Simple Summary
As the stomach is one of the most deformable organs, it poses difficulties in targeting radiotherapy. Through our studies, we have found that a magnetic resonance imaging repositioning prior to daily radiotherapy can guide radiotherapy more precisely while reducing organ-threatening toxicity. The emerging MR-guided radiotherapy, based on the MR-LINAC system, may have the potential to solve the above difficulties due to its unique advantages. The system’s adaptive learning function can greatly improve the accuracy and efficiency of goal descriptions in adaptive planning. Each individualized treatment can be adapted to the patient’s current anatomy, potentially reducing the risk of radiation-related toxicity while increasing the radiation dose. MRI-guided radiotherapy offers a new treatment modality for radiotherapists.
Abstract
It is still very challenging to use conventional radiation therapy techniques to treat stomach tumors, although image-guided radiotherapy, mainly by kV X-ray imaging techniques, has become routine in the clinic. This is because the stomach is one of the most deformable organs, and thus it is vulnerable to respiratory motions, daily diet, and body position changes. In addition, X-ray radiographs and CT volumetric images have low contrast in soft tissues. In contrast, magnetic resonance imaging (MRI) techniques provide good contrast in images of soft tissues. The emerging MR-guided radiotherapy, based on the MR-LINAC system, may have the potential to solve the above difficulties due to its unique advantages. The real-time imaging feature and the high-contrast of soft tissues MR images provided by the MR-LINAC system have facilitated the therapeutic adaptive planning. Online learning capabilities could be used to optimize the automatic delineation of the target organ or tissue prior to each radiotherapy session. This could greatly improve the accuracy and efficiency of the target delineation in adaptive planning. In this clinical case report, we elaborated a workflow for the diagnosis and treatment of two patients with gastric mucosa-associated lymphoid tissue (MALT) lymphoma. One patient underwent MR-guided daily adaptive radiotherapy based on daily automated segmentation using the novel artificial intelligence (AI) technique for gastric delineation.
1. Introduction
Radiation therapy is one of the most effective treatment modalities for lymphoma. The history of radiotherapy for lymphoma represents one of the greatest successes in modern cancer treatment [1,2]. The stomach is a deformable organ, and its shape can easily vary even under prolonged fasting. X-ray imaging techniques, such as kV radiography and cone-beam CT (CBCT), have been broadly used in image-guided radiotherapy (IGRT). Although the X-ray based IGRT techniques have greatly improved the accuracy of patient positioning and the subsequent dose delivery, X-ray images of soft tissues have inferior contrast and are not suitable for stomach imaging. These obstacles have impeded the application of radiation therapy in treating stomach tumors. In fact, inter-fraction dose delivery may be inaccurate, unless an effective and accurate adaptive plan can be made for each treatment. The emerging MR-LINAC system can improve the applicability and accuracy of radiation therapy for upper abdominal tumors [3]. This system combines the functions of linear accelerators (LINACs) used for treatment with magnetic resonance imaging (MRI) scanners to enable online adaptive MRI-guided radiotherapy (MRgRT) [4,5,6,7]. The superior soft-tissue contrast in MR imaging and the possibility of online planning adjustments may allow for precise radiation therapy, which in particular, will be more effective for tumors in deformable organs. In the current reported patient case, we used a clinical MRgRT system consisting of a 1.5 T MR-LINAC (Unity MR-LINAC, Elekta, Stockholm, Sweden) to illustrate the unique ability of MRI guidance to treat a stomach tumor with daily adaptive planning. The adaptive plans were based on daily automated segmentation using the novel artificial intelligence (AI) technique for gastric delineation. The MR-LINAC used consists of a 7 MV linear accelerator and a 1.5 T diagnostic MRI scanner. The images in this study were obtained by making T2W (abdominal sequence parameters: TR = 2000 ms, TE = 206 ms, SNR = 1, ACQ matrix M*P = 232*167) sequences on Elekta Unity MR-LINAC system.
2. Presentation of Case #1
A 53-year-old male patient with a history of gastric bleeding and gastric ulcer for more than 18 years was selected for this study. The patient developed melena on 13 January 2022, without obvious causes. Contrast CT of the chest, abdomen, and pelvis showed that the wall of the gastric antrum was thick with two enlarged lymph nodes. Fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) images revealed high FDG uptake in both the gastric wall of the inhomogeneously thickened greater curvature and the two enlarged lymph nodes. Pathological reports presented mucosa-associated lymphoid tissue (MALT) lymphoma in the greater curvature at the junction of the astral body, after performing immunohistochemistry of CD20+, CD43+, CD3+, BCL2+, CD5 minority+, CD23 minority-, CD38 minority+, CyclinD1 minority+, Lambda individual+, BCL-6-, Kappa-, Ki-67+10%. The patient was diagnosed with stage IIE gastric MALT lymphoma according to Ann Arbor staging. The patient received four cycles of rituximab, 600 mg, and then involved site radiation therapy (ISRT) on the whole stomach. The patient underwent a 4D CT scan on an empty stomach. Before the CT simulation, anisodamine (dose, 10 mg) was administered to reduce gastric motility. The entire stomach (clinic target volume, CTV) was delineated in the 4D CT scans, and the images were combined into an internal CTV (iCTV). The combined iCTV included the enlarged metastatic lymph nodes and the planning target volume (PTV), which presented a 5 mm external expansion from iCTV. The patient was treated with ISRT at 30 Gy in 15 fractions. Then, the patient was treated with the Unity MR-LINAC. Prior to each radiotherapy fraction, the patient was kept on an empty stomach and was administered anisodamine (dose of 10 mg). The empty stomach was delineated with automated segmentation using the AI technique, and then the resulting image was reviewed and modified by an attending radiation oncologist. The MRI image of the whole stomach after anisodamine administration was clearer than the images taken without administering the drug (Figure 1). The images obtained with the AI technology using deep learning algorithms showed that the delineation accuracy of the whole stomach gradually improved. The Dice similarity coefficient (DSC) increased gradually (Figure 2). The DSC measures the volumetric overlap of two sets of data and was obtained with the following Equation (1), which calculates the quotient of similarity between two volumetric sets with a value between 0 and 1. In this formula (Equation (1)), A and B are the target area volumes obtained by AI and manually corrected, respectively. A DSC of 1 means perfect segmentation, whereas a DSC of 0 means no overlap at all.
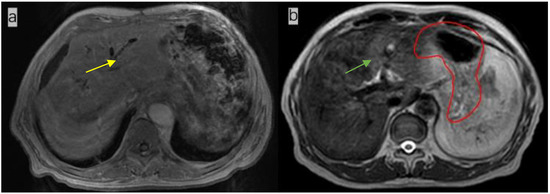
Figure 1.
(a). Magnetic resonance image (MRI) of a patient (not the patient treated in this study) not treated with anisodamine; notice that the outline of the stomach is blurred (yellow arrow); (b) MR image of the patient treated with anisodamine; notice that the outline of the stomach is clearer (green arrow; the red line indicates the contours of the whole stomach).
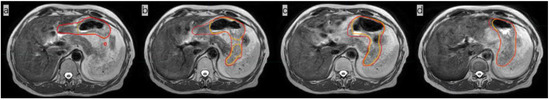
Figure 2.
Comparison of images of the whole stomach on the same plane after the 1st, 5th, 10th, and 15th fractions, showing the radiation oncologist’s delineation (red line) and the delineation obtained with AI (orange line). The dice similarity coefficient (DSC) of the three-dimensional stomach images indicated by these four outlines are 0.8311 (a), 0.9185 (b), 0.9193 (c), and 0.9427 (d), respectively.
The patient was then assigned an online MR-based adaptive plan and underwent radiotherapy. The intrafraction variation during radiotherapy remained stable on two MRI imaging sets before and after the treatment (Figure 3).
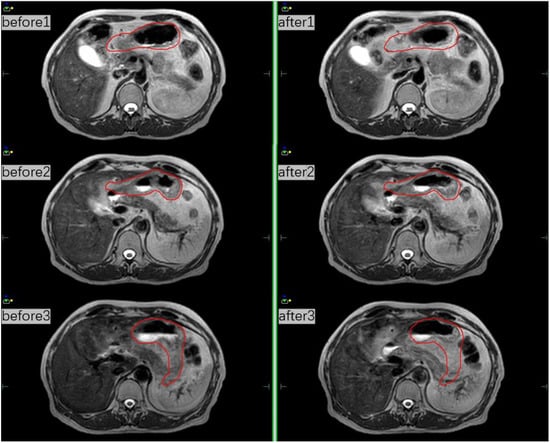
Figure 3.
MRI scans before and after treatment at three different fractions and the red line indicates the whole stomach.
We calculated the DSC after stomach delineation of real-time MR imaging and simulation CT imaging. Interestingly, we found that the patient’s inter-fractional stomach variation changed greatly. With CBCT online guidance, even after expanding the PTV by 1 cm as usual, there remain 8/15 axis planes that could not cover the entire stomach; therefore, the target, i.e., the entire stomach, would be missed (Figure 4) At the same time, we could monitor the organs around the stomach, such as liver, left kidney, and spinal cord, which were irradiated daily, and compared their state them with that of the same organs after a mean dose in the CT simulation. (Table 1) The liver and duodenum were on average less exposed to toxic doses in each MRI-guided treatment than in the CT simulation (liver: 840.7 cGy < 929.1 cGy, duodenum: 874.9 cGy < 1025.1 cGy). Although the average doses received by the spinal cord and the left kidney were slightly higher in MRI-guided therapy than in CT simulation (spinal cord:483.3 cGy > 470.8 cGy, left kidney: 297.7 cGy > 277.7 cGy), they were far lower than their respective dose constraints (the maximum dose constraint for the spinal cord is 4500 cGy, and the average kidney dose constraint is less than 1800 cGy) [8].
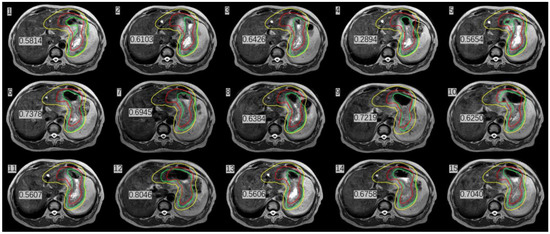
Figure 4.
Stomach delineation DSC (number in Figure) of real-time MR imaging and simulation CT imaging the (red line delineates the stomach on the simulation images, the green line delineates the stomach on the daily MRI images, and the yellow line delineates the stomach considering 1 cm external expansion from the iCTV).

Table 1.
Dose of OAR in CT simulation and MRI-guided therapy.
3. Presentation of Case #2
A 70-year-old female patient presented with anorexia in September, 2021. A gastroscopy showed a 5*5 cm bulge in the middle curve of the gastric body. FDG-PET/CT showed a high FDG-avid gastric mass with slightly FDG-avid lymph nodes. The pathology report showed an extra-lymph node marginal zone indicative of a MALT lymphoma. Immunohistochemistry to measure biomarkers showed the following results: CD20+, CD79a+, CD3-, CD7-, CD5-, CD10-, BCL-6-small amount+, BCL-2 partial+, Mum-1-, C-Mum-1-, C-myc-, CyclinD1-, P53+ about 60% and Ki-67+ about 10%. A bone marrow biopsy showed active bone marrow proliferation with no obvious lymphoma cell involvement. The patient was diagnosed with stage IIE gastric MALT lymphoma according to Ann Arbor staging. The patient received four cycles of rituximab and then ISRT on the whole stomach and the involved lymph nodes, administering ISRT at 30 Gy/15 fractions. The CTV included whole stomach and the enlarged metastatic lymph nodes and was expanded by 1 cm to generate the internal target volume (ITV) so to cover the stomach movement. CBCT was daily performed before radiotherapy to adjust the setup error. However, using daily on board CBCT imaging, we found that the stomach images were poor and did not allow carrying out the adaptive plan for this patient with gastric MALT lymphoma (Figure 5).
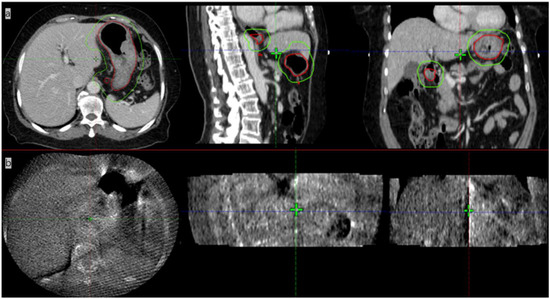
Figure 5.
Images of patient #2 with contrast CT simulation (a) and CBCT imaging (b). The red line in (a) shows the stomach, and the green line contours the PTV.
The basic profiles of the two patients are listed in Table 2.

Table 2.
Comparison of the two patients’ information.
4. Discussion and Review of the Literature
The adaptive planning based on daily variability is nowadays becoming a trend in radiation therapy because, in principle, it could reduce the inaccuracy of inter-fractional dose delivery [9]. The emerging MRI-guided radiation therapy systems, such as the Elekta Unity System, allow for continuous real-time MRI guidance during the whole treatment. With the first patient being treated on this hybrid device in 2017, MR-guided combined RT systems for online therapy are now commercialized and gaining popularity and are expected to become of common use in the near future [10]. MRI-guided radiotherapy and tracking offer unique advantages, such as greatly improved soft tissue targeting and delineation of organs at risk (OAR), finer tracking of small margins, and minimization or elimination of surrogates. During treatment, MRI-guided radiation therapy systems allow a better visualization of the targets, avoidance of soft tissue structures, and adaptation to the anatomy of moving structures [11]. Acquiring MRI data as part of an MRI-guided workflow may provide potentially actionable information before and during treatment about the course of the treatment [12]. In this paper, the patient with MALT lymphoma underwent MR-guided daily adaptive radiotherapy based on daily automated segmentation using AI for gastric delineation. We showed that the MRI-guided system could provide precise radiotherapy, in particular, for moving targets.
IGRT has become routine as a standard configuration in modern radiation therapy. Imaging the patient prior to treatment can help reduce the impact of setup errors, thus improving the dose delivery accuracy [13]. Currently, most modern radiotherapy systems are equipped with kV CBCT to visualize anatomical structures and identify the target volumes. Although CBCT is highly effective, it provides images with poor quality for soft tissues—including abdominal organs such as the liver, stomach, and pancreas—which makes it very difficult to accurately identify the soft-tissue targets and surrounding OARs. In the case of our gastric MALT lymphoma patient shown in Figure 5, it was not feasible to use CBCT for image guidance because of its poor image quality. A larger PTV margin with at least 1 cm had to be delineated, but this would cause higher toxicity to OARs. In addition, a 1 cm margin could not cover the entire empty stomach even if anisodamine (dose 10 mg) was administered to reduce gastric motility (Figure 4) [14] (pp. 174–177). As shown in Figure 4, even after expanding the PTV by 1 cm as usual with CBCT online guidance, there remain 8/15 axis planes that could not cover the entire stomach. Therefore, the target, i.e., the entire stomach, would be missed in regular clinical practice without MRI image guidance. Poor CBCT imaging of the stomach makes it more challenging to incorporate corrections for certain differences in routine patient anatomy, as well as to use approaches based on adaptive treatment protocols. The MR-LINAC system overcomes the limitations of traditional IGRT, especially for soft tissues requiring the precise definition of the target and of OARs. MR-LINAC improves IGRT by enhancing soft-tissue contrast in MR images without delivering concomitant radiation doses. MR-LINAC brings a unique emerging workflow that can help radiation therapy practitioners improve their skills and understand MR anatomy. The case reported here illustrates that MR-LINAC could visualize the stomach clearly, especially after administering anisodamine. Meanwhile, an attractive option for MR-LINAC might be to re-optimize or adjust the therapeutic plan before each radiation treatment. Diagnostic-quality MR images can be acquired based on the actual patient anatomy before and during treatment and then used in an online adaptive workflow. Compared to CBCT, online 1.5 T MRI provides better target visualization on an MR-LINAC and aids online adaptive treatment strategies, including daily replanning. Although online adaptation techniques guided by CBCT are improving, the quality of stomach images obtained by CBCT is likely to remain poor. In contrast, in MRI-guided therapy, increased soft-tissue contrast can more accurately visualize day-to-day variations and may allow the delineation of a smaller PTV margin, thus reducing OAR toxicity [15].
Lois A. Daamen and colleagues reported 25 cases of common upper abdominal tumors (excluding gastric cancer) using 1.5 T MR-LINAC radiotherapy systems and showed that therapy could be guided by online adaptive MRI. Retrospective data from 44 patients with unresectable pancreatic cancer showed that the increasing tumor dose delivered with this MRI-guided approach improved the overall survival [16]. Currently, there are fewer reports on the use of MR-LINACs to treat stomach tumors. For patients with gastric cancer, MR-guided radiotherapy may be an appropriate option, which uses real-time MR guidance and an adaptive planning system and was shown to allow the radiation dose to be safely delivered to gastric focal lesions [17]. Our case showed that inter-fractional gastric motion is constant, and MRI-based treatment systems with integrated treatment planning software can adjust the treatment plans during radiotherapy to accommodate daily changes in the position of these organs and track these structures in real time during radiotherapy, which could avoid tumor underdose as well as the overdose of critical structures. The stomach is one of the most deformable organs, and magnetic resonance accelerators can adjust the treatment plan according to the actual anatomical structure every day. This technique has broad application prospects.
The main limitation of MRI-guided adaptive planning is the fact that it requires the delineation of the target volume including tumor and OAR daily, which is time-consuming for radiation oncologists. However, automated segmentation using AI is an effective way to the delineate target volume quickly. Our case showed that automated segmentation using AI deep learning could delineate the target volume more and more accurately and efficiently.
In summary, MRI-guided radiation therapy is a new, rapidly evolving technology with an improved risk/benefit ratio and cost-effectiveness. Even on moving targets, such as the stomach, radiation oncologists can provide highly precise treatments, with direct visualization of the tumor and nearby healthy tissues. Each individualized treatment can be tailored to the patient’s current anatomy, potentially reducing the risk of radiation-related toxicity while increasing the radiation dose. MRI-guided radiation therapy offers radiation oncologists a new treatment modality. Future controlled trials with large samples are needed to determine if longer patient’s survival is achieved with MRI accelerator-guided radiotherapy than with conventional radiotherapy.
Author Contributions
Conceptualization, Y.S., Y.Z. and J.Y.; methodology, Y.S. and J.Y.; software, Z.L. and H.W.; validation, Y.S., Y.Z. and J.Y.; formal analysis, Y.S.; investigation, Y.S.; resources, Y.Z. and J.Y.; data curation, Y.S.; original draft preparation, Y.S., Y.Z. and J.Y.; review and editing, Y.S., Z.L., H.W., Y.Z. and J.Y.; visualization, Y.S., Y.Z. and J.Y.; supervision, Y.Z. and J.Y.; project administration, Y.Z. and J.Y.; funding acquisition, Y.Z. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the following grants: National Natural Science Foundation of China (Grant No. 81871895), Young Taishan Scholars and Academic Promotion Program of Shandong First Medical University (Grant No. 2019RC003).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. All diagnostic and therapeutic procedures were performed according to the current standard of care and after receiving the patients’ informed consent. No diagnostic or therapeutic acts were performed for the purpose of research. This is only a report of two cases treated according to the standard of care; a formal approval from the Ethic Committee is not required.
Informed Consent Statement
The patients provided a written consent to participate in this study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank the patients and their families who participated in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the data collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.-H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Primers 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Bowzyk Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-Hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef] [PubMed]
- Daamen, L.A.; de Mol van Otterloo, S.R.; van Goor, I.W.J.M.; Eijkelenkamp, H.; Erickson, B.A.; Hall, W.A.; Heerkens, H.D.; Meijer, G.J.; Molenaar, I.Q.; van Santvoort, H.C.; et al. Online adaptive MR-guided stereotactic radiotherapy for unresectable malignancies in the upper abdomen using a 1.5T MR-linac. Acta Oncol. 2022, 61, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kontaxis, C.; Bol, G.H.; Lagendijk, J.J.W.; Raaymakers, B.W. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys. Med. Biol. 2015, 60, 7485–7497. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.; Kashani, R.; Robinson, C.; Curcuru, A.; DeWees, T.; Bradley, J.; Green, O.; Michalski, J.; Mutic, S.; Parikh, P.; et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 126, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaymakers, B.W.; Lagendijk, J.J.W.; Overweg, J.; Kok, J.G.M.; Raaijmakers, A.J.E.; Kerkhof, E.M.; van der Put, R.W.; Meijsing, I.; Crijns, S.P.M.; Benedosso, F.; et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: Proof of concept. Phys. Med. Biol. 2009, 54, N229–N237. [Google Scholar] [CrossRef] [PubMed]
- Lagendijk, J.J.W.; Raaymakers, B.W.; van Vulpen, M. The magnetic resonance imaging-linac system. Semin. Radiat. Oncol. 2014, 24, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Kagadis, G.C.; McNutt, T.R.; Moiseenko, V.; Mutic, S. Vision 20/20: Automation and advanced computing in clinical radiation oncology. Med. Phys. 2014, 41, 010901. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Hörner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, J.S.; Rosenberg, S.A.; Bassetti, M.F. MRI-guided adaptive radiotherapy for liver tumours: Visualising the future. Lancet Oncol. 2020, 21, e74–e82. [Google Scholar] [CrossRef]
- Stephen, R.M.; Jha, A.K.; Roe, D.J.; Trouard, T.P.; Galons, J.-P.; Kupinski, M.A.; Frey, G.; Cui, H.; Squire, S.; Pagel, M.D.; et al. Diffusion MRI with Semi-Automated Segmentation Can Serve as a Restricted Predictive Biomarker of the Therapeutic Response of Liver Metastasis. Magn. Reson. Imaging 2015, 33, 1267–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffray, D.A.; Siewerdsen, J.H.; Wong, J.W.; Martinez, A.A. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1337–1349. [Google Scholar] [CrossRef]
- Videtic, G.; Vassil, A.; Neil, W. Handbook of Treatment Planning in Radiation Oncology, 3rd ed.; Springer Publishing Company, LLC: New York, NY, USA, 2021; pp. 174–177. [Google Scholar]
- Winkel, D.; Werensteijn-Honingh, A.M.; Eppinga, W.S.C.; Intven, M.P.W.; Hes, J.; Snoeren, L.M.W.; Visser, S.A.; Bol, G.H.; Raaymakers, B.W.; Jürgenliemk-Schulz, I.M.; et al. Dosimetric feasibility of hypofractionation for SBRT treatment of lymph node oligometastases on the 1.5T MR-linac. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 154, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Jiang, N.; Rosenberg, S.A.; Olsen, J.R.; Roach, M.C.; Wan, L.; Portelance, L.; Mellon, E.A.; Bruynzeel, A.; Lagerwaard, F.; et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019, 8, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.-J.; Jeon, S.H.; Chie, E.K. A Case Report of Salvage Radiotherapy for a Patient with Recurrent Gastric Cancer and Multiple Comorbidities Using Real-time MRI-guided Adaptive Treatment System. Cureus 2018, 10, e2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).