Longitudinal Measurements of Inflammatory Indices During Treatment for Locally Advanced Rectal Cancer and Associations with Smoking, Ethnicity and Pathological Response
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Adjuvant Therapy Received
3.3. Overall Trends of Inflammatory Markers During Neoadjuvant and Adjuvant Treatment
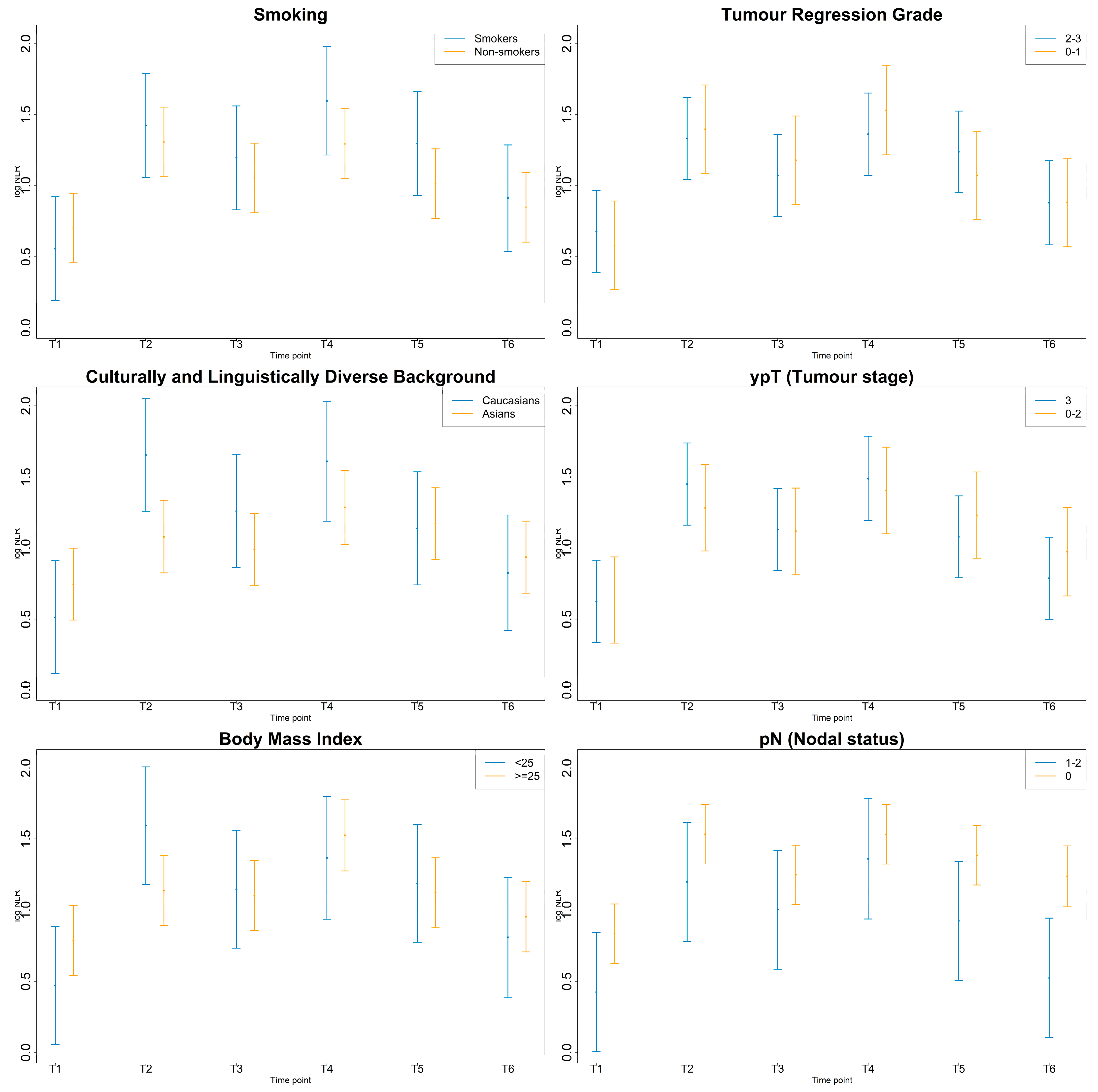
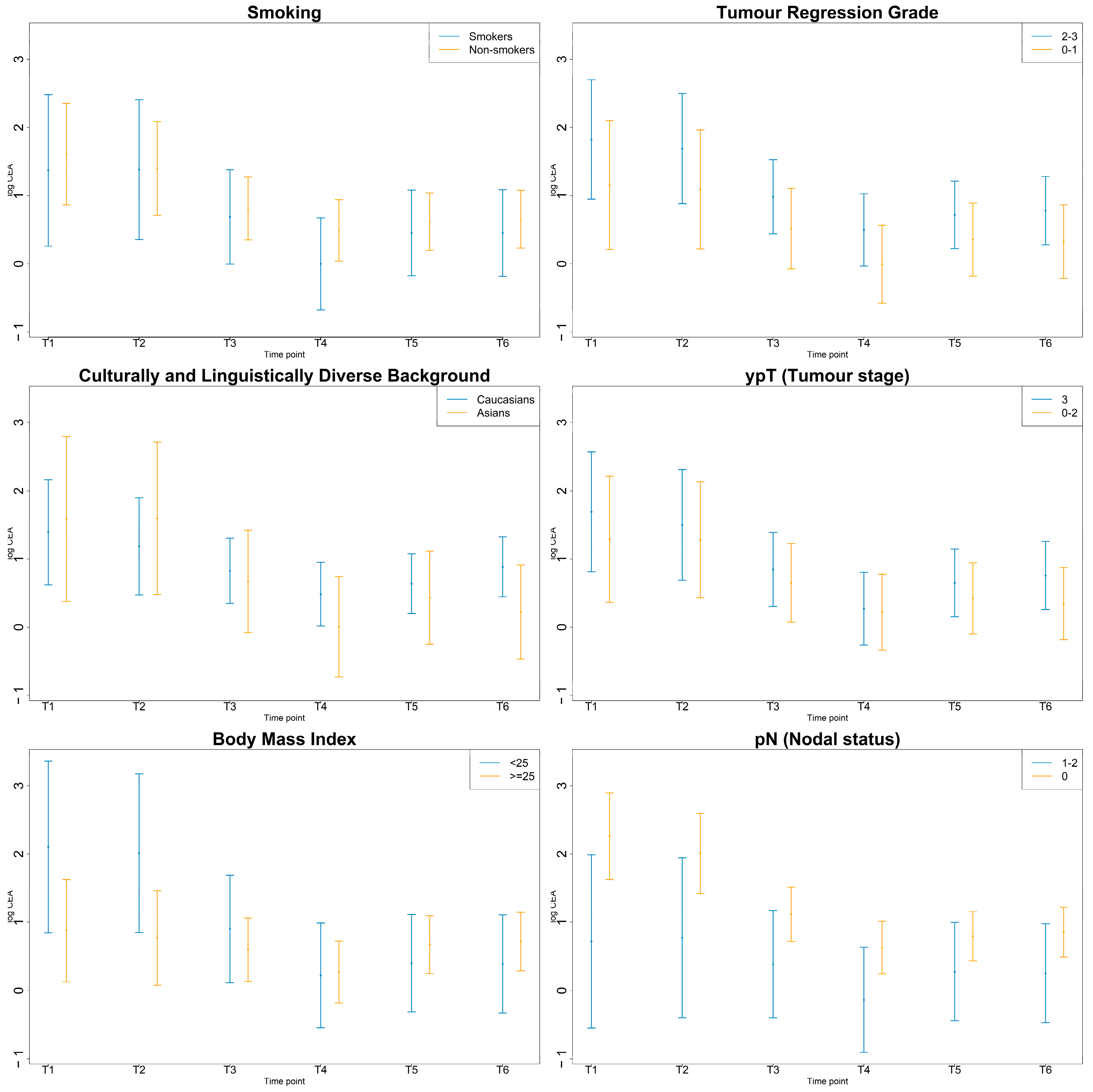
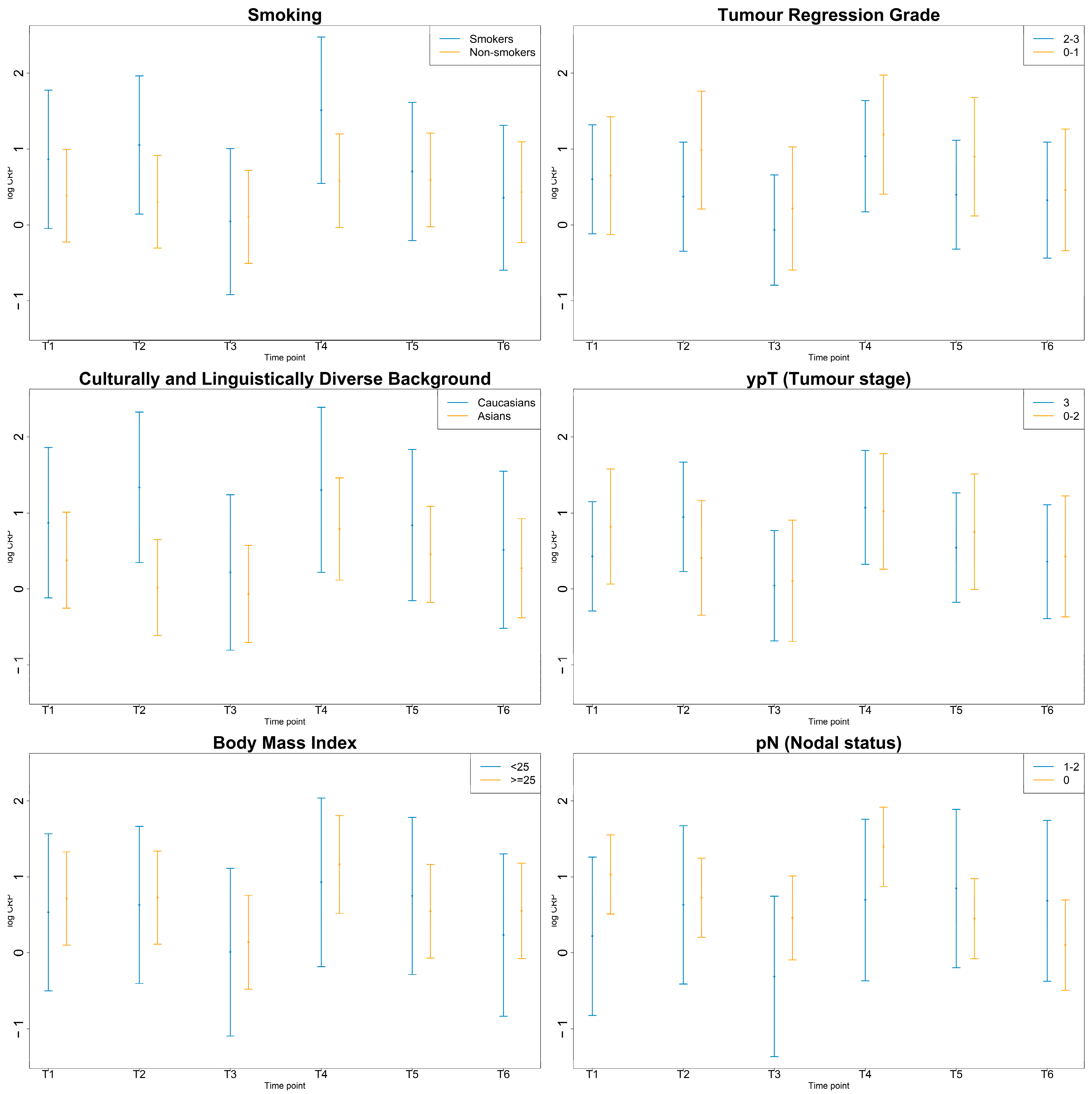
3.4. Inflammatory Markers by Smoking, CALD, BMI Status and Pathologic Response
3.4.1. NLR
3.4.2. PLR
3.4.3. CEA
3.4.4. CRP
3.4.5. Albumin and Fibrinogen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LARC | Locally advanced rectal cancer |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| CRP | C-reactive protein |
| CEA | Carcinoembryonic antigen |
| CALD | Culturally and linguistically diverse |
| BMI | Body mass index |
References
- Dolan, R.D.; McSorley, S.T.; Horgan, P.G.; Laird, B.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2017, 116, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Wieder, T.; Brenner, E.; Braumüller, H.; Bischof, O.; Röcken, M. Cytokine-induced senescence for cancer surveillance. Cancer Metastasis Rev. 2017, 36, 357–365. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; He, X.; Yuan, Y.; Wei, X. Targeting inflammation as cancer therapy. J. Hematol. Oncol. 2024, 17, 13. [Google Scholar] [CrossRef]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of Locally Advanced Rectal Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Colloca, G.; Venturino, A.; Guarneri, D. Neutrophil-to-lymphocyte ratio predicts survival of patients with rectal cancer receiving neo-adjuvant chemoradiation followed by radical resection: A meta-analysis. Expert Rev. Anticancer Ther. 2023, 23, 421–429. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, K.; Park, H.J. Meta-Analysis on the Neutrophil-Lymphocyte Ratio in Rectal Cancer Treated with Preoperative Chemoradiotherapy: Prognostic Value of Pre- and Post-Chemoradiotherapy Neutrophil-Lymphocyte Ratio. Front. Oncol. 2022, 12, 778607. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Bartolotta, P.; Azzolina, D.; Gregori, D.; Fiscon, V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: Systematic review and meta-analysis. Langenbeck’s Arch. Surg. 2023, 408, 85. [Google Scholar] [CrossRef]
- Hamid, H.K.S.; Emile, S.H.; Davis, G.N. Prognostic Significance of Lymphocyte-to-Monocyte and Platelet-to-Lymphocyte Ratio in Rectal Cancer: A Systematic Review, Meta-analysis, and Meta-regression. Dis. Colon Rectum 2022, 65, 178–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xu, M.; Chen, K.; Li, S.; Guan, G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci. Rep. 2020, 10, 8017. [Google Scholar] [CrossRef] [PubMed]
- Wallin, U.; Rothenberger, D.; Lowry, A.; Luepker, R.; Mellgren, A. CEA—A predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis. Colon Rectum 2013, 56, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-L.; Yang, S.-H.; Liang, W.-Y.; Kuo, Y.-J.; Lin, J.-K.; Lin, T.-C.; Chen, W.-S.; Jiang, J.-K.; Wang, H.-S.; Chang, S.-C.; et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat. Oncol. 2013, 8, 43. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, H.R.; Kim, Y.J. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis. Colon Rectum 2013, 56, 698–703. [Google Scholar] [CrossRef]
- Hu, H.; Huang, J.; Lan, P.; Wang, L.; Huang, M.; Wang, J.; Deng, Y. CEA clearance pattern as a predictor of tumor response to neoadjuvant treatment in rectal cancer: A post-hoc analysis of FOWARC trial. BMC Cancer 2018, 18, 1145. [Google Scholar] [CrossRef]
- Kim, C.W.; Yu, C.S.; Yang, S.-S.; Kim, K.H.; Yoon, Y.S.; Yoon, S.N.; Lim, S.-B.; Kim, J.C. Clinical significance of pre-to post-chemoradiotherapy s-CEA reduction ratio in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Ann. Surg. Oncol. 2011, 18, 3271–3277. [Google Scholar] [CrossRef]
- Saito, G.; Sadahiro, S.; Ogimi, T.; Miyakita, H.; Okada, K.; Tanaka, A.; Suzuki, T. Relations of Changes in Serum Carcinoembryonic Antigen Levels before and after Neoadjuvant Chemoradiotherapy and after Surgery to Histologic Response and Outcomes in Patients with Locally Advanced Rectal Cancer. Oncology 2017, 94, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, H.; Qiu, J.; Wu, S.; Gan, Y.; Shao, L.; Lin, C.; Hong, L.; Wu, J. Analysis of risk characteristics for early progression and late progression in locally advanced rectal cancer patients: A large population-based and validated study. Support. Care Cancer 2024, 32, 340. [Google Scholar] [CrossRef]
- Lu, S.; Liu, Z.; Zhou, X.; Wang, B.; Li, F.; Ma, Y.; Wang, W.; Ma, J.; Wang, Y.; Wang, H.; et al. Preoperative Fibrinogen-Albumin Ratio Index (FARI) is a Reliable Prognosis and Chemoradiotherapy Sensitivity Predictor in Locally Advanced Rectal Cancer Patients Undergoing Radical Surgery Following Neoadjuvant Chemoradiotherapy. Cancer Manag. Res. 2020, 12, 8555–8568. [Google Scholar] [CrossRef]
- Tawfik, B.; Mokdad, A.A.; Patel, P.M.; Li, H.C.; Huerta, S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anti-Cancer Drugs 2016, 27, 879–883. [Google Scholar] [CrossRef]
- Toiyama, Y.; Oki, S.; Okugawa, Y.; Ide, S.; Yasuda, H.; Fujikawa, H.; Yoshiyama, S.; Hiro, J.; Ohi, M.; Inoue, Y.; et al. Clinical Impact of Preoperative Albumin-Globulin Ratio in Patients with Rectal Cancer Treated with Preoperative Chemoradiotherapy. Oncology 2018, 95, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Toiyama, Y.; Okugawa, Y.; Oki, S.; Yasuda, H.; Fujikawa, H.; Yoshiyama, S.; Hiro, J.; Kobayashi, M.; Ohi, M.; et al. Clinical Significance of C-Reactive Protein-to- Albumin Ratio with Rectal Cancer Patient Undergoing Chemoradiotherapy Followed by Surgery. Anticancer Res. 2017, 37, 5797–5804. [Google Scholar] [CrossRef]
- Farmer, H.R.; Wray, L.A.; Xian, Y.; Xu, H.; Pagidipati, N.; Peterson, E.D.; Dupre, M.E. Racial Differences in Elevated C-Reactive Protein Among US Older Adults. J. Am. Geriatr. Soc. 2019, 68, 362–369. [Google Scholar] [CrossRef]
- Stepanikova, I.; Bateman, L.B.; Oates, G.R. Systemic Inflammation in Midlife: Race, Socioeconomic Status, and Perceived Discrimination. Am. J. Prev. Med. 2017, 52, S63–S76. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.-S.; Ip, E.; Ng, W.; Chua, W.; Asghari, R.; Roohullah, A.; Descallar, J.; Henderson, C.; Spring, K.; de Souza, P.; et al. Health-Related Quality of Life during Chemoradiation in Locally Advanced Rectal Cancer: Impacts and Ethnic Disparities. Cancers 2019, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Christofaro, D.G.D.; Ritti-Dias, R.M.; Tebar, W.R.; Werneck, A.O.; Bittencourt, M.S.; Cucato, G.G.; Santos, R.D. Are C-reactive protein concentrations affected by smoking status and physical activity levels? A longitudinal study. PLoS ONE 2023, 18, e0293453. [Google Scholar] [CrossRef]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette Smoking and Variations in Systemic Immune and Inflammation Markers. JNCI J. Natl. Cancer Inst. 2014, 106, dju294. [Google Scholar] [CrossRef]
- Sharp, L.; McDevitt, J.; Brown, C.; Carsin, A.-E.; Comber, H. Association between smoking at diagnosis and cause-specific survival in patients with rectal cancer: Results from a population-based analysis of 10,794 cases. Cancer 2017, 123, 2543–2550. [Google Scholar] [CrossRef][Green Version]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef]
- Khonsari, N.M.; Baygi, F.; Tabatabaei-Malazy, O.; Nami, S.M.; Ehsani, A.; Asadi, S.; Qorbani, M. Association of normal weight obesity phenotype with inflammatory markers: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1044178. [Google Scholar]
- 2021 Sydney—South West, Census All Persons QuickStats|Australian Bureau of Statistics. Available online: https://www.abs.gov.au/census/find-census-data/quickstats/2021/127 (accessed on 2 December 2024).
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Gibbons, D.; Hyland, J.M.P.; Treanor, D.; White, A.; E Mulcahy, H.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016, 18, 10. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, H.; Liang, L.; Li, G.; Fan, M.; Wu, Y.; Zhu, J.; Zhang, Z. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat. Oncol. 2014, 9, 295. [Google Scholar] [CrossRef]
- Carruthers, R.; Tho, L.M.; Brown, J.; Kakumanu, S.; McCartney, E.; McDonald, A.C. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Color. Dis. 2012, 14, e701–e707. [Google Scholar] [CrossRef]
- Morais, M.; Fonseca, T.; Machado-Neves, R.; Honavar, M.; Coelho, A.R.; Lopes, J.; Guerreiro, E.; Carneiro, S. Prognostic value of neutrophil-to-lymphocyte ratio (NLR) and platelet-neutrophil (PN) index in locally advanced rectal cancer patients: A retrospective cohort study. Ann. Med. Surg. 2024, 86, 2474–2480. [Google Scholar] [CrossRef]
- Dudani, S.; Marginean, H.; Tang, P.A.; Monzon, J.G.; Raissouni, S.; Asmis, T.R.; Goodwin, R.A.; Gotfrit, J.; Cheung, W.Y.; Vickers, M.M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer 2019, 19, 664. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Yuan, X.; Fu, M.; Qian, H.; Xu, W. Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review). Int. J. Oncol. 2016, 49, 857–867. [Google Scholar] [CrossRef]
- Oberg, H.H.; Wesch, D.; Kalyan, S.; Kabelitz, D. Regulatory interactions between neutrophils, tumor cells and T Cells. Front. Immunol. 2019, 10, 459623. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, M.; Shi, J.; Hua, L.; Gong, Z.; Li, Q.; Shultz, L.D.; Ren, G. Dual roles of neutrophils in metastatic colonization are governed by the host NK cell status. Nat. Commun. 2020, 11, 4387. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.Y.; Cho, H.M.; Shim, B.Y.; Kim, T.H.; Kim, S.Y.; Baek, J.Y.; Oh, J.H.; Nam, T.K.; Yoon, M.S.; et al. Carcinoembryonic antigen has prognostic value for tumor downstaging and recurrence in rectal cancer after preoperative chemoradiotherapy and curative surgery: A multi-institutional and case-matched control study of KROG 14-12. Radiother. Oncol. 2015, 116, 202–208. [Google Scholar] [CrossRef]
- Güden, M.; Tunç Karaman, S.; Basat, O. Evaluation of the relationship between the level of addiction and exhaled carbon monoxide levels with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in smokers. Tob. Induc. Dis. 2022, 20, 52. [Google Scholar] [CrossRef]
- Pedersen, K.M.; Çolak, Y.; Ellervik, C.; Hasselbalch, H.C.; Bojesen, S.E.; Nordestgaard, B.G. Smoking and Increased White and Red Blood Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 965–977. [Google Scholar] [CrossRef]
- Pujani, M.; Chauhan, V.; Singh, K.; Rastogi, S.; Agarwal, C.; Gera, K. The effect and correlation of smoking with platelet indices, neutrophil lymphocyte ratio and platelet lymphocyte ratio. Hematol. Transfus. Cell Ther. 2021, 43, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.C., Jr.; Silverman, N.A.; Chretien, P.B. Effect of Age and Cigarette Smoking on Carcinoembryonic Antigen Levels. JAMA 1976, 235, 1975–1979. [Google Scholar] [CrossRef]
- Fukuda, I.; Yamakado, M.; Kiyose, H. Influence of Smoking on Serum Carcinoembryonic Antigen Levels in Subjects Who Underwent Multiphasic Health Testing and Services. J. Med. Syst. 1998, 22, 89–93. [Google Scholar] [CrossRef]
- Howard, R.; Scheiner, A.; Kanetsky, P.A.; Egan, K.M. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann. Epidemiol. 2019, 38, 11–21.e6. [Google Scholar] [CrossRef]
- Nagar, S.D.; Conley, A.B.; Sharma, S.; Rishishwar, L.; Jordan, I.K.; Mariño-Ramírez, L. Comparing Genetic and Socioenvironmental Contributions to Ethnic Differences in C-Reactive Protein. Front. Genet. 2021, 12, 738485. [Google Scholar] [CrossRef]
- Segal, J.B.; Moliterno, A.R. Platelet Counts Differ by Sex, Ethnicity, and Age in the United States. Ann. Epidemiol. 2006, 16, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pino, M.D.; Richardson, W.S.; Zabaleta, J.; Puttalingaiah, R.T.; Chapple, A.G.; Liu, J.; Kim, Y.; Ponder, M.; DeArmitt, R.; Baiamonte, L.B.; et al. Increased inflammatory low-density neutrophils in severe obesity and effect of bariatric surgery: Results from case-control and prospective cohort studies. eBioMedicine 2022, 77, 103910. [Google Scholar] [CrossRef]
- Juszczyk, K.; Kang, S.; Putnis, S.; Winn, R.; Chen, J.; Aghmesheh, M.; Fylyk, G.; Brungs, D. High body mass index is associated with an increased overall survival in rectal cancer. J. Gastrointest. Oncol. 2020, 11, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kalb, M.; Langheinrich, M.C.; Merkel, S.; Krautz, C.; Brunner, M.; Bénard, A.; Weber, K.; Pilarsky, C.; Grützmann, R.; Weber, G.F. Influence of Body Mass Index on Long-Term Outcome in Patients with Rectal Cancer—A Single Centre Experience. Cancers 2019, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.P.; Lobo, D.N. The clinical significance of hypoalbuminaemia. Clin. Nutr. 2024, 43, 909–914. [Google Scholar] [CrossRef]
- Saal, J.; Bald, T.; Hölzel, M.; Ritter, M.; Brossart, P.; Ellinger, J.; Klümper, N. In the phase III IMmotion151 trial of metastatic renal cell carcinoma the easy-to-implement modified Glasgow prognostic score predicts outcome more accurately than the IMDC score. Ann. Oncol. 2022, 33, 982–984. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; A Dijkstra, E.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]


| N | % | |
|---|---|---|
| Total patients | 29 | 100 |
| Male | 21 | 72 |
| Female | 8 | 28 |
| Age at diagnosis (years) | ||
| Median (range) | 58 (41–82) | |
| Culturally and linguistically diverse background | ||
| Asian | 15 | 52 |
| Caucasian | 14 | 48 |
| Smoking status | ||
| Never smoker | 16 | 55 |
| Current or ex-smoker | 13 | 45 |
| Tumour regression grade | ||
| 0–1 | 16 | 55 |
| 2–3 | 13 | 45 |
| Pathologic tumour stage (ypT) | ||
| 0–2 | 15 | 52 |
| 3 | 14 | 48 |
| Pathologic nodal stage (ypN) | ||
| 0 | 24 | 83 |
| 1–2 | 5 | 17 |
| Recurrent disease | 7 | 24 |
| Adjuvant therapy | ||
| Capecitabine | 24 | 83 |
| FOLFOX (5-fluorouracil and oxaliplatin) | 2 | 7 |
| CAPOX (capecitabine and oxaliplatin) | 1 | 3 |
| T1 | T2 | T3 | T4 | T5 | T6 | |
|---|---|---|---|---|---|---|
| Neutrophil-to-lymphocyte ratio | 2.4 (0.9–5.4) | 3.9 (1.4–10.6) | 3.0 (1.6–6.3) | 4.6 (1.6–9.6) | 3.7 (1.1–7.3) | 3.4 (1.3–6.2) |
| Platelet-to-lymphocyte ratio | 150 (76–264) | 277 (88–556) | 255 (123–426) | 349 (166–624) | 273 (112–495) | 243 (124–407) |
| Carcinoembryonic antigen (μg/L) | 4.5 (1.1–105) | 3.7 (1.1–79.4) | 2.7 (0.6–15.1) | 1.8 (0.6–15.1) | 2.3 (0.6–7) | 2.4 (0.6–7.5) |
| Albumin (g/L) | 44 (40–50) | 43 (38–47) | 44 (39–50) | 43 (33–52) | 45 (37–49) | 43 (36–51) |
| C-reactive protein (mg/L) | 2.3 (0.4–16) | 1.5 (0.2–62.2) | 1.4 (0.4–15.3) | 4.1 (0.4–19.8) | 1.6 (0.3–39.5) | 1.3 (0.4–8.5) |
| Fibrinogen (g/L) | 3.8 (2.8–6.3) | 3.7 (2.6–5.5) | 3.8 (2.6–4.8) | 4.5 (2.7–7.9) | 3.6 (2.6–6) | 3.5 (2.2–5.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, N.; Descallar, J.; Chua, W.; Ng, W.; Ip, E.; Henderson, C.; Roberts, T.L.; Lim, S.H.-S. Longitudinal Measurements of Inflammatory Indices During Treatment for Locally Advanced Rectal Cancer and Associations with Smoking, Ethnicity and Pathological Response. Radiation 2025, 5, 15. https://doi.org/10.3390/radiation5020015
Huang N, Descallar J, Chua W, Ng W, Ip E, Henderson C, Roberts TL, Lim SH-S. Longitudinal Measurements of Inflammatory Indices During Treatment for Locally Advanced Rectal Cancer and Associations with Smoking, Ethnicity and Pathological Response. Radiation. 2025; 5(2):15. https://doi.org/10.3390/radiation5020015
Chicago/Turabian StyleHuang, Nancy, Joseph Descallar, Wei Chua, Weng Ng, Emilia Ip, Christopher Henderson, Tara L. Roberts, and Stephanie Hui-Su Lim. 2025. "Longitudinal Measurements of Inflammatory Indices During Treatment for Locally Advanced Rectal Cancer and Associations with Smoking, Ethnicity and Pathological Response" Radiation 5, no. 2: 15. https://doi.org/10.3390/radiation5020015
APA StyleHuang, N., Descallar, J., Chua, W., Ng, W., Ip, E., Henderson, C., Roberts, T. L., & Lim, S. H.-S. (2025). Longitudinal Measurements of Inflammatory Indices During Treatment for Locally Advanced Rectal Cancer and Associations with Smoking, Ethnicity and Pathological Response. Radiation, 5(2), 15. https://doi.org/10.3390/radiation5020015






