Assessment of Tumor Infiltrating Lymphocytes in Predicting Stereotactic Ablative Radiotherapy (SABR) Response in Unresectable Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data Source
2.2. Patient Population
2.3. Treatment Response Evaluation (Ground Truth Labels for Modeling)
2.4. Clinicopathologic Parameters
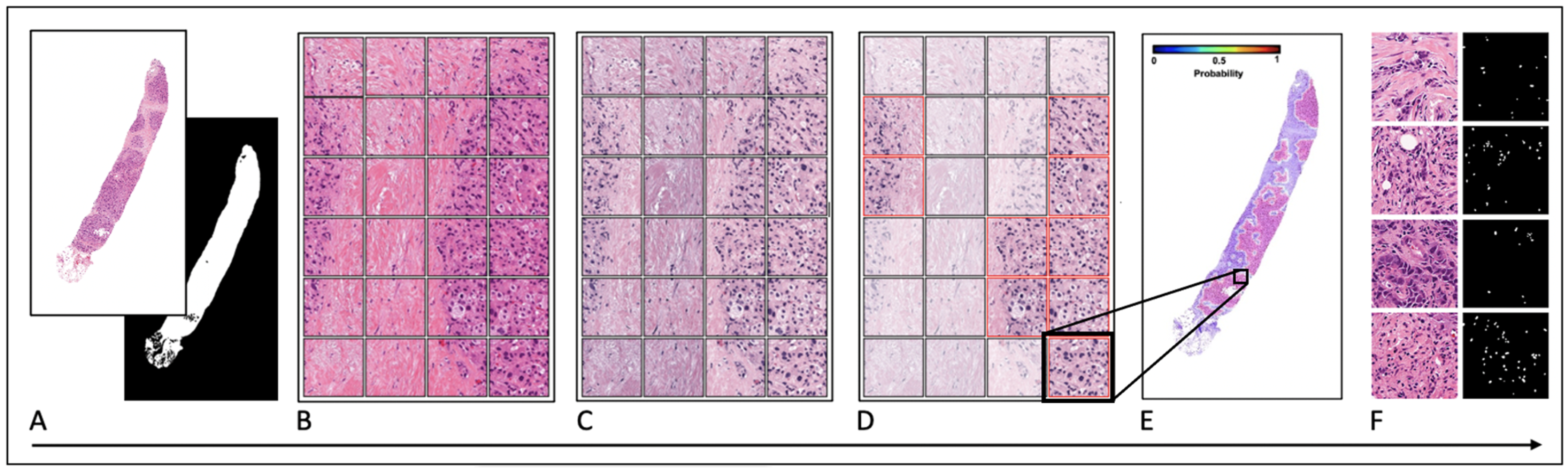
2.5. Tissue Preparation and Digital Pathology
2.6. Quantitative Image Analysis and Feature Extraction
2.7. Statistical Analysis
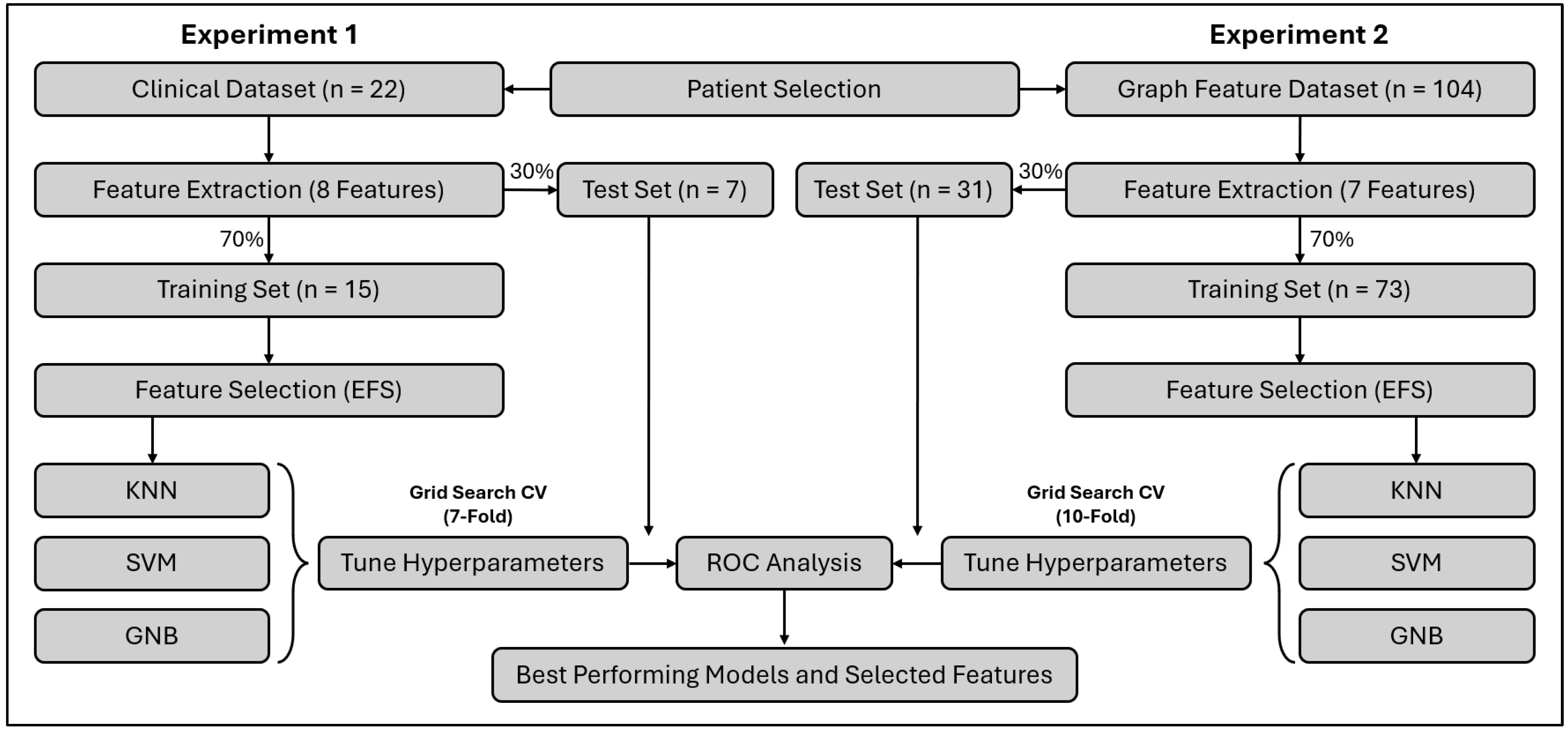
2.8. Machine Learning Prediction Modeling
3. Results
3.1. Patients and Dataset
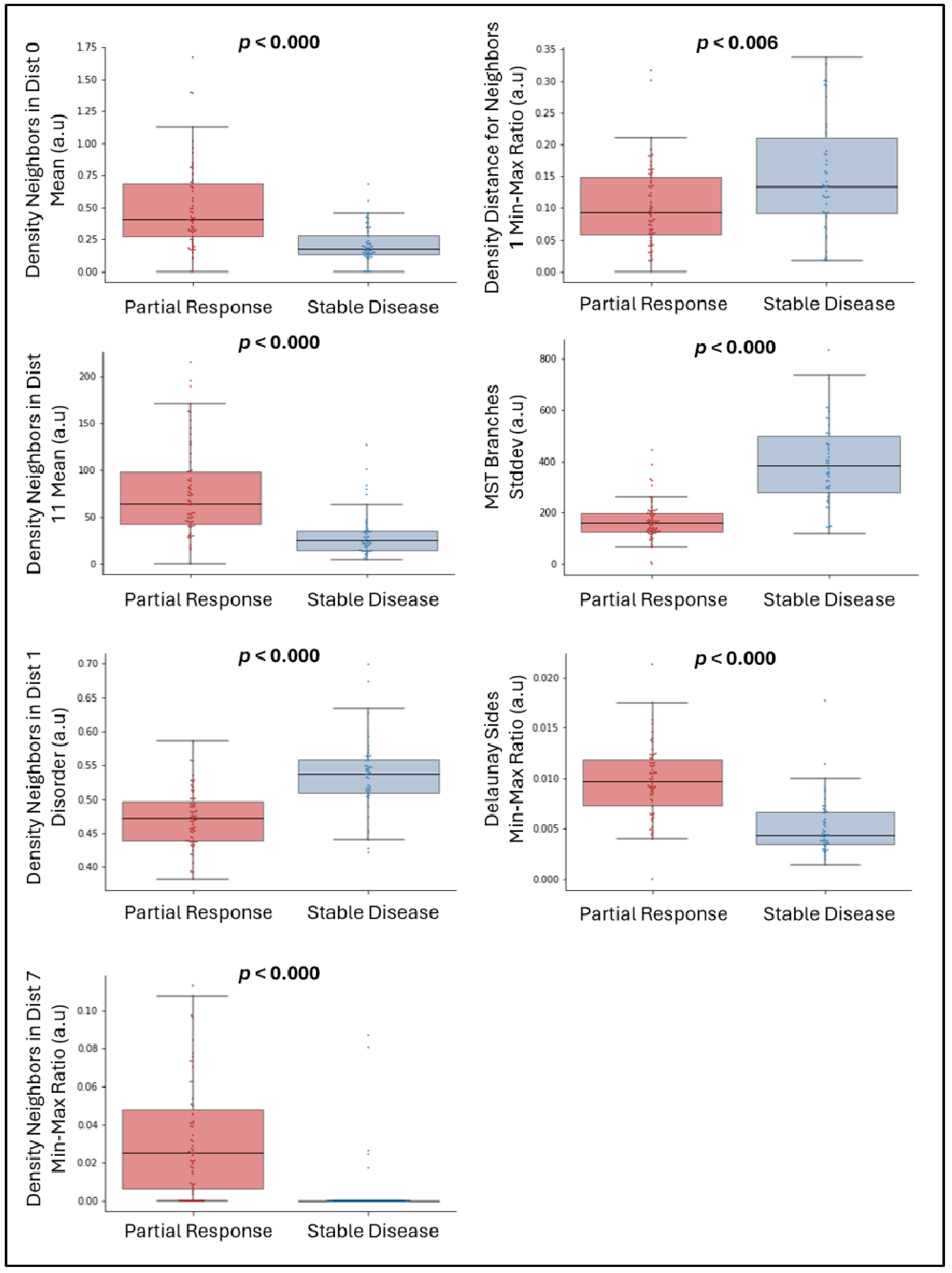
3.2. Significant Univariate Features
3.3. Multiparametric Clinical Models
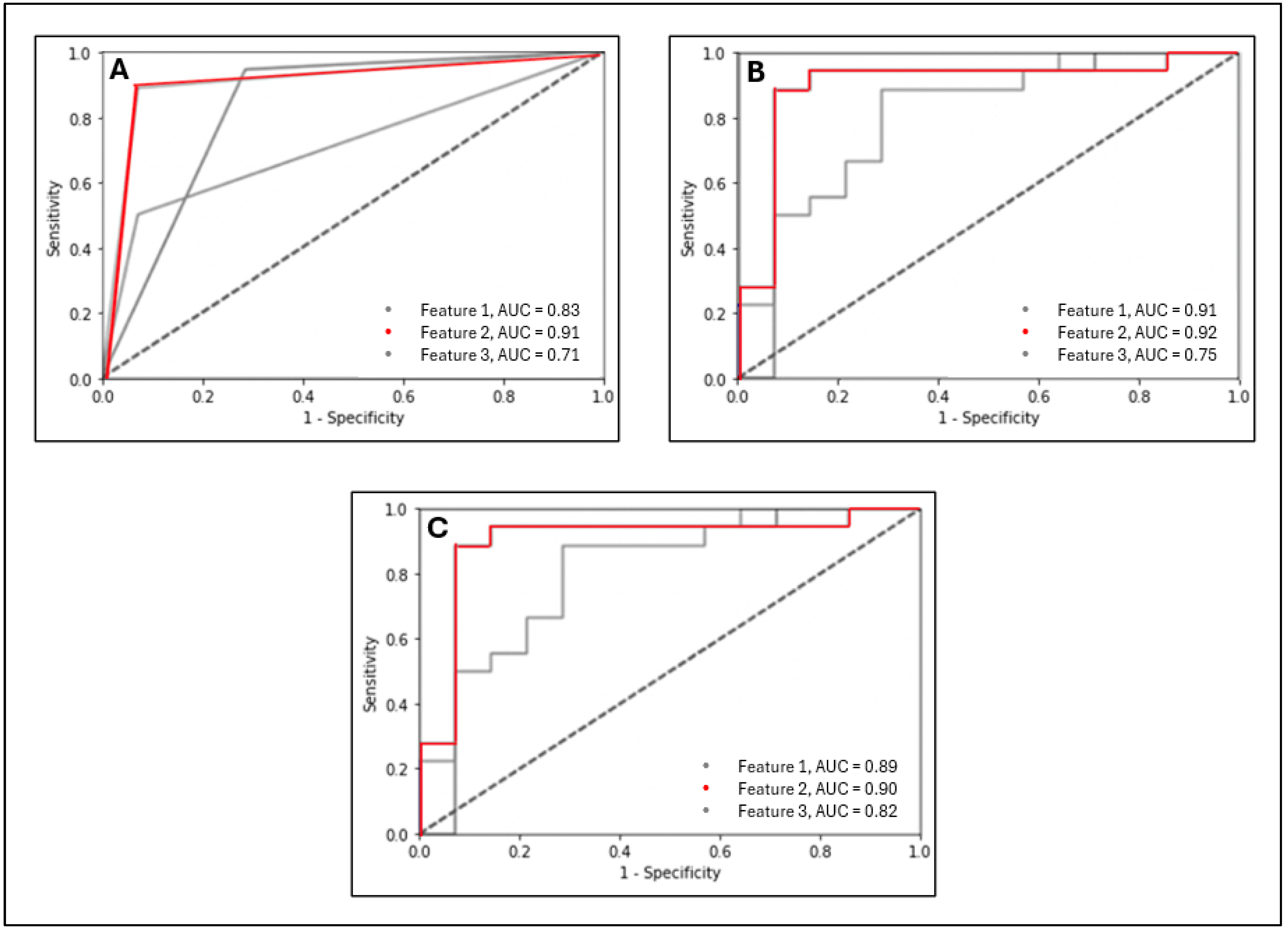
3.4. Single-Feature Graph Models
3.5. Multiple Feature Graph Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.C.; Ueno, N.T. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer 2012, 19, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E. Metastatic Breast Cancer: The Treatment Challenge. Clin. Breast Cancer 2008, 8, 224–233. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.C.; Loprinzi, C.L.; Haddad, T.C. Updates in the Evaluation and Management of Breast Cancer. Mayo Clin. Proc. 2018, 93, 794–807. [Google Scholar] [CrossRef]
- Ruiterkamp, J.; Ernst, M.F. The role of surgery in metastatic breast cancer. Eur. J. Cancer 2011, 47, S6–S22. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.C.; Da Silva, F.M.L.; Do Carmo, I.M.L.; Bonaccorsi, B.V.; Hahn, S.M.; Faroni, L.D. Is there a role for salvage radiotherapy in locally advanced breast cancer refractory to neoadjuvant chemotherapy? Breast 2017, 31, 192–196. [Google Scholar] [CrossRef]
- Jardel, P.; Kammerer, E.; Villeneuve, H.; Thariat, J. Stereotactic radiation therapy for breast cancer in the elderly. Transl. Cancer Res. 2020, 9, S86–S96. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The Tumour Microenvironment after Radiotherapy: Mechanisms of Resistance and Recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Lhuillier, C.; Rudqvist, N.-P.; Yamazaki, T.; Zhang, T.; Charpentier, M.; Galluzzi, L.; Dephoure, N.; Clement, C.C.; Santambrogio, L.; Zhou, X.K.; et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Investig. 2021, 131, e138740. [Google Scholar] [CrossRef]
- Lugade, A.; Moran, J.; Gerber, S.; Rose, R.; Frelinger, J.; Lord, E. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516–7523. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Pusztai, L.; Karn, T.; Safonov, A.; Abu-Khalaf, M.M.; Bianchini, G. New Strategies in Breast Cancer: Immunotherapy. Clin. Cancer Res. 2016, 22, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic Effects of Ablative Radiation on Local Tumor Require CD8+ T Cells: Changing Strategies for Cancer Treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Ko, E.C.; Benjamin, K.T.; Formenti, S.C. Generating antitumor immunity by targeted radiation therapy: Role of dose and fractionation. Adv. Radiat. Oncol. 2018, 3, 486–493. [Google Scholar] [CrossRef]
- Krombach, J.; Hennel, R.; Brix, N.; Orth, M.; Schoetz, U.; Ernst, A.; Schuster, J.; Zuchtriegel, G.; Reichel, C.A.; Bierschenk, S.; et al. Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. OncoImmunology 2019, 8, e1523097. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; White, M.; Tan, J.; Siva, S.; Karroum, L.; David, S. SABR in oligometastatic breast cancer: Current status and future directions. Breast 2021, 60, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lagree, A.; Shiner, A.; Alera, M.A.; Fleshner, L.; Law, E.; Law, B.; Lu, F.-I.; Dodington, D.; Gandhi, S.; Slodkowska, E.A.; et al. Assessment of Digital Pathology Imaging Biomarkers Associated with Breast Cancer Histologic Grade. Curr. Oncol. 2021, 28, 4298–4316. [Google Scholar] [CrossRef]
- Otsu, N. A Tlreshold Selection Method from Gray-Level Histograms. Automatica 1975, 11, 23–27. [Google Scholar]
- OpenCV: Structural Analysis and Shape Descriptors. Available online: https://docs.opencv.org/3.4/d3/dc0/group__imgproc__shape.html#ga107a78bf7cd25dec05fb4dfc5c9e765f (accessed on 13 February 2024).
- Dillencourt, M.B.; Samet, H.; Tamminen, M. A general approach to connected-component labeling for arbitrary image representations. J. ACM 1992, 39, 253–280. [Google Scholar] [CrossRef]
- He, L.; Ren, X.; Gao, Q.; Zhao, X.; Yao, B.; Chao, Y. The connected-component labeling problem: A review of state-of-the-art algorithms. Pattern Recognit. 2017, 70, 25–43. [Google Scholar] [CrossRef]
- Graham, S.; Vu, Q.D.; Raza, S.E.A.; Azam, A.; Tsang, Y.W.; Kwak, J.T.; Rajpoot, N. Hover-Net: Simultaneous segmentation and classification of nuclei in multi-tissue histology images. Med. Image Anal. 2019, 58, 101563. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Kumar, N.; Patil, A.; Kurian, N.C.; Rane, S.; Graham, S.; Vu, Q.D.; Zwager, M.; Raza, S.E.A.; Rajpoot, N.; et al. MoNuSAC2020: A Multi-Organ Nuclei Segmentation and Classification Challenge. IEEE Trans. Med. Imaging 2021, 40, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.; Agner, S.; Madabhushi, A.; Feldman, M.; Tomaszewski, J. Automated Grading of Breast Cancer Histopathology Using Spectral Clustering with Textural and Architectural Image Features. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; Volume 29, p. 499, ISBN 978-1-4244-2002-5. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P.; et al. SMOTE: Synthetic Minority over-Sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Scott, J.G.; Berglund, A.; Schell, M.J.; Mihaylov, I.; Fulp, W.J.; Yue, B.; Welsh, E.; Caudell, J.J.; Ahmed, K.; Strom, T.S.; et al. A genome-based model for adjusting radiotherapy dose (GARD): A retrospective, cohort-based study. Lancet Oncol. 2017, 18, 202–211. [Google Scholar] [CrossRef]
- Tanić, M.; Krivokuća, A.; Čavić, M.; Mladenović, J.; Plesinac Karapandžić, V.; Beck, S.; Radulović, S.; Susnjar, S.; Janković, R. Molecular signature of response to preoperative radiotherapy in locally advanced breast cancer. Radiat. Oncol. 2018, 13, 193. [Google Scholar] [CrossRef]
- Thomas, G.; Eisenhauer, E.; Bristow, R.G.; Grau, C.; Hurkmans, C.; Ost, P.; Guckenberger, M.; Deutsch, E.; Lacombe, D.; Weber, D.C.; et al. The European Organisation for Research and Treatment of Cancer, State of Science in radiation oncology and priorities for clinical trials meeting report. Eur. J. Cancer 2020, 131, 76–88. [Google Scholar] [CrossRef]
- Vasmel, J.E.; Vreuls, C.P.H.; Manson, Q.F.; Charaghvandi, R.K.; van Gorp, J.; van Leeuwen, A.M.G.; van Diest, P.J.; Verkooijen, H.M.; van den Bongard, H.J.G.D. Tumor-Infiltrating Lymphocytes in Low-Risk Patients with Breast Cancer Treated with Single-Dose Preoperative Partial Breast Irradiation. Int. J. Radiat. Oncol. 2021, 109, 1325–1331. [Google Scholar] [CrossRef]
- Tramm, T.; Vinter, H.; Vahl, P.; Özcan, D.; Alsner, J.; Overgaard, J. Tumor-infiltrating lymphocytes predict improved overall survival after post-mastectomy radiotherapy: A study of the randomized DBCG82bc cohort. Acta Oncol. Stockh. Swed. 2022, 61, 153–162. [Google Scholar] [CrossRef]
- Kovács, A.; Stenmark Tullberg, A.; Werner Rönnerman, E.; Holmberg, E.; Hartman, L.; Sjöström, M.; Lundstedt, D.; Malmström, P.; Fernö, M.; Karlsson, P. Effect of Radiotherapy After Breast-Conserving Surgery Depending on the Presence of Tumor-Infiltrating Lymphocytes: A Long-Term Follow-Up of the SweBCG91RT Randomized Trial. J. Clin. Oncol. 2019, 37, 1179–1187. [Google Scholar] [CrossRef]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193.e7. [Google Scholar] [CrossRef]
- Amgad, M.; Stovgaard, E.S.; Balslev, E.; Thagaard, J.; Chen, W.; Dudgeon, S.; Sharma, A.; Kerner, J.K.; Denkert, C.; Yuan, Y.; et al. Report on computational assessment of Tumor Infiltrating Lymphocytes from the International Immuno-Oncology Biomarker Working Group. npj Breast Cancer 2020, 6, 16. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, W. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Ahlers, S.M.; Stratman, C.; Aridor, O.; Pantanowitz, L.; Fine, J.L.; Kuzmishin, J.A.; Montalto, M.C.; Parwani, A.V. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. J. Pathol. Inform. 2014, 5, 33. [Google Scholar] [CrossRef]
- Milano, M.T.; Katz, A.W.; Schell, M.C.; Philip, A.; Okunieff, P. Descriptive Analysis of Oligometastatic Lesions Treated With Curative-Intent Stereotactic Body Radiotherapy. Int. J. Radiat. Oncol. 2008, 72, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, E.; Silipigni, S.; Pantano, F.; Matteucci, P.; Carrafiello, S.; Marrocco, M.; Alaimo, R.; Palumbo, V.; Fiore, M.; Orsaria, P.; et al. BOMB trial: First results of stereotactic radiotherapy to primary breast tumor in metastatic breast cancer patients. Front. Oncol. 2023, 13, 1062355. [Google Scholar] [CrossRef]
- Chmura, S.J.; Winter, K.A.; Woodward, W.A.; Borges, V.F.; Salama, J.K.; Al-Hallaq, H.A.; Matuszak, M.; Milano, M.T.; Jaskowiak, N.T.; Bandos, H.; et al. NRG-BR002: A phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J. Clin. Oncol. 2022, 40, 1007. [Google Scholar] [CrossRef]
- Courdi, A.; Ortholan, C.; Hannoun-Lévi, J.-M.; Ferrero, J.-M.; Largillier, R.; Balu-Maestro, C.; Chapellier, C.; Ettore, F.; Birtwisle-Peyrottes, I. Long-term results of hypofractionated radiotherapy and hormonal therapy without surgery for breast cancer in elderly patients. Radiother. Oncol. 2006, 79, 156–161. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Khan, A.M.; Yuan, Y. Biopsy variability of lymphocytic infiltration in breast cancer subtypes and the ImmunoSkew score. Sci. Rep. 2016, 6, 36231. [Google Scholar] [CrossRef]
| Baseline Characteristics | Arm, No. (%) | |||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||
| Clinical Dataset (n = 22) | Graph Feature Dataset (n = 104) | |||
| Median Age, years | 83.5 | 84.5 | ||
| Sex | ||||
| Female | 21 (95) | 97 (93) | ||
| Male | 1 (5) | 7 (7) | ||
| Local Regional Control | Partial Response (PR) (n = 12) | Stable Disease (SD) (n = 10) | Partial Response (PR) (n = 60) | Stable Disease (SD) (n = 44) |
| SABR Fractionation (Gy/#) | ||||
| 35/5 | 2 (9) | 2 (9) | 2 (2) | 8 (8) |
| 36/4 | 4 (18) | 2 (9) | 22 (21) | 9 (9) |
| 40/4 | 3 (14) | 0 (0) | 15 (14) | 0 (0) |
| 40/5 | 1 (4) | 3 (14) | 4 (4) | 16 (15) |
| 44/4 | 2 (9) | 3 (14) | 17 (16) | 11 (11) |
| Molecular Subtype | ||||
| Luminal HER2− | 9 (41) | 5 (23) | 47 (45) | 20 (19) |
| HER2+ | 1 (4) | 2 (9) | 3 (3) | 14 (14) |
| TNBC | 2 (9) | 3 (14) | 10 (10) | 10 (9) |
| Pre-Tx. Mean Tumor Diam, mm | 31.0 | 26.6 | ||
| Post-Tx. Mean Tumor Diam, mm | 11.7 | 24.4 | ||
| Test Set | ||||||
|---|---|---|---|---|---|---|
| Model | Feature | Acc (%) | Sn (%) | Sp (%) | AUC | |
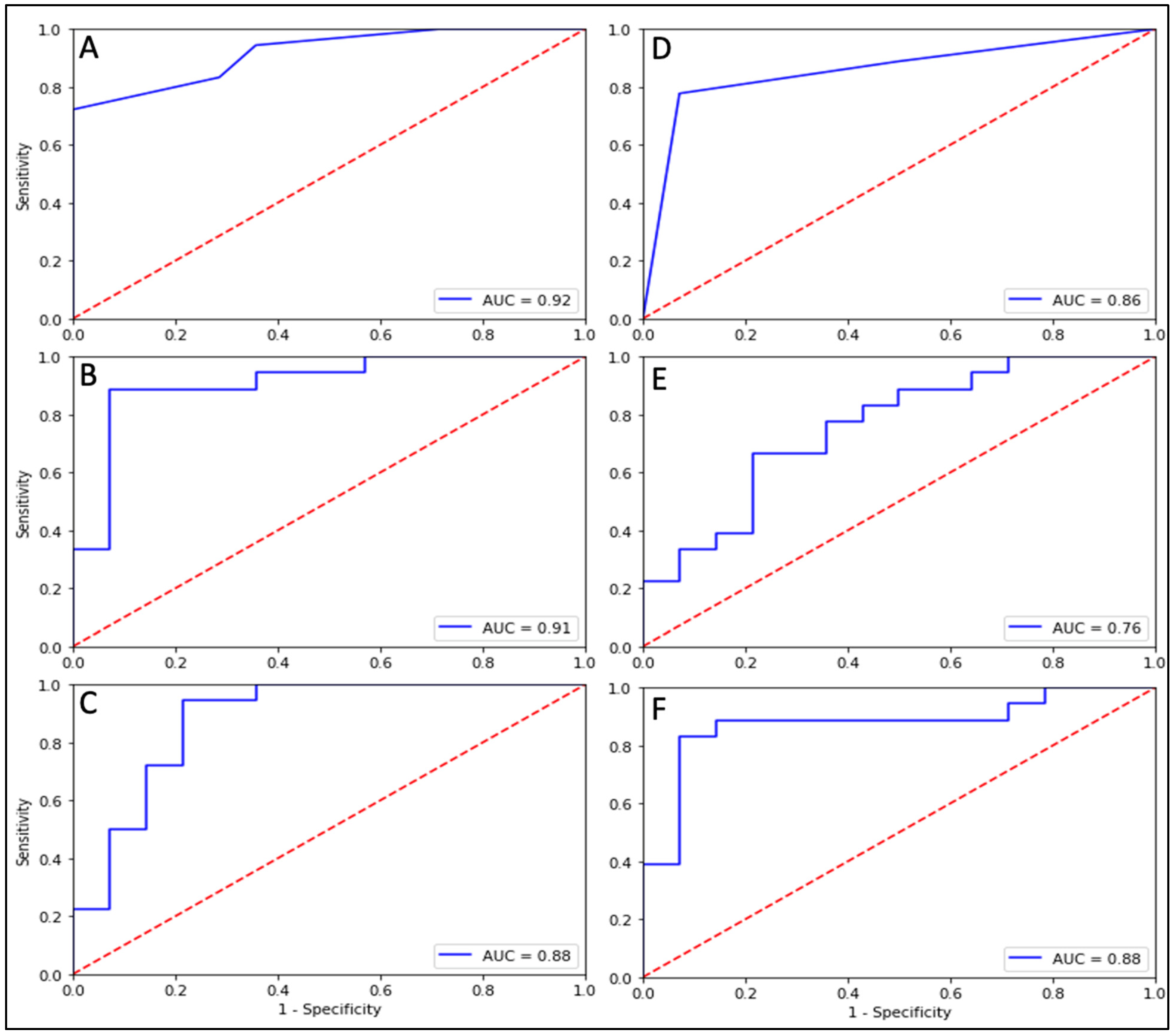
| Multiparametric Clinical Models | KNN | 7, 8 | 42.9 | 50.0 | 33.3 | 0.42 |
| 2, 3, 5 | 57.1 | 75.0 | 33.3 | 0.62 | ||
| SVM | 4, 7 | 42.9 | 75.0 | 0.00 | 0.38 | |
| 2, 3, 6 | 57.1 | 75.0 | 33.3 | 0.58 | ||
| GNB | 3,4 | 28.6 | 25.0 | 33.3 | 0.50 | |
| 3,4,6 | 42.9 | 25.0 | 66.7 | 0.42 | ||
| Single-Feature Graph Models | KNN | 1 | 84.4 | 94.4 | 71.4 | 0.83 |
| 2 | 90.6 | 88.9 | 92.8 | 0.91 | ||
| 3 | 68.8 | 50.0 | 92.8 | 0.71 | ||
| 4 | 81.2 | 83.3 | 78.5 | 0.81 | ||
| 5 | 75.0 | 66.7 | 85.7 | 0.76 | ||
| 6 | 78.1 | 72.2 | 85.7 | 0.79 | ||
| 7 | 56.2 | 50.0 | 64.2 | 0.57 | ||
| SVM | 1 | 90.6 | 94.4 | 85.7 | 0.89 | |
| 2 | 87.5 | 94.4 | 78.5 | 0.90 | ||
| 3 | 62.5 | 38.9 | 92.8 | 0.82 | ||
| 4 | 78.1 | 77.8 | 78.5 | 0.82 | ||
| 5 | 81.2 | 77.8 | 85.7 | 0.79 | ||
| 6 | 75.0 | 66.7 | 85.7 | 0.80 | ||
| 7 | 56.2 | 66.7 | 42.8 | 0.62 | ||
| GNB | 1 | 90.6 | 88.9 | 92.8 | 0.91 | |
| 2 | 84.4 | 94.4 | 71.4 | 0.92 | ||
| 3 | 62.5 | 38.9 | 92.8 | 0.75 | ||
| 4 | 75.0 | 66.7 | 85.7 | 0.82 | ||
| 5 | 68.8 | 50.0 | 92.8 | 0.86 | ||
| 6 | 75.0 | 62.5 | 85.7 | 0.79 | ||
| 7 | 62.5 | 83.3 | 35.7 | 0.64 | ||
| Multiparametric Graph Models | KNN | 2, 7 | 81.2 | 77.8 | 85.7 | 0.92 |
| 2, 5, 7 | 84.4 | 77.8 | 92.8 | 0.86 | ||
| SVM | 3, 5 | 62.5 | 38.9 | 92.8 | 0.91 | |
| 5, 6, 7 | 84.4 | 61.1 | 78.5 | 0.76 | ||
| GNB | 2, 3 | 81.2 | 83.3 | 78.5 | 0.88 | |
| 1, 2, 7 | 84.4 | 88.9 | 78.5 | 0.88 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielecki, M.; Saednia, K.; Lu, F.-I.; Kagan, S.; Vesprini, D.; Jerzak, K.J.; Salgado, R.; Karshafian, R.; Tran, W.T. Assessment of Tumor Infiltrating Lymphocytes in Predicting Stereotactic Ablative Radiotherapy (SABR) Response in Unresectable Breast Cancer. Radiation 2025, 5, 11. https://doi.org/10.3390/radiation5020011
Bielecki M, Saednia K, Lu F-I, Kagan S, Vesprini D, Jerzak KJ, Salgado R, Karshafian R, Tran WT. Assessment of Tumor Infiltrating Lymphocytes in Predicting Stereotactic Ablative Radiotherapy (SABR) Response in Unresectable Breast Cancer. Radiation. 2025; 5(2):11. https://doi.org/10.3390/radiation5020011
Chicago/Turabian StyleBielecki, Mateusz, Khadijeh Saednia, Fang-I Lu, Shely Kagan, Danny Vesprini, Katarzyna J. Jerzak, Roberto Salgado, Raffi Karshafian, and William T. Tran. 2025. "Assessment of Tumor Infiltrating Lymphocytes in Predicting Stereotactic Ablative Radiotherapy (SABR) Response in Unresectable Breast Cancer" Radiation 5, no. 2: 11. https://doi.org/10.3390/radiation5020011
APA StyleBielecki, M., Saednia, K., Lu, F.-I., Kagan, S., Vesprini, D., Jerzak, K. J., Salgado, R., Karshafian, R., & Tran, W. T. (2025). Assessment of Tumor Infiltrating Lymphocytes in Predicting Stereotactic Ablative Radiotherapy (SABR) Response in Unresectable Breast Cancer. Radiation, 5(2), 11. https://doi.org/10.3390/radiation5020011






