Early and Transient Formation of Highly Acidic pH Spikes in Water Radiolysis under the Combined Effect of High Dose Rate and High Linear Energy Transfer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Multitrack Chemistry Simulation of High-LET and High-Dose-Rate Effects in Water Irradiated by Fast Incident Protons
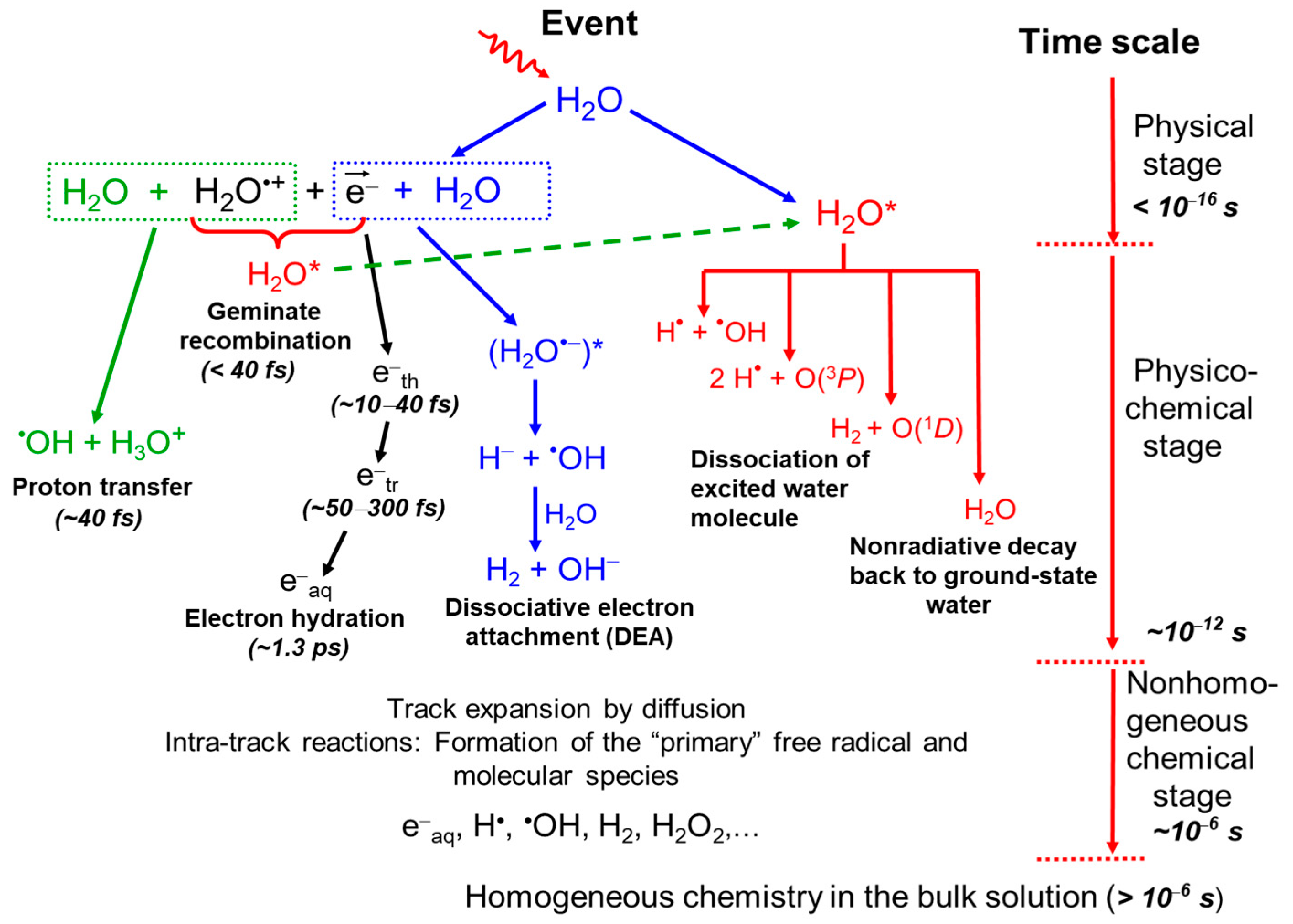
2.1. Radiolysis of Pure, Deaerated Water: Time Scale of Events and Formation of Primary Radical and Molecular Products
2.2. Simulating the Effect of LET with Incident Protons of Different Energies
2.3. Modeling the Effect of Dose Rate: The “Instantaneous Pulse” (or Dirac) Model
2.4. Monte Carlo Multitrack Chemistry Simulations: The IONLYS-IRT Code
3. Results and Discussion
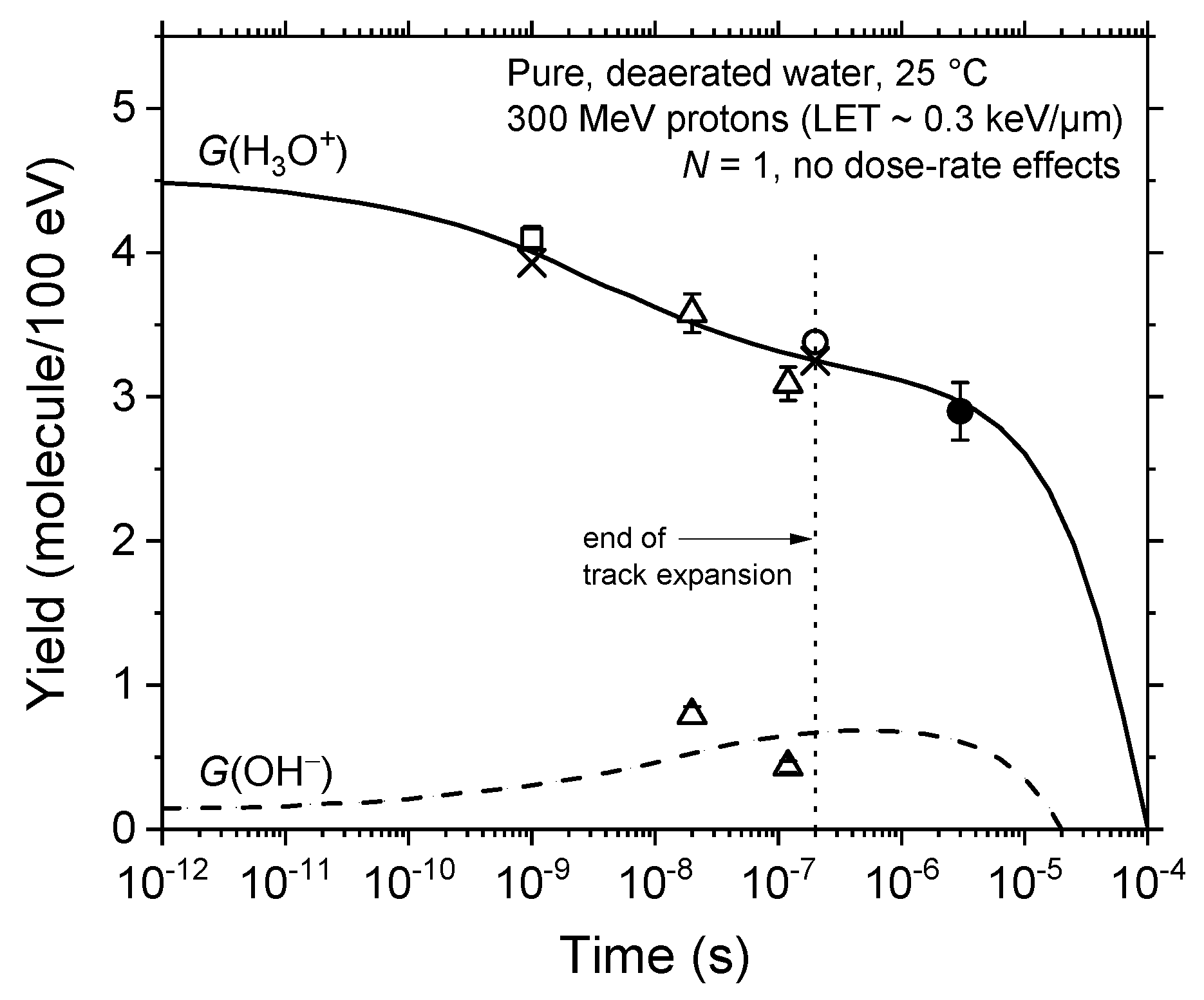
3.1. Time Evolution of the Yields of H3O+ and OH− in the Absence of Dose-Rate Effects
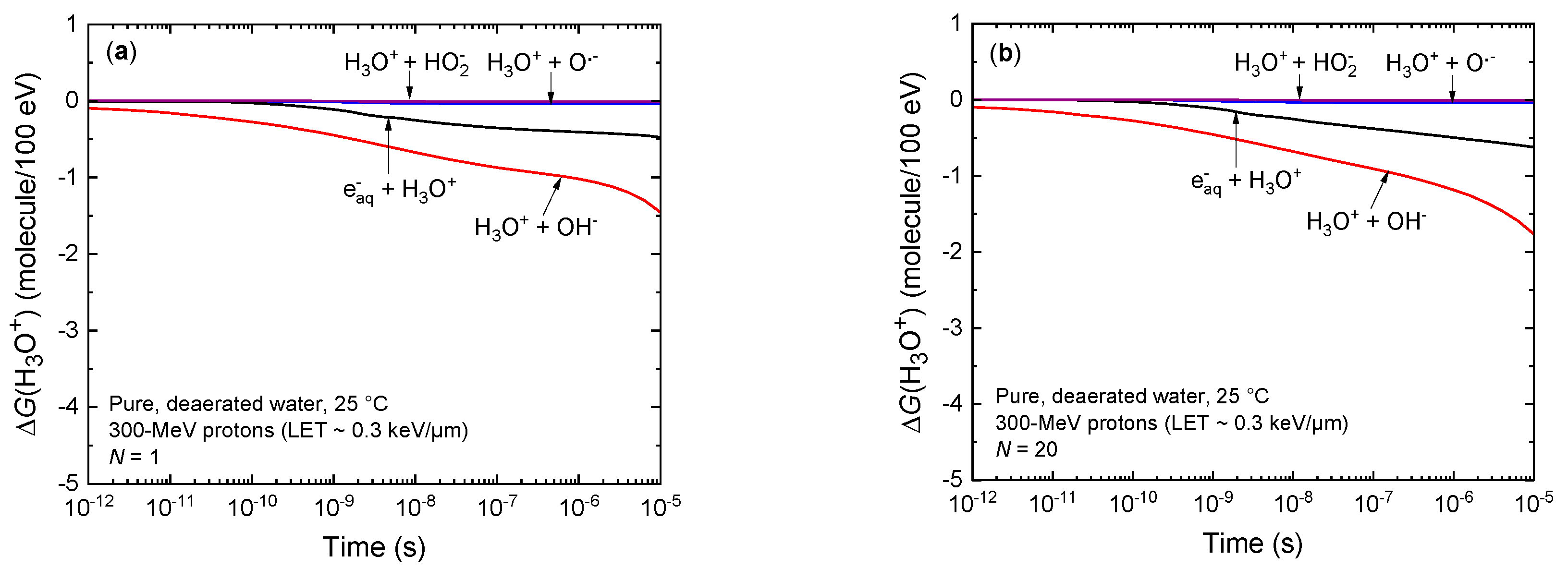
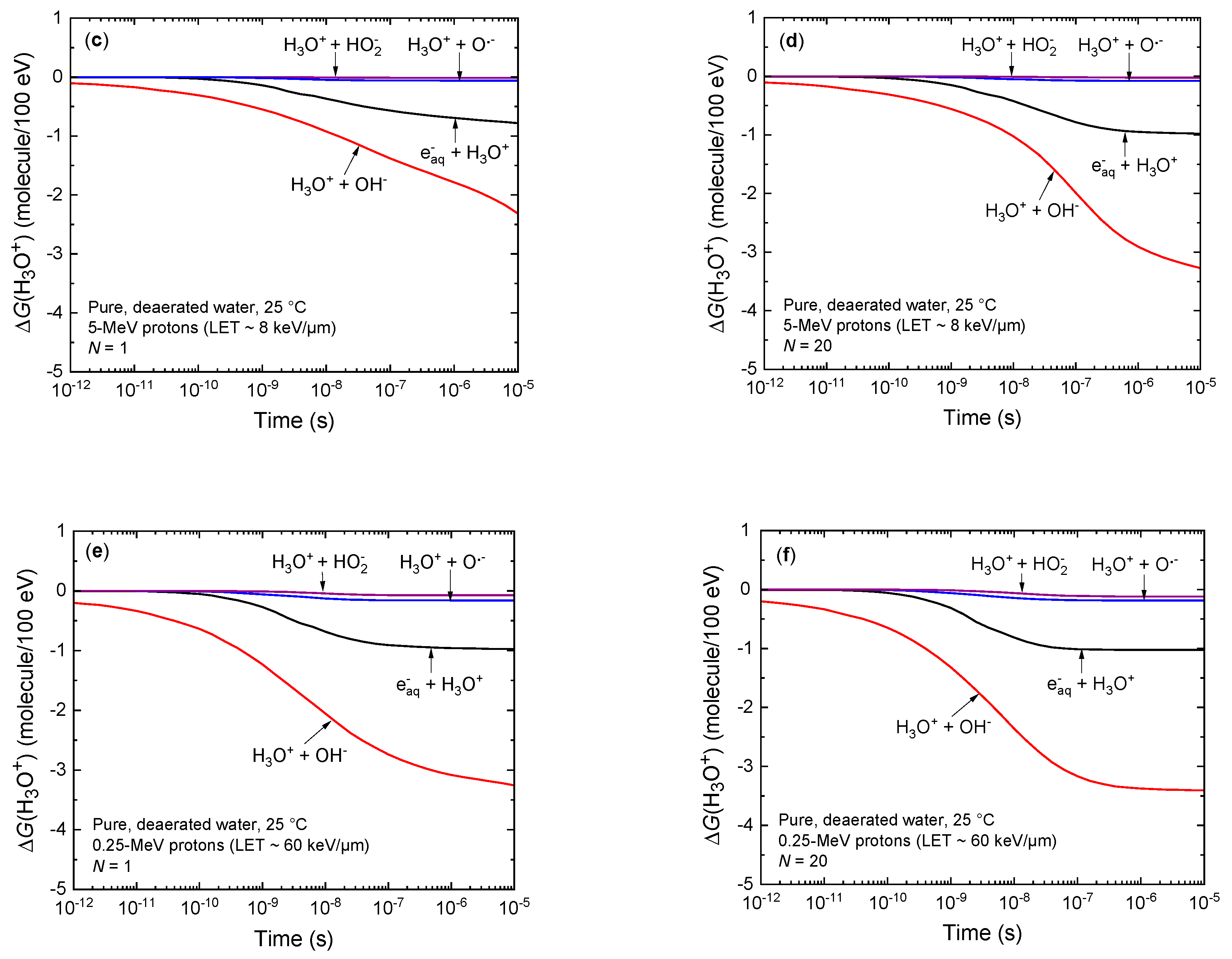
3.2. Temporal Variation of the Yields of H3O+ and OH− under Irradiation Conditions Combining Both High-Dose-Rate and High-LET Radiation
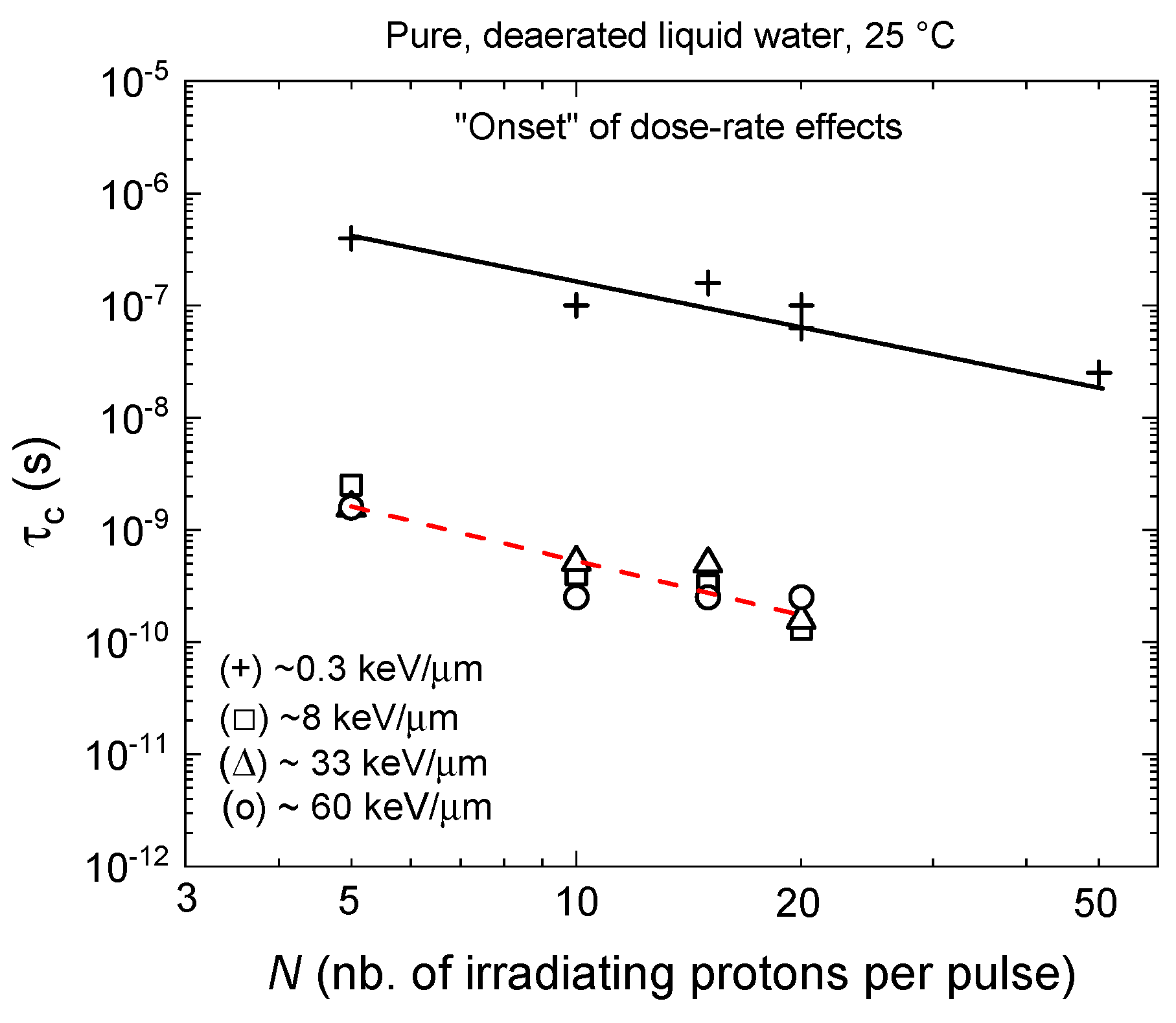
3.3. The Onset of Dose-Rate Effects
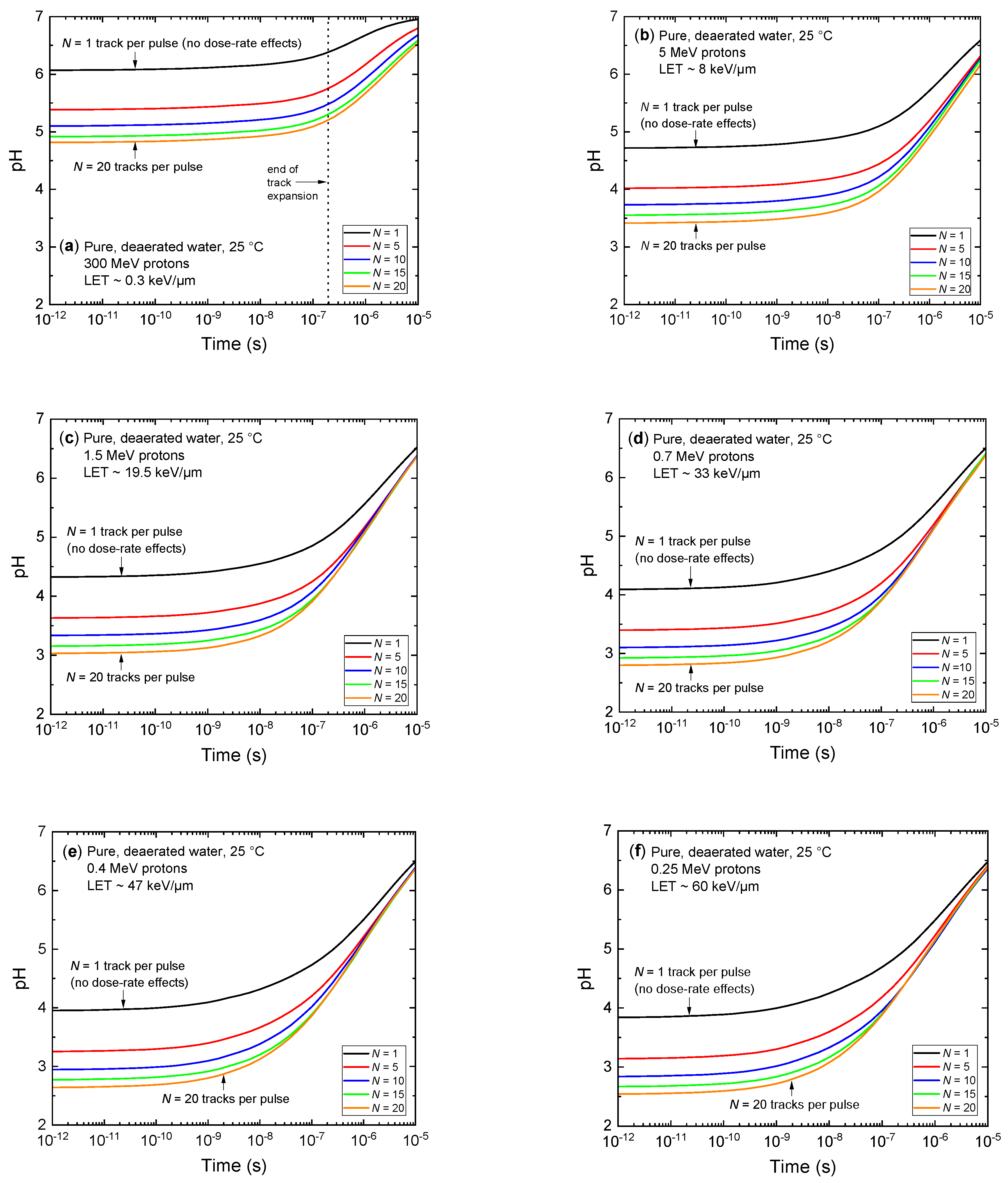
3.4. Ultrafast, Transient Acidic pH Response
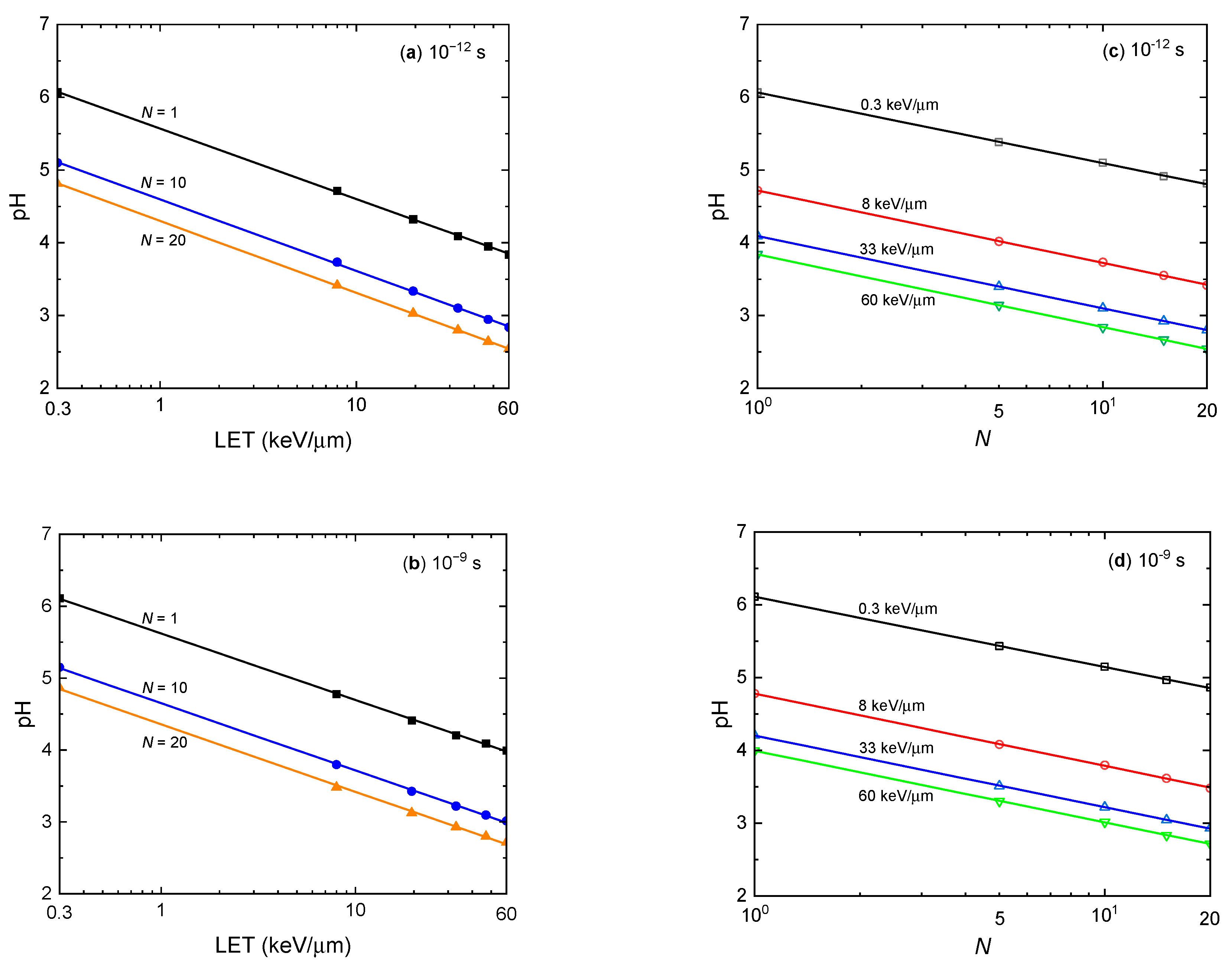
3.5. Correlation between pH, LET, and Dose Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair. A Chemical Perspective; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Becker, D.; Adhikary, A.; Sevilla, M.D. Physicochemical mechanisms of radiation-induced DNA damage. In Charged Particle and Photon Interactions with Matter. Recent Advances, Applications, and Interfaces; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2011; Chapter 19; pp. 503–541. [Google Scholar]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P. Radiation-induced damage in DNA. In Radiation Chemistry: Present Status and Future Trends; Jonah, C.D., Rao, B.S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Chapter 21; pp. 585–622. [Google Scholar]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Redox reactions of antioxidants: Contributions from radiation chemistry of aqueous solutions. In Charged Particle and Photon Interactions with Matter. Recent Advances, Applications, and Interfaces; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2011; Chapter 22; pp. 595–622. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Penabeï, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Assessment of cystamine’s radioprotective/antioxidant ability under high-dose-rate irradiation: A Monte Carlo multi-track chemistry simulation study. Antioxidants 2023, 12, 776. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Favaudon, V.; Fouillade, C.; Vozenin, M.-C. La radiothérapie FLASH pour épargner les tissus sains. Médecine/Sciences 2015, 31, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-high dose rate (FLASH) radiotherapy: Silver bullet or fool’s gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef]
- Wardman, P. Radiotherapy using high-intensity pulsed radiation beams (FLASH): A radiation-chemical perspective. Radiat. Res. 2020, 194, 607–617. [Google Scholar] [CrossRef]
- Marcu, L.G.; Bezak, E.; Peukert, D.D.; Wilson, P. Translational research in FLASH radiotherapy—From radiobiological mechanisms to in vivo results. Biomedicines 2021, 9, 181. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nature Rev. Clin. Oncol. 2022, 19, 791–803. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Chang, C.-W.; Charyyev, S.; Zhou, J.; Bradley, J.D.; Liu, T.; Yang, X. A potential revolution in cancer treatment: A topical review of FLASH radiotherapy. J. Appl. Clin. Med. Phys. 2022, 23, e13790. [Google Scholar] [CrossRef] [PubMed]
- Kacem, H.; Almeida, A.; Cherbuin, N.; Vozenin, M.-C. Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation. Int. J. Radiat. Biol. 2022, 98, 506–516. [Google Scholar] [CrossRef]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W., Jr.; Vozenin, M.-C.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Hageman, E.; Che, P.-P.; Dahele, M.; Slotman, B.J.; Sminia, P. Radiobiological aspects of FLASH radiotherapy. Biomolecules 2022, 12, 1376. [Google Scholar] [CrossRef]
- Jay-Gerin, J.-P. Ultra-high dose-rate (FLASH) radiotherapy: Generation of early, transient, strongly acidic spikes in the irradiated tumor environment. Cancer Radiother. 2020, 24, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. Generation of ultrafast, transient, highly acidic pH spikes in the radiolysis of water at very high dose rates: Relevance for FLASH radiotherapy. Can. J. Chem. 2022, 100, 272–279. [Google Scholar] [CrossRef]
- Loh, Z.-H.; Doumy, G.; Arnold, C.; Kjellsson, L.; Southworth, S.H.; Al Haddad, A.; Kumagai, Y.; Tu, M.-F.; Ho, P.J.; March, A.M.; et al. Observation of the fastest chemical processes in the radiolysis of water. Science 2020, 367, 179–182. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Garrett, B.C.; Dixon, D.A.; Camaioni, D.M.; Chipman, D.M.; Johnson, M.A.; Jonah, C.D.; Kimmel, G.A.; Miller, J.H.; Rescigno, T.N.; Rossky, P.J.; et al. Role of water in electron-initiated processes and radical chemistry: Issues and scientific advances. Chem. Rev. 2005, 105, 355–390. [Google Scholar] [CrossRef]
- Cobut, V.; Frongillo, Y.; Jay-Gerin, J.-P.; Patau, J.P. A Monte Carlo calculation of subexcitation and vibrationally-relaxing electron spectra in irradiated liquid water. Radiat. Phys. Chem. 1992, 40, 589–591. [Google Scholar] [CrossRef]
- LaVerne, J.A.; Pimblott, S.M. Electron energy-loss distributions in solid, dry DNA. Radiat. Res. 1995, 141, 208–215. [Google Scholar] [CrossRef]
- Meesungnoen, J.; Jay-Gerin, J.-P.; Filali-Mouhim, A.; Mankhetkorn, S. Low-energy electron penetration range in liquid water. Radiat. Res. 2002, 158, 657–660. [Google Scholar] [CrossRef] [PubMed]
- LaForge, A.C.; Michiels, R.; Bohlen, M.; Callegari, C.; Clark, A.; von Conta, A.; Coreno, M.; Di Fraia, M.; Drabbels, M.; Huppert, M.; et al. Real-time dynamics of the formation of hydrated electrons upon irradiation of water clusters with extreme ultraviolet light. Phys. Rev. Lett. 2019, 122, 133001. [Google Scholar] [CrossRef]
- Low, P.J.; Chu, W.; Nie, Z.; Mohd Yusof, M.S.B.; Prezhdo, O.V.; Loh, Z.-H. Observation of a transient intermediate in the ultrafast relaxation dynamics of the excess electron in strong-field-ionized liquid water. Nature Commun. 2022, 13, 7300. [Google Scholar] [CrossRef] [PubMed]
- Lea, D.E. Actions of Radiations on Living Cells; Cambridge University Press: Cambridge, UK, 1946; Chapter 2. [Google Scholar]
- Lea, D.E. The action of radiations on dilute aqueous solutions: The spatial distribution of H and OH. Br. J. Radiol. 1947, 1, 59–64. [Google Scholar]
- Morrison, P. Radiation in living matter: The physical processes. In Symposium on Radiobiology. The Basic Aspects of Radiation Effects on Living Systems; Nickson, J.J., Ed.; Wiley: New York, NY, USA, 1952; pp. 1–12. [Google Scholar]
- Elliot, A.J.; Bartels, D.M. The Reaction Set, Rate Constants and g-Values for the Simulation of the Radiolysis of Light Water over the Range 20 to 350 °C Based on Information Available in 2008; Report No. 153-127160-450-001; Atomic Energy of Canada Limited: Mississauga, ON, Canada, 2009. [Google Scholar]
- Kanike, V.; Meesungnoen, J.; Jay-Gerin, J.-P. Transient acid pH effect in tracks in the radiolysis of water: Does this effect contribute to biological damage caused by ionizing radiation? Austin J. Nucl. Med. Radiother. 2015, 2, 1011. [Google Scholar]
- Kanike, V.; Meesungnoen, J.; Jay-Gerin, J.-P. Acid spike effect in spurs/tracks of the low/high linear energy transfer radiolysis of water: Potential implications for radiobiology. RSC Adv. 2015, 5, 43361–43370. [Google Scholar] [CrossRef]
- LaVerne, J.A. Track effects of heavy ions in liquid water. Radiat. Res. 2000, 153, 487–496. [Google Scholar] [CrossRef]
- Smith, D.R.; Stevens, W.H. Radiation-induced hydrolysis of acetal: Evidence for the reaction of H3O+ ions in spurs in the radiolysis of water. Nature 1963, 200, 66–67. [Google Scholar] [CrossRef]
- Anbar, M.; Thomas, J.K. Pulse radiolysis studies of aqueous sodium chloride solutions. J. Phys. Chem. 1964, 68, 3829–3835. [Google Scholar] [CrossRef]
- Pikaev, A.K.; Kabakchi, S.A.; Zansokhova, A.A. Yields and reactions of hydrogen ions on radiolysis of water and aqueous solutions. Faraday Discuss. Chem. Soc. 1977, 63, 112–123. [Google Scholar] [CrossRef]
- Byakov, V.M.; Stepanov, S.V. The mechanism for the primary biological effects of ionizing radiation. Phys. Usp. 2006, 49, 469–487. [Google Scholar] [CrossRef]
- Kanike, V. “Acid-Spike” Effect in Spurs/Tracks of the Low/High Linear Energy Transfer Radiolysis of Water: Potential Implications for Radiobiology and Nuclear Industry. Master’s Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2016. Available online: https://savoirs.usherbrooke.ca/handle/11143/9711 (accessed on 17 November 2016).
- Islam, M.M.; Kanike, V.; Meesungnoen, J.; Lertnaisat, P.; Katsumura, Y.; Jay-Gerin, J.-P. In Situ generation of ultrafast transient “acid spikes” in the 10B(n,α)7Li radiolysis of water. Chem. Phys. Lett. 2018, 693, 210–215. [Google Scholar] [CrossRef]
- Schneider, N.M.; Norton, M.M.; Mendel, B.J.; Grogan, J.M.; Ross, F.M.; Bau, H.H. Electron-water interactions and implications for liquid cell electron microscopy. J. Phys. Chem. C 2014, 118, 22373–22382. [Google Scholar] [CrossRef]
- Platzman, R.L. The physical and chemical basis of mechanisms in radiation biology. In Radiation Biology and Medicine. Selected Reviews in the Life Sciences; Claus, W.D., Ed.; Addison-Wesley: Reading, MA, USA, 1958; pp. 15–72. [Google Scholar]
- Kuppermann, A. Theoretical foundations of radiation chemistry. J. Chem. Educ. 1959, 36, 279–285. [Google Scholar] [CrossRef]
- Meesungnoen, J.; Jay-Gerin, J.-P. Radiation chemistry of liquid water with heavy ions: Monte Carlo simulation studies. In Charged Particle and Photon Interactions with Matter. Recent Advances, Applications, and Interfaces; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2011; Chapter 14; pp. 355–400. [Google Scholar]
- Ferradini, C.; Jay-Gerin, J.-P. Radiolysis of water and aqueous solutions: History and present state of the science. Can. J. Chem. 1999, 77, 1542–1575. (In French) [Google Scholar] [CrossRef]
- Magee, J.L. Radiation chemistry. Annu. Rev. Nucl. Sci. 1953, 3, 171–192. [Google Scholar] [CrossRef]
- Freeman, G.R. Basics of radiation chemistry. In The Study of Fast Processes and Transient Species by Electron Pulse Radiolysis; Baxendale, J.H., Busi, F., Eds.; Reidel Publishing: Dordrecht, The Netherlands, 1982; pp. 19–34. [Google Scholar]
- Plante, I.L.; Filali-Mouhim, A.; Jay-Gerin, J.-P. SIMULRAD: A JAVA interface for a Monte Carlo simulation code to visualize in 3D the early stages of water radiolysis. Radiat. Phys. Chem. 2005, 72, 173–180. [Google Scholar] [CrossRef]
- Mozumder, A. Fundamentals of Radiation Chemistry; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Sanguanmith, S.; Meesungnoen, J.; Muroya, Y.; Lin, M.; Katsumura, Y.; Jay-Gerin, J.-P. On the spur lifetime and its temperature dependence in the low linear energy transfer radiolysis of water. Phys. Chem. Chem. Phys. 2012, 14, 16731–16736. [Google Scholar] [CrossRef]
- Buxton, G.V. Radiation chemistry of the liquid state: (1) Water and homogeneous aqueous solutions. In Radiation Chemistry. Principles and Applications; Farhataziz, Rodgers, M.A.J., Eds.; VCH Publishers: New York, NY, USA, 1987; pp. 321–349. [Google Scholar]
- Bjergbakke, E.; Hart, E.J. Oxygen formation in the γ-ray irradiation of Fe2+-Cu2+ solutions. Radiat. Res. 1971, 45, 261–273. [Google Scholar] [CrossRef]
- Pastina, B.; LaVerne, J.A. Effect of molecular hydrogen on hydrogen peroxide in water radiolysis. J. Phys. Chem. A 2001, 105, 9316–9322. [Google Scholar] [CrossRef]
- Ferradini, C. Heterogeneous aspect of radiolytic phenomena (Article in French). In Actions Biologique et Chimique des Radiations Ionisantes; Tilquin, B., Ed.; Éditions Ciaco: Brussels, Belgium, 1990; Volume 1, pp. 52–63. [Google Scholar]
- Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. Linear energy transfer dependence of transient yields in water irradiated by 150 keV–500 MeV protons in the limit of low dose rates. Can. J. Chem. 2020, 98, 427–433. [Google Scholar] [CrossRef]
- Watt, D.E. Quantities for Dosimetry of Ionizing Radiations in Liquid Water; Taylor & Francis: London, UK, 1996. [Google Scholar]
- Stopping Powers and Ranges for Protons and Alpha Particles; ICRU Report No. 49; International Commission on Radiation Units and Measurements: Bethesda, DC, USA, 1993.
- Mustaree, S.; Meesungnoen, J.; Butarbutar, S.L.; Causey, P.; Stuart, C.R.; Jay-Gerin, J.-P. Self-radiolysis of tritiated water. 3. The •OH scavenging effect of bromide ions on the yield of H2O2 in the radiolysis of water by 60Co γ-rays and tritium β-particles at room temperature. RSC Adv. 2014, 4, 43572–43581. [Google Scholar] [CrossRef]
- Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. A computer modeling study of water radiolysis at high dose rates. Relevance to FLASH radiotherapy. Radiat. Res. 2021, 195, 149–162. [Google Scholar] [CrossRef]
- Sultana, A.; Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. On the transient radiolytic oxygen depletion in the ultra-high (FLASH) dose-rate radiolysis of water in a cell-like environment: Effect of e−aq and •OH competing scavengers. Radiat. Res. 2022, 197, 566–567. [Google Scholar] [CrossRef]
- Alanazi, A.; Sultana, A.; Meesungnoen, J.; Jay-Gerin, J.-P. The effect of linear energy transfer on the early, transient radiolytic oxygen depletion in the radiolysis of water by high-dose-rate irradiating protons. Can. J. Chem. 2023, 101, 70–80. [Google Scholar] [CrossRef]
- Sultana, A.; Meesungnoen, J.; Jay-Gerin, J.-P. High-dose-rate effects in the radiolysis of water at elevated temperatures. Can. J. Chem. 2021, 99, 594–602. [Google Scholar] [CrossRef]
- The Dosimetry of Pulsed Radiation; ICRU Report No. 34; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1982.
- Cobut, V.; Frongillo, Y.; Patau, J.P.; Goulet, T.; Fraser, M.-J.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water—I. Physical and physicochemical aspects. Radiat. Phys. Chem. 1998, 51, 229–243. [Google Scholar]
- Frongillo, Y.; Goulet, T.; Fraser, M.-J.; Cobut, V.; Patau, J.P.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water—II. Nonhomogeneous chemistry. Radiat. Phys. Chem. 1998, 51, 245–254. [Google Scholar]
- Tachiya, M. Theory of diffusion-controlled reactions: Formulation of the bulk reaction rate in terms of the pair probability. Radiat. Phys. Chem. 1983, 21, 167–175. [Google Scholar] [CrossRef]
- Pimblott, S.M.; Pilling, M.J.; Green, N.J.B. Stochastic models of spur kinetics in water. Radiat. Phys. Chem. 1991, 37, 377–388. [Google Scholar] [CrossRef]
- Goulet, T.; Fraser, M.-J.; Frongillo, Y.; Jay-Gerin, J.-P. On the validity of the independent reaction times approximation for the description of the nonhomogeneous kinetics of liquid water radiolysis. Radiat. Phys. Chem. 1998, 51, 85–91. [Google Scholar] [CrossRef]
- Plante, I. Développement de Codes de Simulation Monte Carlo de la Radiolyse de l’Eau par des Électrons, Ions Lourds, Photons et Neutrons. Applications à Divers Sujets d’Intérêt Expérimental. Ph.D. Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Tippayamontri, T.; Sunuchakan, S.; Meesungnoen, J.; Sunaryo, G.R.; Jay-Gerin, J.-P. Fast neutron radiolysis of the ferrous sulfate (Fricke) dosimeter: Monte Carlo simulations. In Recent Research Developments in Physical Chemistry; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, Kerala, India, 2009; Volume 10, pp. 143–211. [Google Scholar]
- Mirsaleh Kohan, L.; Sanguanmith, S.; Meesungnoen, J.; Causey, P.; Stuart, C.R.; Jay-Gerin, J.-P. Self-radiolysis of tritiated water. 1. A comparison of the effects of 60Co γ-rays and tritium β-particles on water and aqueous solutions at room temperature. RSC Adv. 2013, 3, 19282–19299. [Google Scholar] [CrossRef]
- Čerček, B.; Kongshaug, M. Hydrogen ion yields in the radiolysis of neutral aqueous solutions. J. Phys. Chem. 1969, 73, 2056–2058. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.C.; Fowles, P.; Sammon, D.C.; Stringer, B. Pulse radiolytic induced transient electrical conductance in liquid solutions. Part 1. Technique and the radiolysis of water. Trans. Faraday Soc. 1970, 66, 1498–1508. [Google Scholar] [CrossRef]
- Anderson, R.F.; Vojnovic, B.; Michael, B.D. The radiation-chemical yields of H3O+ and OH− as determined by nanosecond conductimetric measurements. Radiat. Phys. Chem. 1985, 26, 301–303. [Google Scholar] [CrossRef]
- Schmidt, K.H.; Ander, S.M. Formation and recombination of H3O+ and hydroxide in irradiated water. J. Phys. Chem. 1969, 73, 2846–2852. [Google Scholar] [CrossRef]
- Kuppermann, A. Diffusion kinetics in radiation chemistry. In Actions Chimiques et Biologiques des Radiations; Haïssinsky, M., Ed.; Masson: Paris, France, 1961; Volume 5, pp. 85–166. [Google Scholar]
- Hummel, A. Radiation Chemistry: The Chemical Effects of Ionizing Radiation and their Applications; Interfaculty Reactor Institute-Technische Universiteit Delft (IRI-FUT): Delft, The Netherlands, 1995. [Google Scholar]
- Schardt, D.; Elsässer, T.; Schulz-Ertner, D. Heavy-ion tumor therapy: Physical and radiobiological benefits. Rev. Mod. Phys. 2010, 82, 383–425. [Google Scholar] [CrossRef]
- Baverstock, K.F.; Burns, W.G. Primary production of oxygen from irradiated water as an explanation for decreased radiobiological oxygen enhancement at high LET. Nature 1976, 260, 316–318. [Google Scholar] [CrossRef]
- Draganić, I.G.; Draganić, Z.D. The Radiation Chemistry of Water; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Negendank, W.; Edelmann, L. The State of Water in the Cell; Scanning Microscopy International: Chicago, IL, USA, 1988. [Google Scholar]
- Swietach, P.; Vaughan-Jones, R.D. Relationship between intracellular pH and proton mobility in rat and guinea-pig ventricular myocytes. J. Physiol. 2005, 566, 793–806. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bepari, M.I.; Meesungnoen, J.; Jay-Gerin, J.-P. Early and Transient Formation of Highly Acidic pH Spikes in Water Radiolysis under the Combined Effect of High Dose Rate and High Linear Energy Transfer. Radiation 2023, 3, 165-182. https://doi.org/10.3390/radiation3030014
Bepari MI, Meesungnoen J, Jay-Gerin J-P. Early and Transient Formation of Highly Acidic pH Spikes in Water Radiolysis under the Combined Effect of High Dose Rate and High Linear Energy Transfer. Radiation. 2023; 3(3):165-182. https://doi.org/10.3390/radiation3030014
Chicago/Turabian StyleBepari, Md Ibrahim, Jintana Meesungnoen, and Jean-Paul Jay-Gerin. 2023. "Early and Transient Formation of Highly Acidic pH Spikes in Water Radiolysis under the Combined Effect of High Dose Rate and High Linear Energy Transfer" Radiation 3, no. 3: 165-182. https://doi.org/10.3390/radiation3030014
APA StyleBepari, M. I., Meesungnoen, J., & Jay-Gerin, J.-P. (2023). Early and Transient Formation of Highly Acidic pH Spikes in Water Radiolysis under the Combined Effect of High Dose Rate and High Linear Energy Transfer. Radiation, 3(3), 165-182. https://doi.org/10.3390/radiation3030014








