COVID-19 Update: The Golden Time Window for Pharmacological Treatments and Low Dose Radiation Therapy
Abstract
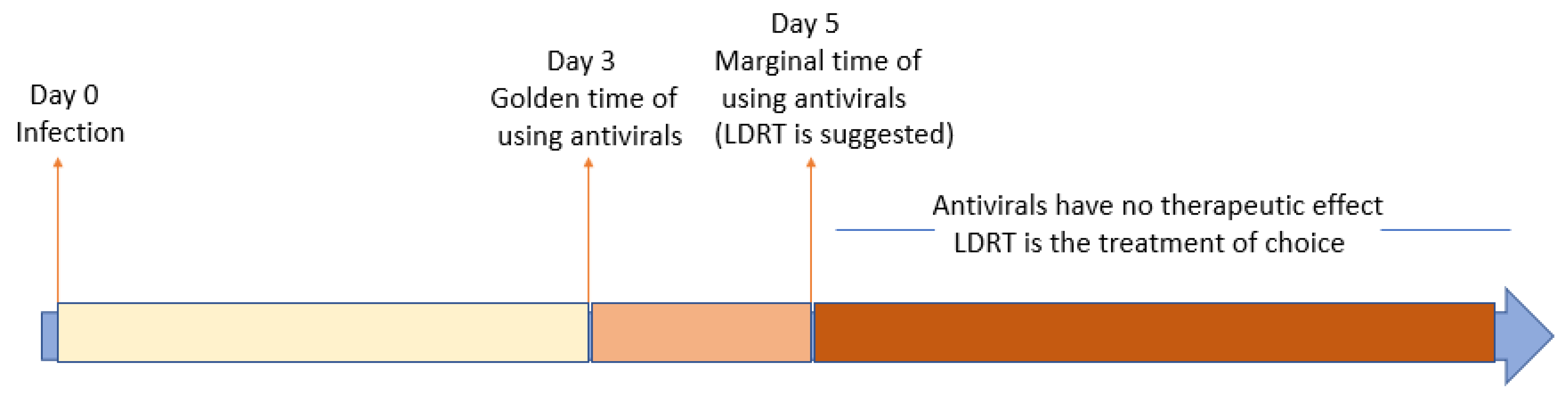
:Simple Summary
Abstract
1. Introduction
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahebnasagh, A.; Avan, R.; Saghafi, F.; Mojtahedzadeh, M.; Sadremomtaz, A.; Arasteh, O.; Tanzifi, A.; Faramarzi, F.; Negarandeh, R.; Safdari, M.; et al. Pharmacological treatments of COVID-19. Pharmacol. Rep. 2020, 72, 1446–1478. [Google Scholar] [CrossRef] [PubMed]
- García-Lledó, A.; Gómez-Pavón, J.; del Castillo, J.G.; Hernández-Sampelayo, T.; Martín-Delgado, M.C.; Sánchez, F.J.M.; Martínez-Sellés, M.; García, J.M.M.; Guillén, S.M.; Rodríguez-Artalejo, F.J.; et al. Pharmacological treatment of COVID-19: An opinion paper. Rev. Esp. Quim. 2022, 35, 115–130. (In English) [Google Scholar] [CrossRef] [PubMed]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updates 2021, 59, 100794. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.M.; Haddadi, N.; Nassif, N.T.; Lin, Y. Targeting the SphK-S1P-SIPR Pathway as a Potential Therapeutic Approach for COVID-19. Int. J. Mol. Sci. 2020, 21, 7189. (In English) [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P. Pathogenesis and treatment of cytokine storm in COVID-19. Turk. J. Biol. 2021, 45, 372–389. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, S.; Barlavie, Y.; Niv, Y.; Arad, D.; Lev, S. Accessing unproven interventions in the COVID-19 pandemic: Discussion on the ethics of ‘compassionate therapies’ in times of catastrophic pandemics. J. Med. Ethics 2021, 2020, 106783. (In English) [Google Scholar] [CrossRef] [PubMed]
- Scavone, C.; Brusco, S.; Bertini, M.; Sportiello, L.; Rafaniello, C.; Zoccoli, A.; Berrino, L.; Racagni, G.; Rossi, F.; Capuano, A. Current pharmacological treatments for COVID-19: What’s next? Br. J. Pharmacol. 2020, 177, 4813–4824. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2020, 384, 497–511. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Iliescu, F.S.; Mihaescu, G.; Cucu, A.I.; Ionescu, O.N.; Popescu, M.; Simion, M.; Burlibasa, L.; Tica, M.; Chifiriuc, M.C.; et al. Advances in the Rapid Diagnostic of Viral Respiratory Tract Infections. Front. Cell. Infect. Microbiol. 2022, 12, 11. (In English) [Google Scholar] [CrossRef]
- WHO COVID-19 Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 14 April 2022).
- Baric, R.S. Emergence of a Highly Fit SARS-CoV-2 Variant. N. Engl. J. Med. 2020, 383, 2684–2686. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef]
- Colson, P.; Devaux, C.A.; Lagier, J.C.; Gautret, P.; Raoult, D. A Possible Role of Remdesivir and Plasma Therapy in the Selective Sweep and Emergence of New SARS-CoV-2 Variants. J. Clin. Med. 2021, 10, 3276. (In English) [Google Scholar] [CrossRef]
- Torneri, A.; Libin, P.; Vanderlocht, J.; Vandamme, A.-M.; Neyts, J.; Hens, N. A prospect on the use of antiviral drugs to control local outbreaks of COVID-19. BMC Med. 2020, 18, 191. [Google Scholar] [CrossRef]
- Tracking SARS-CoV-2 Variants. 2022. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 14 April 2022).
- Mortazavi, S.A.; Bevelacqua, J.J.; Welsh, J.S.; Masoumi, S.J.; Zarandi, B.F.B.B.; Ghadimi-Moghadam, A.; Haghani, M.; Mortazavi, S.M.J. The Paradox of COVID-19 in Sub-Saharan Africa: Why It Is More Unethical Not to Investigate Low Dose Radiotherapy for COVID-19. J. Biomed. Phys. Eng. 2022. Available online: https://jbpe.sums.ac.ir/article_48063.html (accessed on 14 April 2022). (In English).
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Ghadimi-Moghadam, A.; Haghani, M.; Bevelacqua, J.; Jafarzadeh, A.; Kaveh-Ahangar, A.; Mortazavi, S.; Ghadimi-Moghadam, A.; Mortazavi, S. COVID-19 tragic pandemic: Concerns over unintentional “directed accelerated evolution” of novel Coronavirus (SARS-CoV-2) and introducing a modified treatment method for ARDS. J. Biomed. Phys. Eng. 2020, 10, 241–246. [Google Scholar]
- Mortazavi, A.; Mortazavi, S.M.J.; Sihver, L. Selective Pressure-Free Treatments for COVID-19. Radiation 2021, 1, 18–32. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Kefayat, A.; Cai, J. Low-dose radiation as a treatment for COVID-19 pneumonia: A threat or real opportunity? Med. Phys. 2020, 47, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, A.R.; Bevelacqua, J.J.; Mortazavi, S.A.R.; Mortazavi, S.M.J. COVID-19: Introducing low dose radiation as an effective treatment for pneumonia that shouldn’t induce selective pressure and new mutations. J. Biomed. Phys. Eng. 2020, 10, 247. [Google Scholar]
- Cuttler, J.M.; Bevelacqua, J.J.; Mortazavi, S. Unethical not to Investigate Radiotherapy for COVID-19. Dose-Response 2020, 18, 1559325820950104. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortazavi, S.M.J.; Zarandi, B.F.B.B.; Jafarzadeh, A.; Mortazavi, S.A.; Sihver, L. COVID-19 Update: The Golden Time Window for Pharmacological Treatments and Low Dose Radiation Therapy. Radiation 2022, 2, 268-272. https://doi.org/10.3390/radiation2030020
Mortazavi SMJ, Zarandi BFBB, Jafarzadeh A, Mortazavi SA, Sihver L. COVID-19 Update: The Golden Time Window for Pharmacological Treatments and Low Dose Radiation Therapy. Radiation. 2022; 2(3):268-272. https://doi.org/10.3390/radiation2030020
Chicago/Turabian StyleMortazavi, Seyed Mohammad Javad, B. F. Bahaaddini Baigy Zarandi, Abdollah Jafarzadeh, S. Alireza Mortazavi, and Lembit Sihver. 2022. "COVID-19 Update: The Golden Time Window for Pharmacological Treatments and Low Dose Radiation Therapy" Radiation 2, no. 3: 268-272. https://doi.org/10.3390/radiation2030020
APA StyleMortazavi, S. M. J., Zarandi, B. F. B. B., Jafarzadeh, A., Mortazavi, S. A., & Sihver, L. (2022). COVID-19 Update: The Golden Time Window for Pharmacological Treatments and Low Dose Radiation Therapy. Radiation, 2(3), 268-272. https://doi.org/10.3390/radiation2030020







