Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Patients
2.3. Data Collection
2.4. Statistical Analysis
3. Results
Characteristics and Clinical Outcome of Ra-223 Therapy
4. Discussion
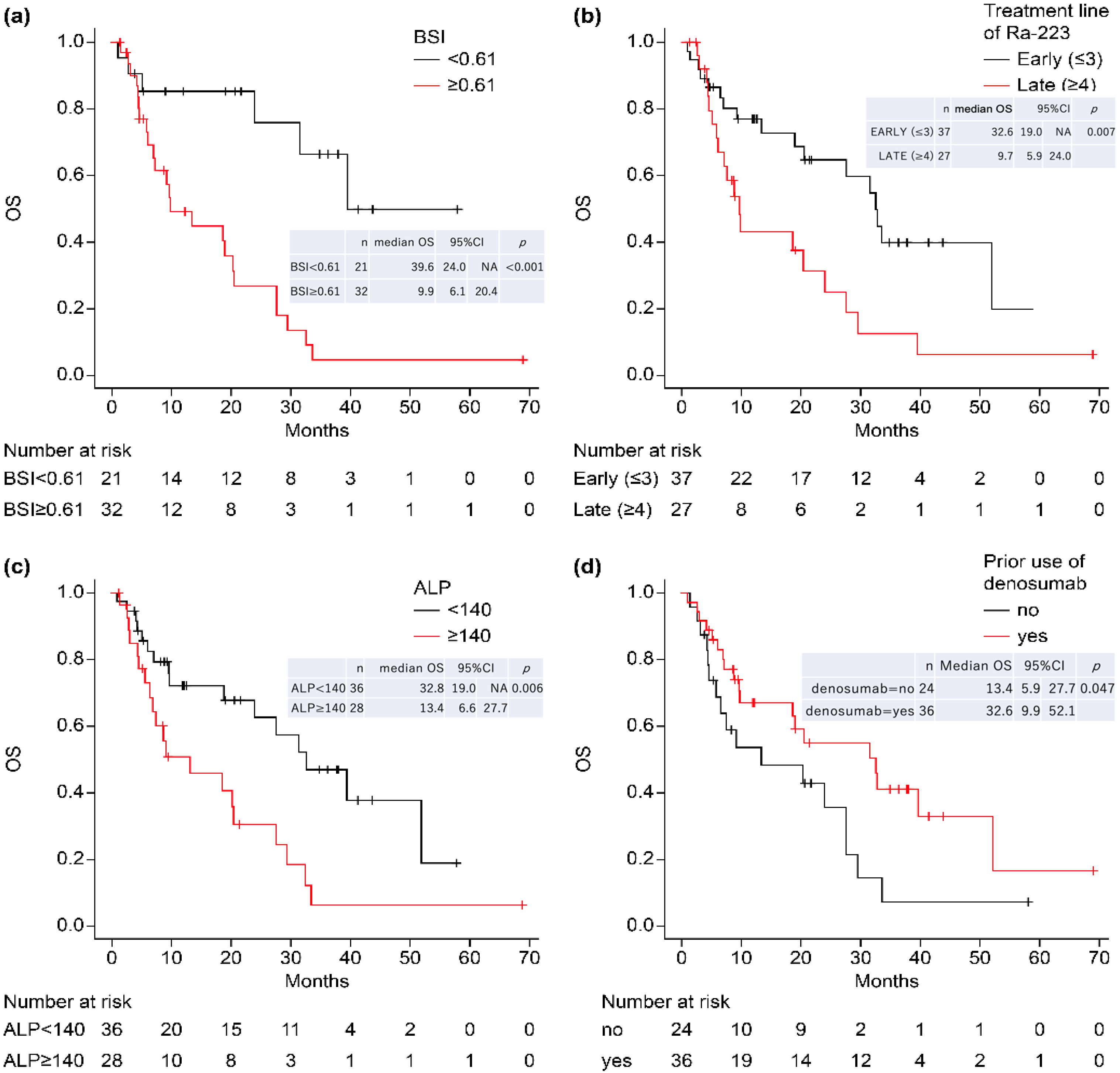
4.1. Relationship between BSI and Other Factors
4.2. Relationship between Baseline ALP and ALP_Rate
4.3. Relationship between Treatment Line and Prognosis
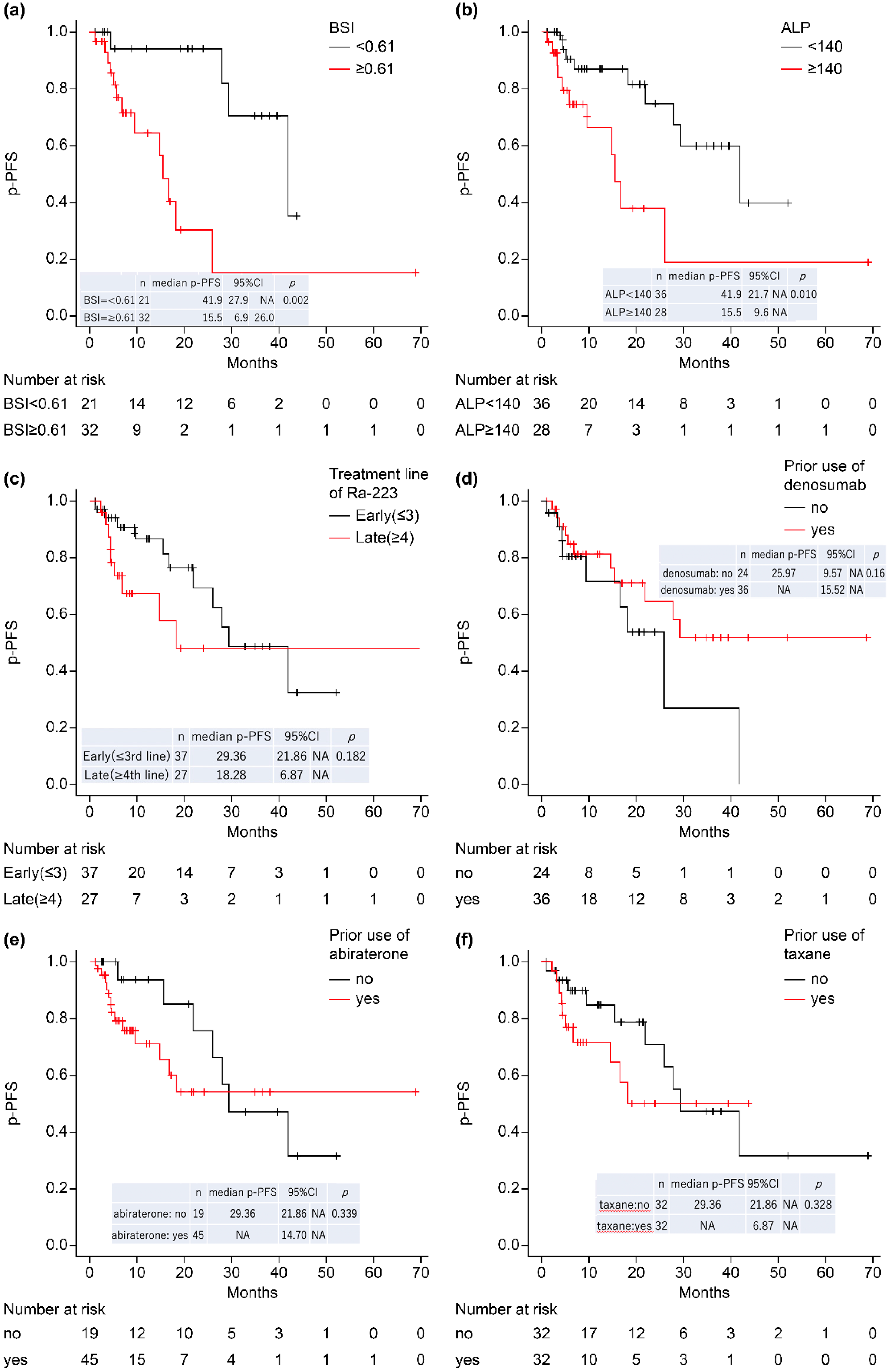
4.4. Time to Pain Progression
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with newly diagnosed metastatic prostate cancer in the “Docetaxel Era”: Data from 917 patients in the control arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schopfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration- resistant prostate cancer progressing after docetaxel treatment: A randomized open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Bruland, O.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Coleman, R.E.; Michalski, J.M.; Nilsson, S.; O’Sullivan, J.M.; Parker, C.; Widmark, A.; Thuresson, M.; Xu, L.; Germino, J.; et al. Hematologic safety of radium-223 dichloride: Baseline prognostic factors associated with myelosuppression in the ALSYMPCA trial. Clin. Genitourin. Cancer 2017, 15, 42–52.e8. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomized trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef]
- Saad, F.; Carles, J.; Gillessen, S.; Heidenreich, A.; Heinrich, D.; Gratt, J.; Lévy, J.; Miller, K.; Nilsson, S.; Petrenciuc, O.; et al. Radium-223 and concomitant therapies in patients with metastatic castration resistant prostate cancer: An international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016, 17, 1306–1316. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Vogelzang, N.J.; Bruland, Ø.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Ukai, R.; Hashimoto, K. Bone scan index can be a useful biomarker of survival outcomes in patients with metastatic castration-resistant prostate cancer treated with radium-223. Cancer Rep. 2019, 2, e1203. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Scher, H.I.; Chen, M.H.; McLeod, D.G.; Carroll, P.R.; Moul, J.W.; D’Amico, A.V. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J. Clin. Oncol. 2005, 23, 6556–6560. [Google Scholar] [CrossRef]
- Anand, A.; Morris, M.J.; Larson, S.M.; Minarik, D.; Josefsson, A.; Helgstrand, J.T.; Oturai, P.S.; Edenbrandt, L.; Røder, M.A.; Bjartell, A. Automated Bone Scan Index as a quantitative imaging biomarker in metastatic castration-resistant prostate cancer patients being treated with enzalutamide. EJNMMI Res. 2016, 6, 23. [Google Scholar] [CrossRef]
- McKay, R.R.; Jacobus, S.; Fiorillo, M.; Ledet, E.M.; Cotogna, P.M.; Steinberger, A.E.; Jacene, H.A.; Sartor, O.; Taplin, M.E. Radium-223 use in clinical practice and variables associated with completion of therapy. Clin. Genitourin. Cancer 2017, 15, e289–e298. [Google Scholar] [CrossRef]
- Saad, F.; Gillessen, S.; Heinrich, D.; Keizman, D.; O’Sullivan, J.M.; Nilsson, S.; Miller, K.; Wirth, M.; Reeves, J.; Seger, M.; et al. Disease characteristics and completion of treatment in patients with metastatic castration-resistant prostate cancer treated with radium-223 in an international early access program. Clin. Genitourin. Cancer 2019, 17, 348–355.e5. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okuda, Y.; Kanaki, T.; Tanaka, R.; Nagahara, A.; Nakai, Y.; Nakayama, M.; Kakimoto, K.I.; Nishimura, K. Clinical indicators for predicting prognosis after radium-223 administration in castration-resistant prostate cancer with bone metastases. Int. J. Clin. Oncol. 2021, 26, 192–198. [Google Scholar] [CrossRef]
- Oudard, S.; Banu, E.; Scotte, F.; Banu, A.; Medioni, J.; Beuzeboc, P.; Joly, F.; Ferrero, J.M.; Goldwasser, F.; Andrieu, J.M. Prostate-specific antigen doubling time before onset of chemotherapy as a predictor of survival for hormone-refractory prostate cancer patients. Ann. Oncol. 2007, 18, 1828–1833. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Vidal, M.; Delgado, A.; Martinez, C.; Correa, J.J.; Durango, I.C. Overall survival prediction in metastatic castration-resistant prostate cancer treated with radium-223. Int. Braz. J. Urol. 2020, 46, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Enzyme/Reagent Special Committee of Japanese Society of Clinical Chemistry. Reference Material for Request of Public Comment [Regarding the Relationship between Current and Revised JSCC Standard Methods and IFCC Standard Measurement Method]. Available online: http://jscc-jp.gr.jp/home/wp-content/uploads/2018/10/ALP-Public-Comment_HP.pdf (accessed on 1 August 2019). (In Japanese).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2005; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 1 July 2022).
- National Comprehensive Cancer Network. Prostate Cancer (Version 4.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 1 July 2022).
- U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203971lbl.pdf (accessed on 22 August 2022).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/xofigo (accessed on 22 August 2022).
- Japan Society of Clinical Oncology Guideline. 2016. Available online: http://www.jsco-cpg.jp/prostate-cancer/guideline/#XIV_cq01 (accessed on 1 July 2022).
- The Japanese Urological Association Guideline. 2016. Available online: https://www.urol.or.jp/lib/files/other/guideline/23_prostatic_cancer_2016.pdf (accessed on 1 July 2022).
- Fosbøl, M.Ø.; Petersen, P.M.; Kjaer, A.; Mortensen, J. Ra therapy of advanced metastatic castration-resistant prostate cancer: Quantitative assessment of skeletal tumor burden for prognostication of clinical outcome and hematologic toxicity. J. Nucl. Med. 2018, 59, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Cislo, P.; Sartor, O.; Vogelzang, N.J.; Coleman, R.E.; O’Sullivan, J.M.; Reuning-Scherer, J.; Shan, M.; Zhan, L.; Parker, C. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann. Oncol. 2016, 27, 868–874. [Google Scholar] [CrossRef]

| Characteristics | Median | Minimum | Maximum |
|---|---|---|---|
| Follow-up time, median (range) (month) | 10.9 | 1.2 | 73.6 |
| Age, median (range) | 74 | 49 | 87 |
| Height, median (range) (cm) | 164 | 147 | 177 |
| Weight, median (range) (kg) | 60 | 37 | 99 |
| T stage | |||
| T1 | 1 | 2.0% | |
| T2 | 11 | 22.0% | |
| T3 | 28 | 56.0% | |
| T4 | 10 | 20.0% | |
| Not available | (14) | ||
| Gleason score (GS), n (%) | |||
| GS6 | 1 | 1.6% | |
| GS7 | 9 | 14.8% | |
| GS8 | 8 | 13.1% | |
| GS9 | 31 | 50.8% | |
| GS10 | 12 | 19.7% | |
| Not available | (3) | ||
| Lymph node metastasis, n (%) | |||
| Yes | 32 | 56.1% | |
| No | 25 | 43.9% | |
| Not available | (7) | ||
| Number of doses, n (%) | |||
| 1 | 1 | 1.6% | |
| 2 | 6 | 9.4% | |
| 3 | 7 | 10.9% | |
| 4 | 3 | 4.7% | |
| 5 | 5 | 7.8% | |
| 6 | 42 | 65.6% | |
| Treatment line of Ra-223, n (%) | |||
| Early (≤3) | 37 | 57.8% | |
| Late (≥4) | 27 | 42.2% | |
| Pain * at the first dose of Ra-223 administration | |||
| Yes | 24 | 37.5% | |
| No | 40 | 62.5% | |
| PSA * at diagnosis (iPSA) | 195.2 | 4.8 | 6453.6 |
| PSA at baseline | 22.9 | 0.0 | 2193.0 |
| PSA_rate | 116% | −100% | 8332% |
| Increase | 48 | 60.0% | |
| Decrease | 12 | 20.0% | |
| PSADT * (days) | 58.7 | 24.0 | 608.2 |
| LDH at baseline (U/L) | 213.5 | 149.0 | 1720.0 |
| LDH_rate | 3.7% | −31.2% | 914.4% |
| ALP at baseline (U/L) | 113.6 | 35.0 | 1296.8 |
| ALP_rate | −16.7% | −92.0% | 1322.3% |
| Increase | 18 | 28.6% | |
| Decrease | 45 | 71.4% | |
| ALP_rate | −16.9% | −92.0% | 91.1% |
| Calcium (albumin-corrected) (mg/dL) | 9.4 | 8.0 | 13.0 |
| Ca_rate | 0.5% | −33.1% | 19.0% |
| Alb (g/dL) | 3.8 | 2.8 | 4.6 |
| Hb (g/dL) | 12.0 | 8.8 | 15.4 |
| Hb_rate | −7.4% | −61.8% | 19.6% |
| Bone scan index (BSI) | 0.93 | 0.00 | 10.32 |
| Prior use of taxane, n (%) | |||
| Yes | 32 | 50.0% | |
| No | 32 | 50.0% | |
| Prior (or concurrent) use of abiraterone, n (%) | |||
| Prior | 42 | 65.6% | |
| Concurrent | 3 | 4.7% | |
| No | 19 | 29.7% | |
| Prior use of enzalutamide, n (%) | |||
| Yes | 43 | 67.2% | |
| No | 21 | 32.8% | |
| Prior use of bone supportive agent, n (%) | |||
| Bisphosphonate | 6 | 10.0% | |
| Denosumab | 36 | 60.0% | |
| No | 18 | 30.0% | |
| Not available | (4) |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Age ≥ 76 (vs. <76) | 0.905 | 0.457 | 1.793 | 0.775 | ||||
| Weight ≥ 63 (vs. <63) (kg) | 0.764 | 0.381 | 1.532 | 0.448 | ||||
| BSI ≥ 0.61 (vs. <0.61) | 4.805 | 1.918 | 12.040 | <0.001 | 2.848 | 0.846 | 9.591 | 0.091 |
| Number of doses, 6 (vs. ≤5) | 0.181 | 0.088 | 0.372 | <0.001 | 0.155 | 0.026 | 0.943 | 0.043 |
| Treatment line of Ra-223, Late (≥4) vs. Early (≤3) | 2.432 | 1.246 | 4.746 | 0.009 | ||||
| GS = 10 (vs. ≤9) | 5.043 | 1.855 | 13.700 | 0.002 | ||||
| T4 (vs. ≤3) | 2.193 | 0.984 | 4.886 | 0.055 | ||||
| iPSA ≥ 177 (vs. <177) (ng/mL) | 1.129 | 0.575 | 2.220 | 0.724 | ||||
| PSA ≥ 22.9 (vs. <22.9) (ng/mL) | 4.785 | 2.208 | 10.370 | <0.001 | 2.435 | 0.887 | 6.681 | 0.084 |
| PSA_rate ≥ 45 (vs. <45) (%) | 5.858 | 2.504 | 13.700 | <0.001 | 6.052 | 1.671 | 21.910 | 0.006 |
| PSADT ≥ 90 (vs. <90) (days) | 0.397 | 0.193 | 0.820 | 0.012 | ||||
| Alb ≥ 3.8 (vs. <3.8) (g/dL) | 0.369 | 0.185 | 0.735 | 0.005 | ||||
| ALP ≥ 140 (vs. <140) (IU/mL) | 2.457 | 1.259 | 4.795 | 0.008 | ||||
| ALP_rate ≥ −47 (vs. <−47) (%) | 0.393 | 0.193 | 0.797 | 0.01 | ||||
| Ca ≥ 9.6 (vs. <9.6) | 1.456 | 0.754 | 2.813 | 0.264 | ||||
| Ca_rate ≥ −1 (vs. <−1) (%) | 1.095 | 0.552 | 2.172 | 0.794 | ||||
| Hb ≥ 11.4 (vs. <11.4) (g/dL) | 0.248 | 0.123 | 0.499 | <0.001 | 0.595 | 0.137 | 2.585 | 0.488 |
| Hb_rate ≥ −8 (vs. <−8) (%) | 0.401 | 0.205 | 0.787 | 0.008 | 0.236 | 0.087 | 0.643 | 0.005 |
| LDH ≥ 240 (vs. <240) (U/L) | 2.181 | 1.106 | 4.303 | 0.024 | ||||
| LDH_rate ≥ 10 (vs. <10) (%) | 2.26 | 1.16 | 4.41 | 0.016 | ||||
| Prior use of taxane (vs. no) | 2.605 | 1.297 | 5.234 | 0.007 | ||||
| Prior use of abiraterone (vs. no) | 2.530 | 1.163 | 5.502 | 0.019 | ||||
| Prior (or concurrent) use of abiraterone (vs. no) | 2.305 | 1.074 | 4.945 | 0.032 | ||||
| Prior use of enzalutamide (vs. no) | 1.425 | 0.685 | 2.967 | 0.343 | ||||
| Prior use of denosumab (vs. no) | 0.516 | 0.265 | 1.004 | 0.051 | ||||
| BSI_Low (<0.61), n = 21 | BSI_High (≥0.61), n = 32 | ||||||
|---|---|---|---|---|---|---|---|
| Median | IQR (0.25) | IQR (0.75) | Median | IQR (0.25) | IQR (0.75) | p | |
| PSA | 13.06 | 5.52 | 19.40 | 58.45 | 9.15 | 152.80 | 0.009 |
| PSA_rate | 76.3% | −5.8% | 281.0% | 163.2% | 41.2% | 343.4% | 0.224 |
| PSADT | 50.48 | 35.31 | 142.39 | 59.82 | 43.75 | 169.53 | 0.430 |
| ALP | 82.00 | 59.00 | 113.03 | 163.65 | 103.00 | 291.55 | <0.001 |
| ALP_rate | −2.7% | −28.9% | 9.0% | −32.9% | −56.8% | −3.6% | 0.015 |
| LDH | 195.00 | 174.00 | 233.00 | 219.50 | 192.75 | 292.50 | 0.064 |
| LDH_rate | −3.1% | −10.9% | 12.3% | 12.1% | −1.9% | 25.7% | 0.093 |
| Ca | 9.40 | 9.10 | 9.50 | 9.40 | 8.98 | 9.70 | 0.750 |
| Ca_rate | 0.00 | −0.03 | 0.05 | 0.01 | −0.03 | 0.05 | 0.906 |
| Hb | 12.00 | 10.90 | 13.40 | 11.40 | 10.55 | 12.53 | 0.167 |
| Hb_rate | −2.2% | −10.4% | 4.7% | −7.9% | −27.5% | −1.5% | 0.030 |
| Early (≤3rd Line), n = 37 | Late (≥4th Line), n = 27 | ||||||
|---|---|---|---|---|---|---|---|
| Median | IQR (0.25) | IQR (0.75) | Median | IQR (0.25) | IQR (0.75) | p | |
| PSA | 13.06 | 5.73 | 97.56 | 59.10 | 19.00 | 92.10 | 0.021 |
| PSA_rate | 97.5% | 1.6% | 320.2% | 188.0% | 45.0% | 346.2% | 0.179 |
| ALP | 119.00 | 77.00 | 208.80 | 110.92 | 58.90 | 227.46 | 0.331 |
| ALP_rate | −27.8% | −57.1% | −2.7% | −9.6% | −39.6% | 7.9% | 0.087 |
| LDH | 214.00 | 181.00 | 251.00 | 213.00 | 187.50 | 292.00 | 0.446 |
| LDH_rate | 2.2% | −10.0% | 24.9% | 12.5% | −4.1% | 32.9% | 0.224 |
| Hb | 12.50 | 11.30 | 13.00 | 11.00 | 10.50 | 12.20 | 0.020 |
| Hb_rate | −7.9% | −13.3% | 0.0% | −6.3% | −19.3% | 2.4% | 0.961 |
| BSI | 0.98 | 0.22 | 3.73 | 0.93 | 0.44 | 1.93 | 0.979 |
| Pretreatment Type | Early (n = 37) | Late (n = 27) | OR (Late/Early) |
|---|---|---|---|
| Taxane (n = 32) | 8/37 (25%) | 24/27 (88.9%) | 4.1 |
| Abiraterone (n = 45) | 25/37 (67.6%) | 20/27 (74.1%) | 1.1 |
| Enzarutamide (n = 43) | 25/37 (67.6%) | 18/27 (66.7%) | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguma, Y.; Hosono, M.; Okajima, K.; Inoue, E.; Nakamatsu, K.; Doi, H.; Matsuura, T.; Inada, M.; Uehara, T.; Wada, Y.; et al. Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer. Radiation 2022, 2, 273-284. https://doi.org/10.3390/radiation2030021
Oguma Y, Hosono M, Okajima K, Inoue E, Nakamatsu K, Doi H, Matsuura T, Inada M, Uehara T, Wada Y, et al. Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer. Radiation. 2022; 2(3):273-284. https://doi.org/10.3390/radiation2030021
Chicago/Turabian StyleOguma, Yasuo, Makoto Hosono, Kaoru Okajima, Eri Inoue, Kiyoshi Nakamatsu, Hiroshi Doi, Tomohiro Matsuura, Masahiro Inada, Takuya Uehara, Yutaro Wada, and et al. 2022. "Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer" Radiation 2, no. 3: 273-284. https://doi.org/10.3390/radiation2030021
APA StyleOguma, Y., Hosono, M., Okajima, K., Inoue, E., Nakamatsu, K., Doi, H., Matsuura, T., Inada, M., Uehara, T., Wada, Y., Ri, A., Yamamoto, Y., Yoshimura, K., Uemura, H., & Nishimura, Y. (2022). Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer. Radiation, 2(3), 273-284. https://doi.org/10.3390/radiation2030021







