95% on room air. His PaO2/FiO2 (P/F) ratio (which can be used to categorise the severity of ARDS [10]) improved from 200 mmHg/F to 381 mmHg/F forty-eight hours post radiotherapy [Figure 1]. Biochemically, inflammatory markers improved with CRP reducing from 10.4 mg/dL at baseline to 1.4 mg/dL five days after LDRT. Notably, serum BNP dropped from 473 ng/L to 80 ng/L, which may imply a reduction in hypoxia-induced heart strain [Table 1]. Lymphocyte nadir was 0.63 (normal range being 1–4 × 109/L); values below 0.5 are considered to be significantly low [11]. Radiologically, by day 50, the previously seen bilateral interstitial infiltrates were no longer present; however, there did appear to be some persistent residual changes with coarsening of bronchovascular markings but no fibrosis [Figure 2].
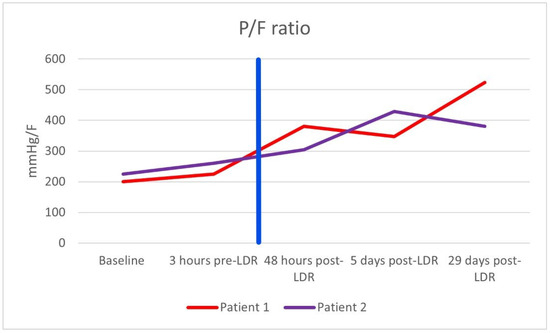
Figure 1.
PaO2/FiO2 (P/F) ratio of both patients. The vertical blue line marks when radiotherapy was administered.

Table 1.
Biochemical results for patient 1 and patient 2 at baseline and 5 days post low-dose radiotherapy (LDR). C-reactive protein (CRP) [normal range 0–5 mg/L], Interleukin-6 (IL-6) [normal range 0–7 pg/mL], D-dimer [normal range 0–7 µg/mL], and B-type natriuretic peptide (BNP) [normal range 0–400 ng/L].
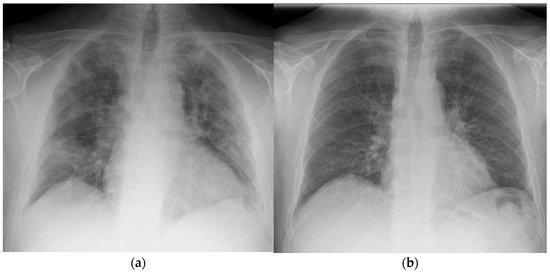
Figure 2.
Chest X-ray taken of patient 1 at (a) day 1 prior to radiotherapy and (b) day 42 post treatment.
3.2. Patient 2
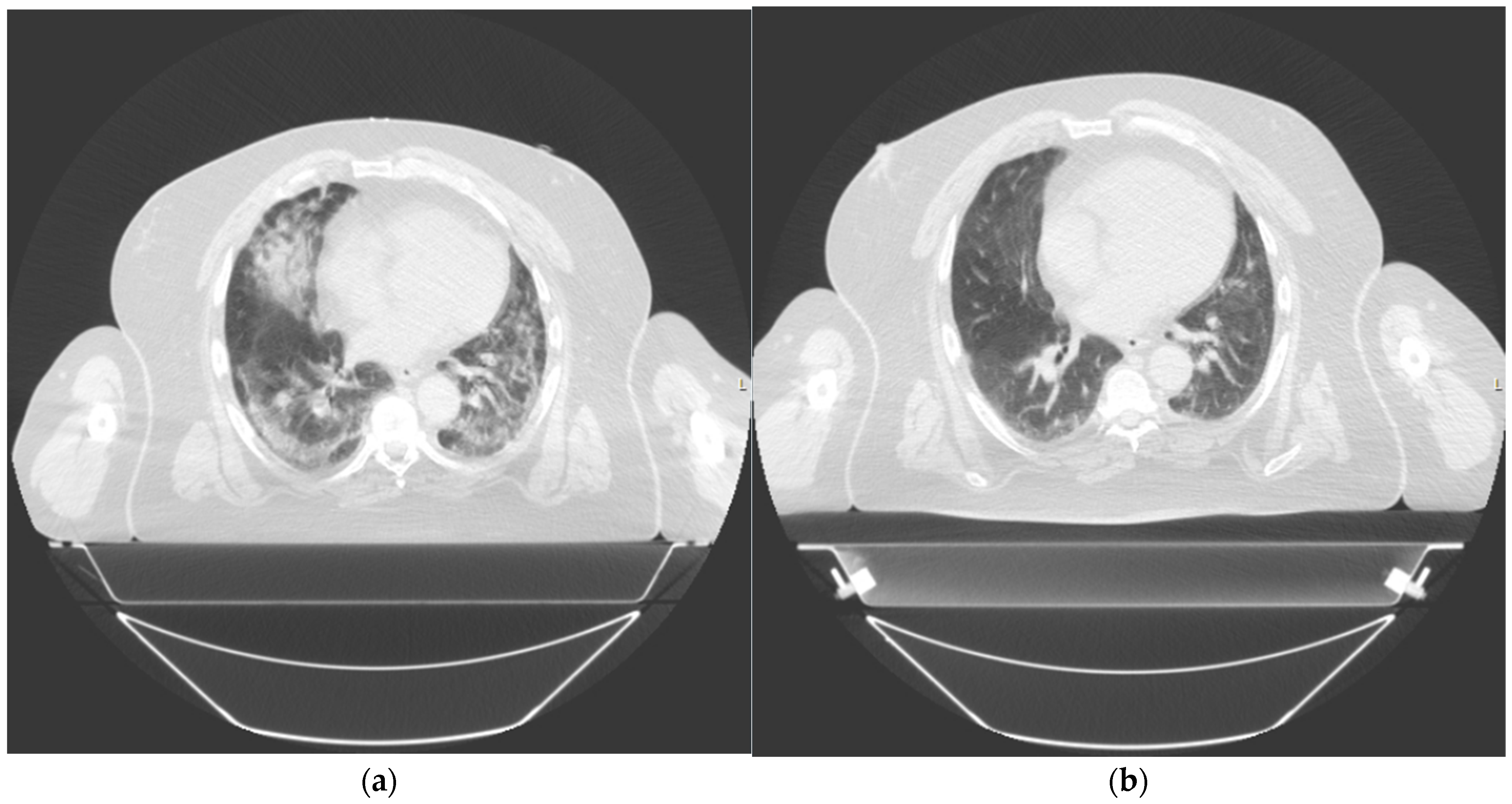
After the single dose of LDRT, we observed clinical, biochemical, and radiological improvement. Prior to LDRT, the patient required 40% oxygen to maintain saturations of 96%. Forty-eight hours after LDRT, oxygen saturations were maintained at 92% on room air. His P/F ratio improved from 225 mmHg/F to 305 mmHg/F. Biochemically, his inflammatory markers improved with CRP dropping from 50.2 mg/dL at baseline to 3.9 mg/dL five days after LDRT. Serum BNP also reduced from 810 ng/L pre-radiotherapy to 439 ng/L five days post radiotherapy. Lymphocyte nadir was 0.81. A CT thorax scan performed at 42 days post radiotherapy no longer demonstrated bilateral pulmonary infiltrates although some patchy subpleural ground-glass changes persisted [Figure 3]. There was no fibrosis noted.
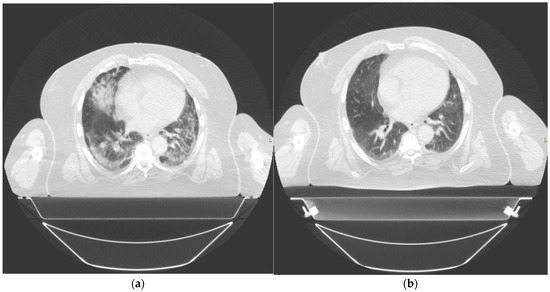
Figure 3.
Thorax computed tomography (CT) taken of patient 2 at (a) day 1 prior to treatment and (b) day 42 post treatment.
4. Discussion
This paper reports on two patients with confirmed COVID-19 who received a single dose of LDRT 0.5 Gy to both lungs with subsequent clinical, biochemical, and radiological improvement. We noted similar rapid improvement in Calabrese et al., 2013 [6], with significant improvement 48 h post radiotherapy.
The trial originally planned to recruit 13 patients; however, it was terminated early due to reduced staff capacity (COVID-19 sickness) and the reluctance of patients to enter the trial due to fear of radiotherapy toxicities, with approximately only 20% acceptance.
We believe that there are two key variables which determine the effectiveness of LDRT in treating COVID-19 pneumonia/ARDS: dose and timing. Systematic reviews suggesting the ineffectiveness of LDRT in treating COVID-19 (reporting no improvement in mortality, clinical course, or radiological appearances) failed to take timing and dose into account when selecting their studies [12,13].
Of the studies focusing on viral pneumonia, the greatest clinical benefit is observed when low-dose radiation is delivered within 7 days of symptom development [14]. It is most likely to work best before the onset of a fibrotic response [15]. Likewise, if LDRT is given too early in the disease course, it may be counterproductive since a controlled proinflammatory state is needed for an effective immune response against COVID-19 [16]. In our trial, patient 1 received LDRT on day 5 of admission; patient 2 on day 2 of admission. Our inclusion criteria specified treatment should be given within 5 days of admission for patients needing supplemental 28–40% oxygen.
Dose is an important consideration when administering LDRT to COVID-19 patients. It has been demonstrated that radiotherapy doses of ≥1 Gy may result in proinflammatory effects and fibrosis; the opposite of the desired result [5]. Single doses below 0.1 Gy may also be pro-inflammatory. Lumniczky et al. (2022) discussed important immune and inflammation-related processes that develop after low-, intermediate-, or high-dose irradiation [17]. It was stated by Ameri et al. (2021) in their trial using different radiation doses, that 0.5 Gy was more effective than 1 Gy [18]. In our trial, both patients received a dose of 0.5 Gy to both lungs.
There are several advantages to using LDRT to treat COVID-19. Firstly, by using radiotherapy rather than steroids or antivirals, one may be able to reduce and possibly prevent selective pressure-induced adaptive mutations [5], which in theory may aid in controlling the pandemic. Furthermore, there have been instances where systemic corticosteroid use has been linked to increasing the risk of fatal fungal infections as a result of immunosuppression [19]. This is not of immediate concern with radiotherapy since this does not induce immunosuppression, but rather, the immune system is shifted to produce an anti-inflammatory response. Practically, LDRT is accessible, cheap, and time effective with one dose being performed in a median of 38 min [8].
There are several key risks which need to be taken into consideration when using LDRT to treat COVID-19:
- (a.)
- Pneumonitis—radiation lung toxicity is extremely rare at the doses given (0.5 Gy) [20].
- (b.)
- Risk of secondary malignancies—several studies have explored radiation risks associated with typical therapeutic doses (>50 Gy total) of localised radiation [21] and very-low-dose whole body radiation [22]. There is, however, no clear evidence to quantify the risk of radiation-induced malignancy when using regional ultra-low-dose radiotherapy as performed with these patients [23]. Using a cut-off age (e.g., ≥50 years) would help to reduce this risk.
- (c.)
- Cardiac toxicity—the risk of cardiac toxicity using LDRT of 0.5 Gy would be considered very low [24].
- (d.)
- Lymphopenia—the bone marrow, spleen, and circulating lymphocytes may be exposed to radiation; thus, there is a risk of potential haematological toxicity and theoretical risk of immunosuppression. This could lead to a deterioration in viral infection or increase the chance of opportunistic infections. In both our patients, the lymphocyte nadir count remained above 0.5 × 109/L [25].
- (e.)
- Cross-infection—to staff and patients, particularly oncology patients within the department who are likely to be immuno- and myelosuppressed. Appropriate protocols must be put in place to prevent cross-infection [2].
5. Conclusions
Low-dose radiotherapy, if given at the right time and dose, may have the potential to be a useful treatment option in the absence or failure of a standard treatment for severe COVID-19 lung disease. We acknowledge as a limitation that the small sample size of this report limits the ability to draw general conclusions. Considering also the small sample size of all studies included in the meta-analyses, both preclinical and early phase clinical work is required to explore the validity of this further. Variables to be addressed include as follows:
- [a]
- Dose: the distinction between 0.5 Gy, which has promising anti-inflammatory effects, and higher doses (≥1 Gy), which may trigger pro-inflammatory and fibrotic responses, must be carefully examined.
- [b]
- Timing of LDRT administration: between early (<7 days post symptom onset) and late (>7 days) treatment, as earlier intervention may prevent the progression to fibrosis and severe acute respiratory distress syndrome (ARDS), while later intervention may have diminished efficacy.
- [c]
- Disease severity (e.g., oxygen requirement, inflammatory marker levels, ARDS presence) should be explored further to define in which clinical scenaria LDRT may offer benefit.
- [d]
- Use of simple radiotherapy methods, such as utilised in our two patients, i.e., parallel-opposed anterior–posterior/posterior–anterior (AP/PA) fields, utilizing 6 MV photons on standard linear accelerators. Approaches should prioritize patient comfort, reduce treatment times, and ensure broader implementation across various healthcare settings, particularly in resource-limited environments.
- [e]
- A structured approach to analysing long-term safety outcomes to provide critical insights into the risk–benefit balance of LDRT (including immunological status, markers of cardiac toxicity, and risk of radiation-induced malignancies).
Author Contributions
Conceptualization, L.E., D.D., A.C. and D.H.; methodology, A.V. and D.H.; formal analysis, L.E. and P.T.; investigation, P.M., G.C. and W.I.; writing—original draft preparation, P.T.; writing—review and editing, L.E., D.C. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the NIHR Lancashire Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, the Department of Health, or the Lancashire Clinical Research Facility. We would also like to acknowledge the charitable funding from Lancashire Teaching Hospitals Charity.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the NHS Health Research Authority (HRA) (REF: 20/HRA/5014). IRAS project ID: 285167.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data beyond that presented in this paper are contained within patient’s medical notes and are therefore not publicly available.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO. Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 17 August 2024).
- Hadjiyiannakis, D.; Dimitroyannis, D.; Eastlake, L.; Peedell, C.; Tripathi, L.; Simcock, R.; Vyas, A.; Deutsch, E.; Chalmers, A. Personal View: Low-Dose Lung Radiotherapy Should be Evaluated as a Treatment for Severe COVID-19 Lung Disease. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, e64–e68. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Hu, C.; Wan, K.; Hu, P.; Wang, R.; Luo, J.; Li, T.; Ping, R.; Hu, Q.; Sun, Y.; et al. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int. Rev. Immunol. 2021, 41, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Shams, S.F.; Mohammadi, S.; Mortazavi, S.A.R.; Sihver, L. Low-Dose Radiation Therapy for COVID-19: A Systematic Review. Radiation 2021, 1, 234–249. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G. How radiotherapy was historically used to treat pneumonia: Could it be useful today? Yale J. Biol. Med. 2013, 86, 555–570. [Google Scholar]
- Kumar, R.; Haresh, K.; Sharma, D.; Gupta, A.; Gupta, S.; Vellaiyan, S.; Rath, G.K. Low-dose radiotherapy for COVID 19: A radioimmuno-logical perspective. J. Cancer Res. Ther. 2021, 17, 295–302. [Google Scholar] [CrossRef]
- Dunlap, N.E.; van Berkel, V.; Cai, L. COVID-19 and low-dose radiation therapy. Radiat. Med. Prot. 2021, 2, 139–145. [Google Scholar] [CrossRef]
- WHO. Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197, Erratum in Lancet Infect. Dis. 2020, 20, e250. [Google Scholar] [CrossRef]
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Cheem, N.; Connollym, B.; Dark, P.; Finney, S.; et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir. Res. 2019, 6, e000420. [Google Scholar] [CrossRef]
- Strati, P.; Shanafelt, T.D. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: Diagnosis, natural history, and risk stratification. Blood 2015, 126, 454–462. [Google Scholar] [CrossRef]
- Pandey, S.R.; Yadav, S.A.; Gautam, S.; Giri, K.; Devkota, A.; Shrestha, S.; Bhandari, S.; Baniya, S.; Adhikari, B.; Adhikari, B.; et al. Effectiveness of low-dose radiation therapy in COVID- 19 patients globally: A systematic review. F1000 Res. 2022, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, K.; Chavoshi, M.; Bayani, R.; Darzikolaee, N.M. Low-Dose Whole Lung Irradiation for Treatment of COVID-19 Pneumonia: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, A. Roentgen therapy of “virus” pneumonia. Am. J. Roentgenol. Radium Ther. 1943, 49, 635–658. [Google Scholar]
- Gupta, S.; Ahuja, R.; Sharma, N.; Singh, P.; Verma, S.; Gupta, M. Low dose lung radiotherapy for COVID-19 pneumonia: A potential treatment. Respir. Med. 2021, 186, 106531. [Google Scholar] [CrossRef]
- Kapoor, R.; Welsh, J.S.; Dhawan, V.; Javadinia, S.A.; Calabrese, E.J.; Dhawan, G. Low-dose radiation therapy (LDRT) for COVID-19 and its deadlier variants. Arch. Toxicol. 2021, 95, 3425–3432. [Google Scholar] [CrossRef]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef]
- Ameri, A.; Ameri, P.; Rahnama, N.; Mokhtari, M.; Sedaghat, M.; Hadavand, F.; Bozorgmehr, R.; Haghighi, M.; Taghizadeh-Hesary, F. Low-Dose Whole-Lung Irradiation for COVID-19 Pneumonia: Final Results of a Pilot Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 859–866. [Google Scholar] [CrossRef]
- Ahmadikia, K.; Hashemi, S.J.; Khodavaisy, S.; Getso, M.I.; Alijani, N.; Badali, H.; Mirhendi, H.; Salehi, M.; Tabari, A.; Ardehali, M.M.; et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021, 64, 798–808. [Google Scholar] [CrossRef]
- Rahi, M.S.; Parekh, J.; Pednekar, P.; Parmar, G.; Abraham, S.; Nasir, S.; Subramaniyam, R.; Jeyashanmugaraja, G.P.; Gunasekaran, K. Radiation-Induced Lung Injury—Current Perspectives and Management. Clin. Pract. 2021, 11, 410–429. [Google Scholar] [CrossRef]
- Journy, N.; Mansouri, I.; Allodji, R.S.; Demoor-Goldschmidt, C.; Ghazi, D.; Haddy, N.; Rubino, C.; Veres, C.; Zrafi, W.S.; Rivera, S.; et al. Volume effects of radiotherapy on the risk of second primary cancers: A systematic review of clinical and epidemiological studies. Radiother. Oncol. 2019, 131, 150–159. [Google Scholar] [CrossRef]
- Douple, E.B.; Mabuchi, K.; Cullings, H.M.; Preston, D.L.; Kodama, K.; Shimizu, Y.; Fujiwara, S.; Shore, R.E. Long-term Radiation-Related Health Effects in a Unique Human Population: Lessons Learned from tIhe Atomic Bomb Survivors of Hiroshima and Nagasaki. Disaster Med. Public Health Prep. 2011, 5 (Suppl. S1), S122–S133. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.G.; Diehn, M.; Cucinotta, F.A.; Weichselbaum, R. Lack of supporting data make the risks of a clinical trial of radiation therapy as a treatment for COVID-19 pneumonia unacceptable. Radiother. Oncol. 2020, 147, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Hufnagle, J.J.; Andersen, S.N.; Maani, E.V. Radiation-Induced Cardiac Toxicity. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nag, S.; Shah, V. Once-a-week lower hemibody irradiation (HBI) for metastatic cancers. Int. J. Radiat. Oncol. 1986, 12, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).