Establishment of the First Orchidarium in Serbia: Strategy for Sustainable Management of Native Orchid Genetic Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Habitat Characteristics and Plant Material Selection
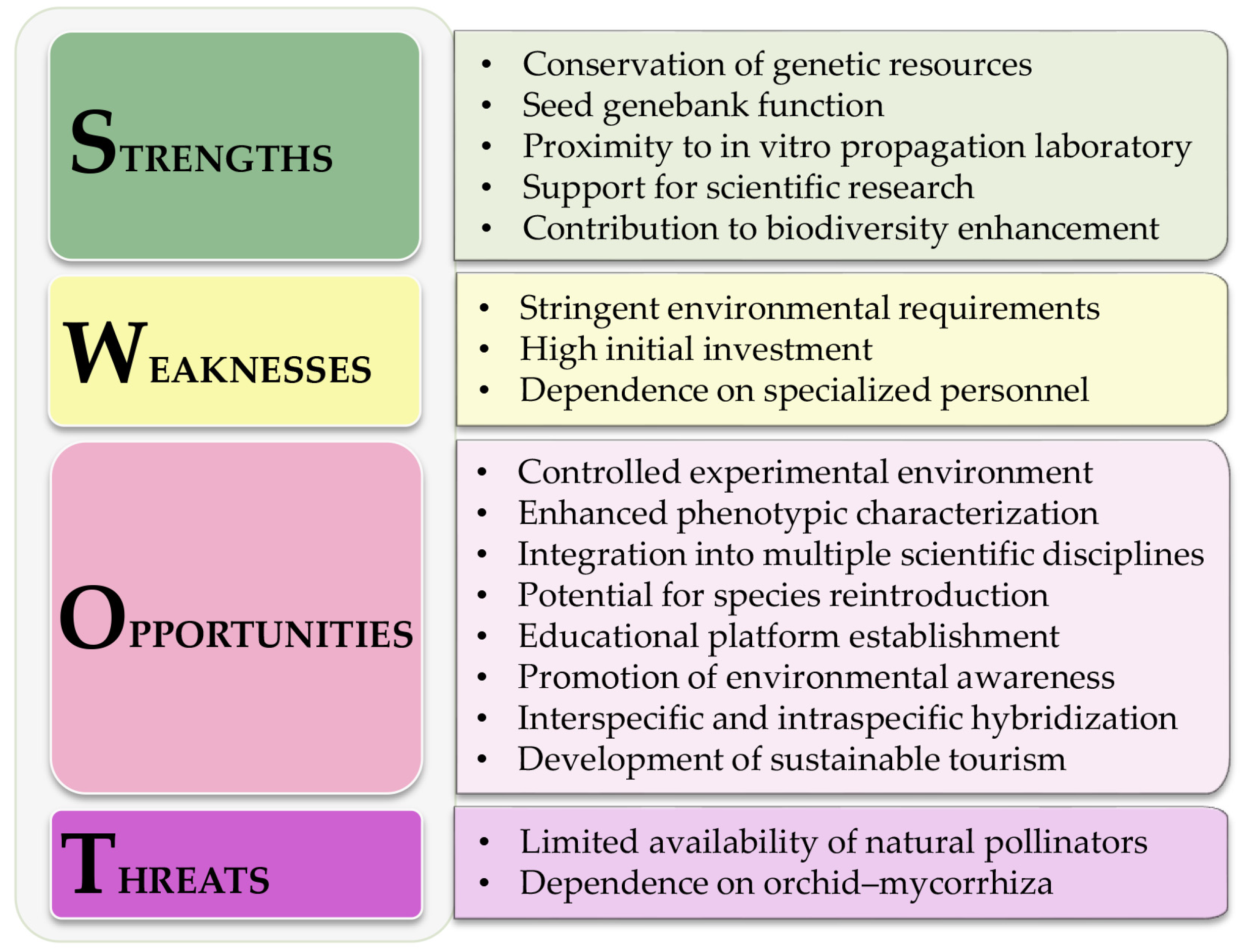
2.2. SWOT Analysis of National Orchidarium Development
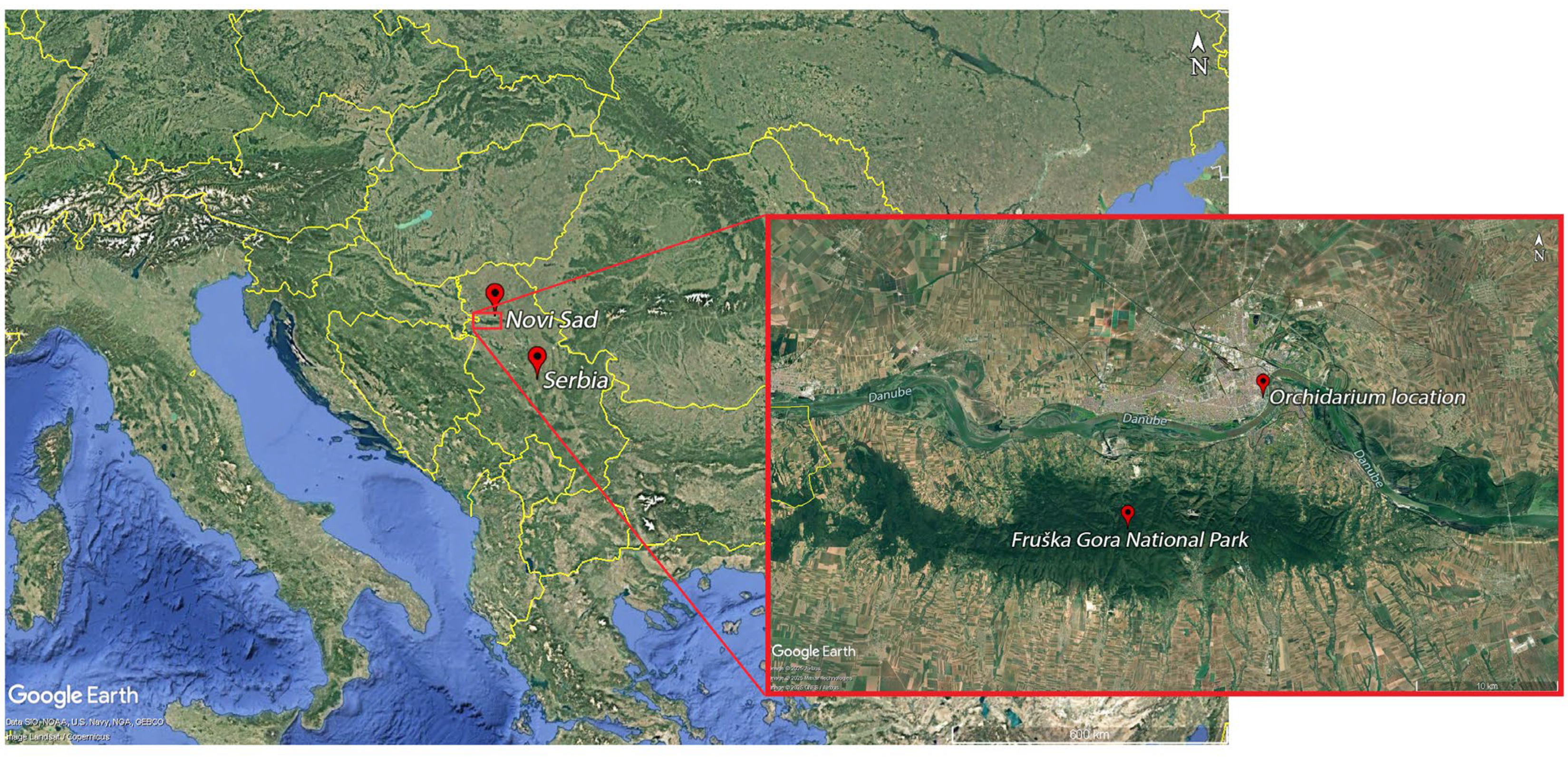
2.3. Project Site Description
2.4. Concept Development and Visualization Tools
3. Results and Discussion
3.1. SWOT Analysis
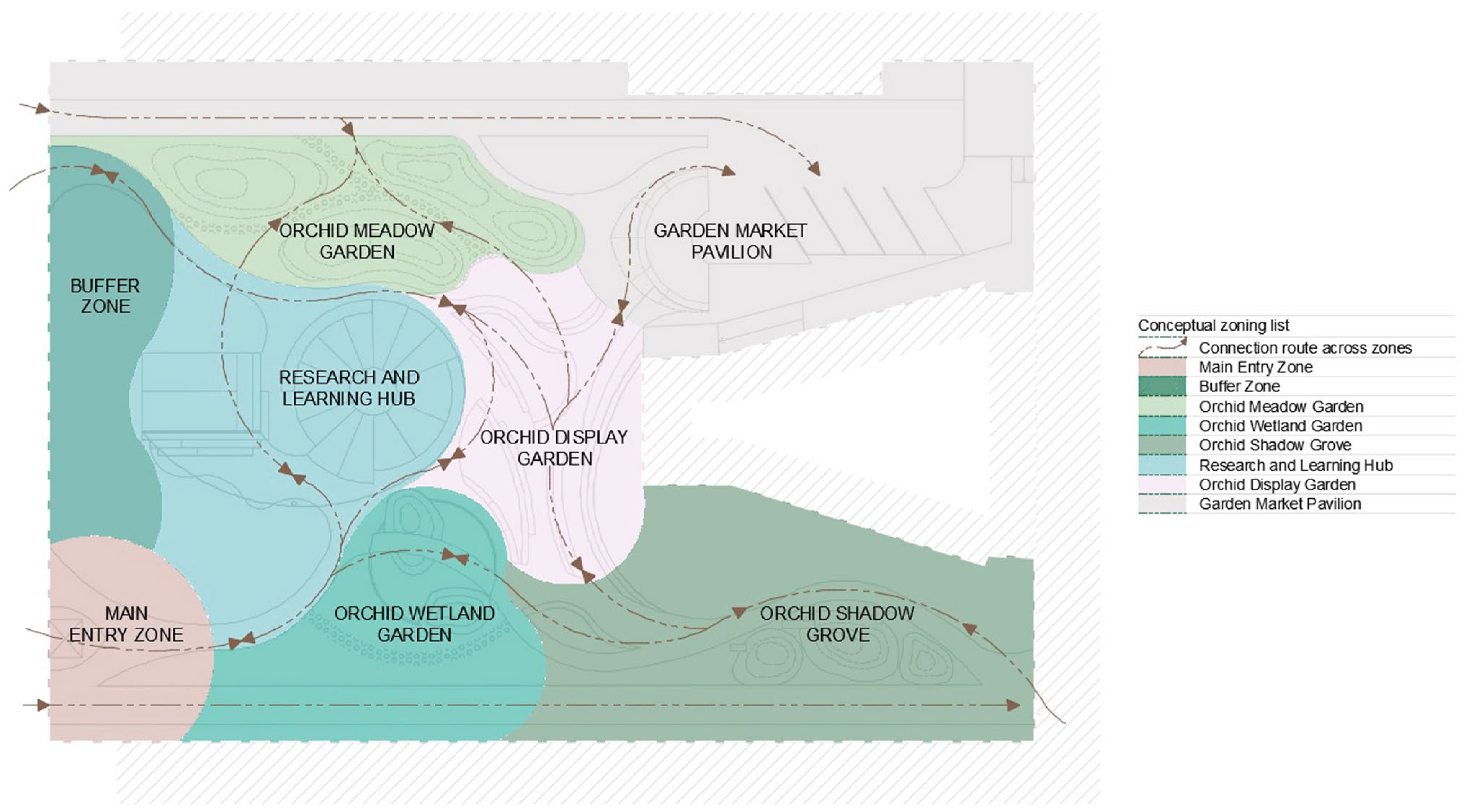
3.2. Conceptual Design of the Orchidarium
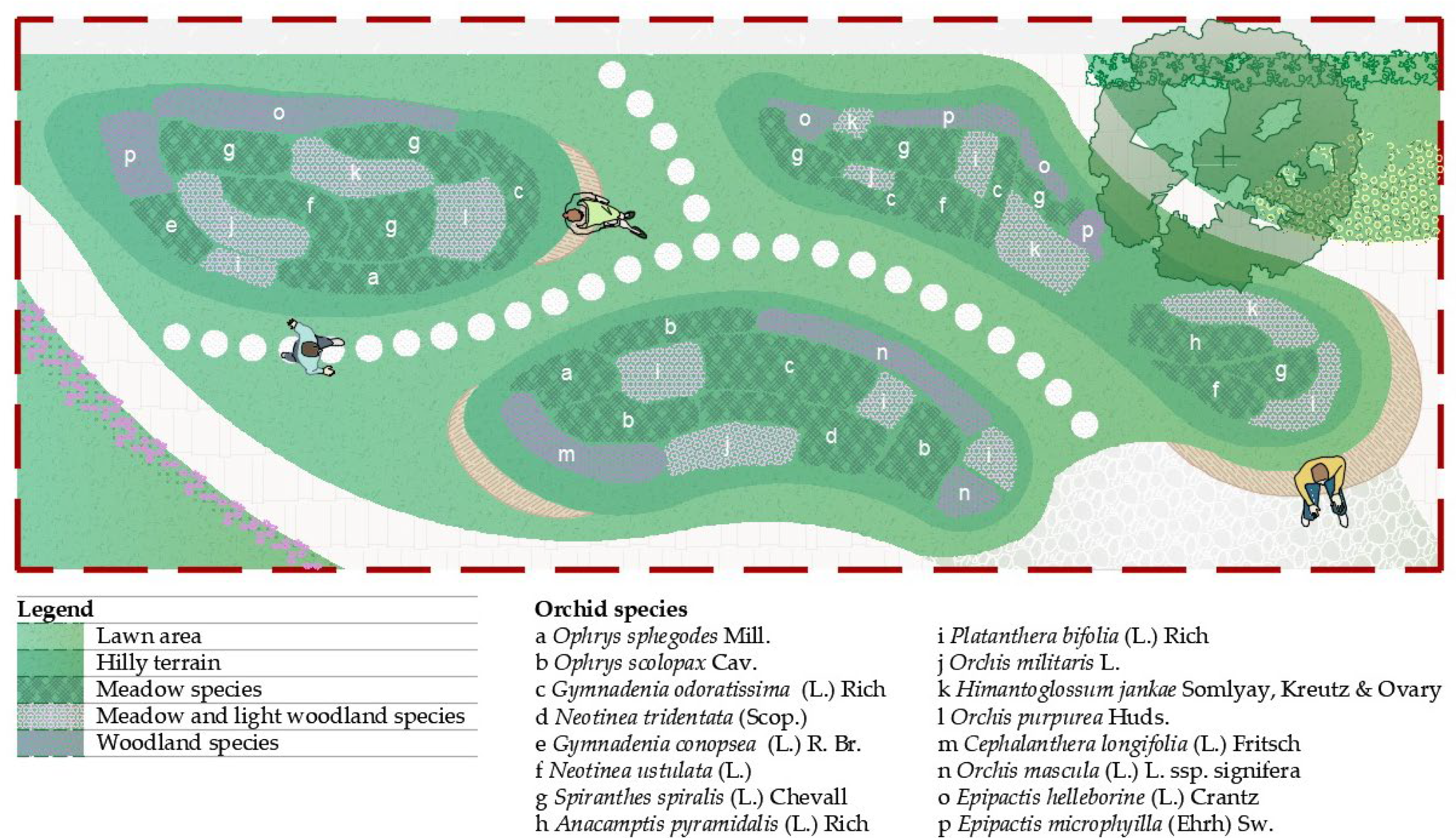
3.2.1. Perimeter Design: Entry and Buffering Elements
3.2.2. Design Reflections of Natural Orchid Habitats
3.2.3. Bridging Nature, Science, and Education
3.2.4. From Wild Habitats to Home Gardens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aina, A.T.; Salau, A.T. The Challenges of Sustainable Development in Nigeria; Nigerian Environmental Study/Action Team (NEST): Rio de Janeiro, Brazil, 1992; pp. 8–16. [Google Scholar]
- Ruiz Serrano, A.; Musumeci, A.; Li, J.J.; Ruiz Serrano, M.; Serrano Barquin, C. Rationality and the exploitation of natural resources: A psychobiological conceptual model for sustainability. Environ. Dev. Sustain. 2024, 27, 13167–13189. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic Garden Solutions to the Plant Extinction Crisis. Plants People Planet 2020, 2, 515–529. [Google Scholar] [CrossRef]
- Faraji, L.; Karimi, M. Botanical gardens as valuable resources in plant sciences. Biodivers. Conserv. 2022, 31, 2905–2926. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sharrock, S. Botanic Gardens Complement Agricultural Gene Bank in Collecting and Conserving Plant Genetic Diversity. Biopreserv. Biobank. 2018, 16, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, M.M.; Dixon, K.W. Propagation science, recovery and translocation of terrestrial orchids. In Orchid Conservation; Dixon, K.W., Kell, S.P., Barrett, R.L., Cribb, P.J., Eds.; Natural History Publications: Kota Kinabalu, Malaysia, 2003; pp. 259–288. [Google Scholar]
- Dixon, K.W. Gardening to conservation—The emerging role of botanic gardens in recovery of endangered species. In Conservation into the 21st Century, Proceedings of the 4th International Botanic Gardens Conservation Congress, Perth, Australia, 25–19 September 1995; Touchell, D.H., Dixon, K.W., Eds.; Kings Park and Botanic Gardens: Perth, Australia, 1995; pp. 169–174. [Google Scholar]
- Chase, M.; Christenhusz, M.; Mirenda, T. The Book of Orchids: A Life-Size Guide to Six Hundred Species from Around the World; Ivy Press: London, UK, 2017. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of Distribution, Abundance and Composition of Forest Terrestrial Orchids. Biodivers. Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Djordjević, V.; Niketić, M.; Stevanović, V. Orchids of Serbia: Taxonomy, Life Forms, Pollination Systems, and Phytogeographical Analysis. In Orchidaceae: Characteristics, Distribution and Taxonomy; Đorđević, V., Ed.; Nova Science Publishers: New York, NY, USA, 2021; pp. 57–163. ISBN 978-1-68507-189-9. [Google Scholar] [CrossRef]
- Djordjević, V.; Lakušić, D.; Novković, I.; Stevanović, V.; Tsiftsis, S. Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types. Plants 2025, 14, 443. [Google Scholar] [CrossRef]
- Dulić, J.; Ljubojević, M.; Savić, D.; Ognjanov, V.; Dulić, T.; Barać, G.; Milović, M. Implementation of SWOT Analysis to Evaluate Conservation Necessity and Utilization of Natural Wealth: Terrestrial Orchids as a Case Study. J. Environ. Plan. Manag. 2020, 63, 2265–2286. [Google Scholar] [CrossRef]
- Seaton, P.T.; Hu, H.; Perner, H.; Pritchard, H.W. Ex Situ Conservation of Orchids in a Warming World. Bot. Rev. 2010, 76, 193–203. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Jacquemyn, H.; Kull, T.; Hutchings, M.J. The Demography of Terrestrial Orchids: Life History, Population Dynamics and Conservation. Bot. J. Linn. Soc. 2020, 192, 315–332. [Google Scholar] [CrossRef]
- Geppert, C.; Perazza, G.; Wilson, R.J.; Bertolli, A.; Prosser, F.; Melchiori, G.; Marini, L. Consistent Population Declines but Idiosyncratic Range Shifts in Alpine Orchids under Global Change. Nat. Commun. 2020, 11, 5835. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial Orchid Conservation in the Age of Extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Liu, H.; Feng, C.L.; Luo, Y.B.; Chen, B.S.; Wang, Z.S.; Gu, H.Y. Potential Challenges of Climate Change to Orchid Conservation in a Wild Orchid Hotspot in Southwestern China. Bot. Rev. 2010, 76, 174–192. [Google Scholar] [CrossRef]
- Paul, J.; Budd, C.; Freeland, J. Conservation Genetics of an Endangered Orchid in Eastern Canada. Conserv. Genet. 2013, 14, 195–204. [Google Scholar] [CrossRef]
- Vogt-Schilb, H.; Munoz, F.; Richard, F.; Schatz, B. Recent Declines and Range Changes of Orchids in Western Europe (France, Belgium and Luxembourg). Biol. Conserv. 2015, 190, 133–141. [Google Scholar] [CrossRef]
- IUCN Red List. Available online: https://www.iucnredlist.org/ (accessed on 13 May 2025).
- Swarts, N.D.; Dixon, K.W. Perspectives on orchid conservation in botanic gardens. Trends Plant Sci. 2009, 14, 590–598. [Google Scholar] [CrossRef]
- New York Botanical Garden. Available online: https://www.nybg.org/ (accessed on 13 May 2025).
- Royal Botanic Gardens, Kew. Available online: https://www.kew.org/ (accessed on 13 May 2025).
- Marie Selby Botanical Gardens. Available online: https://www.selby.org/ (accessed on 13 May 2025).
- National Orchid Garden, Singapore Botanic Gardens. Available online: https://www.nparks.gov.sg/sbg/our-gardens/national-orchid-garden (accessed on 13 May 2025).
- Savić, D. Ekologija, Rasprostiranje i Zaštita Vrsta Familije Orchidaceae na Fruškoj Gori. Master’s Thesis, Faculty of Biology, University of Belgrade, Belgrade, Serbia, 1998. [Google Scholar]
- Dulić, J. Biodiversity and Breeding of Terrestrial Orchids of Fruška Gora. Ph.D. Thesis, Faculty of Agriculture, University of Novi Sad, Novi Sad, Serbia, 2019. [Google Scholar]
- Harp, A.; Harp, S. Orchids of Britain and Ireland; A&C Black Publishers Ltd.: London, UK, 2005. [Google Scholar]
- Wittlinger, L.; Petrikovičová, L. Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local study). Plants 2021, 10, 588. [Google Scholar] [CrossRef]
- Öztürk, D. Morphological, Anatomical and Ecological Studies on Orchis simia (Orchidaceae) Taxon of Eskişehir, Turkey. Eurasian J. Biol. Chem. Sci. 2020, 3, 110–115. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Ecological Indicator Values of Central European Plant Species. In Scripta Geobotanica; Goltze: Göttingen, Germany, 1991; Volume 18, pp. 1–248. [Google Scholar]
- Rusconi, O.; Broennimann, O.; Storrer, Y.; Le Bayon, R.-C.; Guisan, A.; Rasmann, S. Detecting Preservation and Reintroduction Sites for Endangered Plant Species Using a Two-Step Modeling and Field Approach. Conserv. Sci. Pract. 2022, 4, e12800. [Google Scholar] [CrossRef]
- Maleva, M.; Borisova, G.; Chukina, N.; Sinenko, O.; Filimonova, E.; Lukina, N.; Glazyrina, M. Adaptive Morphophysiological Features of Neottia ovata (Orchidaceae) Contributing to Its Natural Colonization on Fly Ash Deposits. Horticulturae 2021, 7, 109. [Google Scholar] [CrossRef]
- Janečková, P.; Wotavová, K.; Schödelbauerová, I.; Jersáková, J.; Kindlmann, P. Relative Effects of Management and Environmental Conditions on Performance and Survival of Populations of a Terrestrial Orchid, Dactylorhiza majalis. Biol. Conserv. 2006, 129, 40–49. [Google Scholar] [CrossRef]
- Qumsiyeh, M.; Handal, E.; Chang, J.; Abualia, K.; Najajreh, M.; Abusarhan, M. Role of Museums and Botanical Gardens in Ecosystem Services in Developing Countries: Case Study and Outlook. Int. J. Environ. Stud. 2017, 74, 340–350. [Google Scholar] [CrossRef]
- Čurčić, M.; Narandžić, T.; Božanić Tanjga, B.; Grubač, M.; Pušić Devai, M.; Šarac, V.; Ljubojević, M. Designing the First Rosarium in Serbia to Fulfill Environmental, Societal, and Economical Purposes. J. Zool. Bot. Gard. 2024, 5, 590–605. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Available online: https://www.hidmet.gov.rs/eng/meteorologija/stanica_sr.php?moss_id=13168 (accessed on 13 May 2025).
- Mounce, R.; Smith, P.; Brockington, S. Ex Situ Conservation of Plant Diversity in the World’s Botanic Gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.P. Biological and Cultural Diversity in the Context of Botanic Garden Conservation Strategies. Plant Divers. 2017, 39, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.B.; Buzatto, C.R.; Farias-Singer, R.; Machado-Neto, N.B.; Custodio, C.C.; Prendergast, K. You Don’t Have to Kill the Orchids—Good Practices in Orchid Research. Iheringia Série Botânica 2024, 79, e20241356. [Google Scholar] [CrossRef]
- Masters, S.; Bogarín, D.; Viruel, J.; van Vugt, R.; van Andel, T.; de Boer, H.J.; Gravendeel, B. Horticultural Hybrid Development of Edible Terrestrial Orchids for Verifiable Sustainable Trade. Front. Sustain. Food Syst. 2025, 9, 1526533. [Google Scholar] [CrossRef]
- Błaszak, M.; Rybska, E.; Tsivitanidou, O.; Constantinou, C.P. Botanical Gardens for Productive Interplay between Emotions and Cognition. Sustainability 2019, 11, 7160. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Recent developments in it the study of orchid mycorrhiza. Plant Soil 2002, 224, 149–163. [Google Scholar] [CrossRef]
- Abadie, J.C.; Puttsepp, U.; Gebauer, G.; Faccio, A.; Bonfanate, P.; Selosse, M.A. Cephalanthera longifolia is mixotrophic: A comparative study between green and non-photosynthetic individuals. Can. J. Bot. 2006, 84, 1462–1477. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Trophic relationships in orchid mycorrhiza-diversity and implications for conservation. Lankesteriana 2007, 7, 334–341. [Google Scholar] [CrossRef]
- Jersakova, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and Evolution of Deceptive Pollination in Orchids. Biol. Rev. 2006, 81, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, W.; Guo, J. How Can an Urban Botanical Garden in a Densely Built-Up Landscape Develop Sustainably with Urban Renewal?—The Case of Shanghai Botanical Garden. J. Zool. Bot. Gard. 2024, 5, 719–732. [Google Scholar] [CrossRef]
- Ching, G.H.; Mahmood, N. The User’s Perceptions of Perdana Botanical Garden in Kuala Lumpur. J. Des. Built Environ. 2016, 16. [Google Scholar] [CrossRef]
- Patzelt, A.; Anderson, A. Planning and Implementing Botanic Garden Design Projects. In From Idea to Realisation—BGCI’s Manual on Planning, Developing and Managing Botanic Gardens; BGCI: Richmond, UK, 2016; pp. 3–26. [Google Scholar][Green Version]
- Villagra-Islas, P. Newer Plant Displays in Botanical Gardens: The Role of Design in Environmental Interpretation. Landsc. Res. 2011, 36, 573–597. [Google Scholar] [CrossRef]
- Oudolf, P.; Kingsbury, N. Planting: A New Perspective; Timber Press: Portland, OR, USA, 2016. [Google Scholar][Green Version]
- Smardon, R.C. Perception and Aesthetics of the Urban Environment: Review of the Role of Vegetation. Landsc. Urban Plan. 1988, 15, 85–106. [Google Scholar] [CrossRef]
- Lienert, J. Habitat Fragmentation Effects on Fitness of Plant Populations—A Review. J. Nat. Conserv. 2004, 12, 53–72. [Google Scholar] [CrossRef]
- Seaton, P.T.; Pritchard, H.W.; Marks, T.R. Aspects of Orchid Conservation: Seed and Pollen Storage and Their Value in Re-Introduction Projects. Univ. J. Plant Sci. 2015, 3, 72–76. [Google Scholar] [CrossRef]
- Shirsath, P. Orchid Market Report 2025 (Global Edition), 8th ed.; Report ID: CMR395533; Cognitive Market Research: Chicago, IL, USA, 2025. [Google Scholar][Green Version]
- Dulić, J.; Ljubojević, M.; Ognjanov, V.; Barać, G.; Dulić, T. In Vitro Germination and Seedling Development of Two European Orchid Species, Himantoglossum jankae Somlyay, Kreutz & Óvári and Spiranthes spiralis (L.) Chevall. Vitr. Cell. Dev. Biol. Plant 2019, 55, 418–424. [Google Scholar] [CrossRef]
- Ostojić, J.; Ljubojević, M.; Narandžić, T.; Pušić, M. In Vitro Culture Conditions for Asymbiotic Germination and Seedling Development of Anacamptis pyramidalis (L.) Rich. and Gymnadenia conopsea (L.) R. Br. S. Afr. J. Bot. 2022, 150, 829–839. [Google Scholar] [CrossRef]
- Dulić, J.; Ljubojević, M.; Prlainović, I.; Barać, G.; Narandžić, T.; Ognjanov, V. Germination and Protocorm Formation of Ophrys sphegodes Mill—In Vitro Protocol for a Rare Orchid Species. Contemp. Agric. 2018, 67, 196–201. [Google Scholar] [CrossRef]
- Vukajlović, Đ.Đ.; van Veghel, H.; Đurović, S.Đ. Economic Justification for Floriculture Development in Serbia. Econ. Agric. 2017, 64, 469–482. [Google Scholar] [CrossRef]
- Kauth, P.J.; Vendrame, W.A.; Kane, M.E. In Vitro Seed Culture and Seedling Development of Calopogon tuberosus. Plant Cell Tiss. Organ Cult. 2006, 85, 91–102. [Google Scholar] [CrossRef]
- Hinsley, A.; Veríssimo, D.; Roberts, D.L. Heterogeneity in Consumer Preferences for Orchids in International Trade and the Potential for the Use of Market Research Methods to Study Demand for Wildlife. Biol. Conserv. 2015, 190, 80–86. [Google Scholar] [CrossRef]


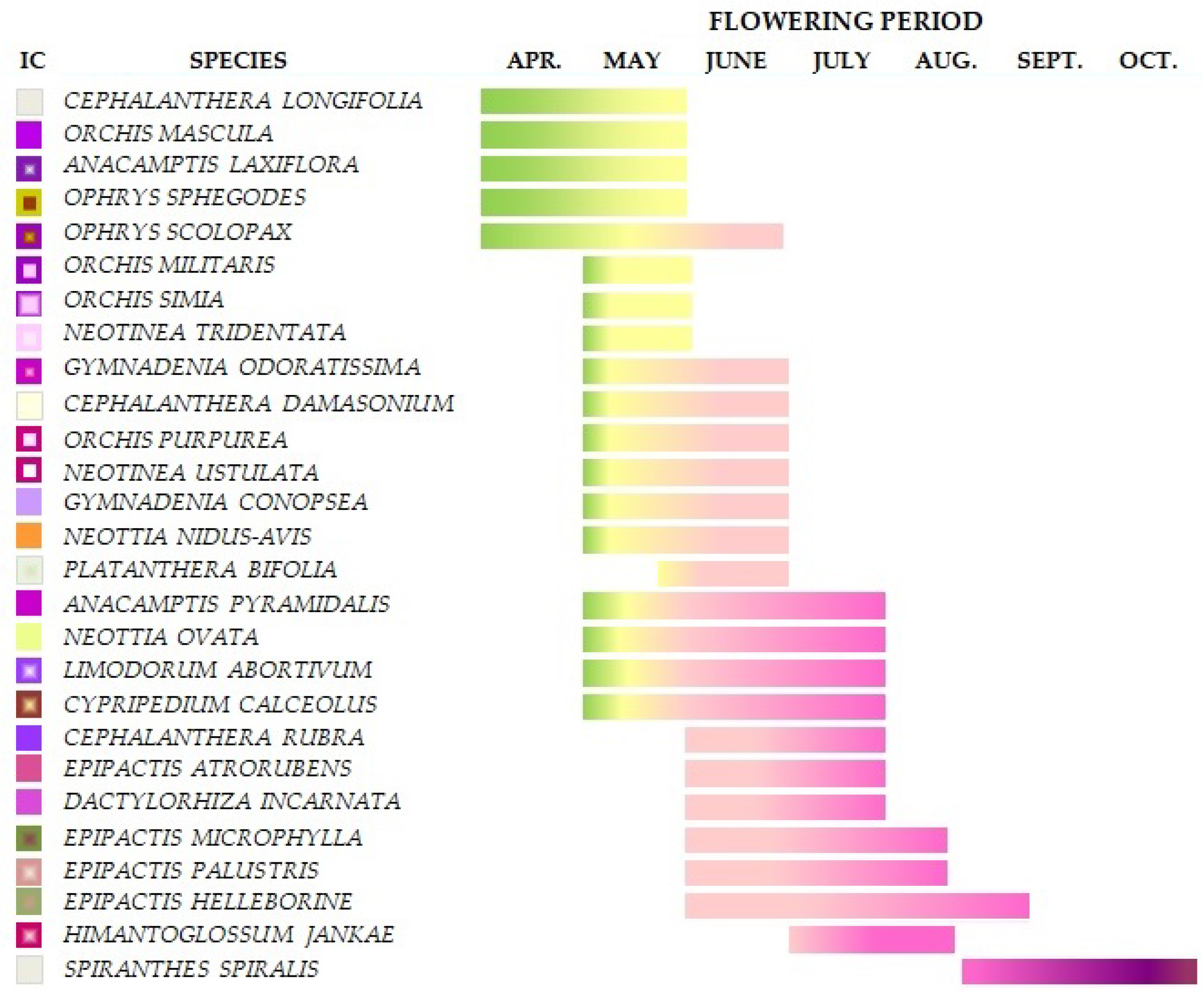




| Species | Vegetation Type | Soil Reaction | References | ||
|---|---|---|---|---|---|
| Light Woodland | Meadow | Wet Meadow | |||
| Epipactis helleborine (L.) Crantz | + | Ac–N | [27,28] | ||
| Neottia nidus-avis (L.) Rich. | + | Ac | [27] | ||
| Platanthera bifolia (L.) Rich. | + | + | Ac–N | [27,29] | |
| Cephalanthera damasonium (Mill.) Druce | + | Ac–N | [29] | ||
| Cephalanthera rubra (L.) Rich. | + | Ac–N | [29] | ||
| Cephalanthera longifolia (L.) Fritsch | + | Ac–SAl | [27] | ||
| Orchis mascula (L.) L. ssp. Signifera (Vest) Soó | + | Ac–SAl | [27] | ||
| Orchis purpurea Huds. | + | + | Ac–N | [27,29] | |
| Orchis simia Lam. | + | SAc–SAl | [30] | ||
| Limodorum abortivum (L.) Sw. | + | Ac | [27,29] | ||
| Orchis militaris L. | + | + | N–SAl | [27] | |
| Anacamptis laxiflora (Lam.) R.M. Bateman, Pridgeon & M.W. Chase | + | - | [26] | ||
| Epipactis microphylla (Ehrh.) Sw. | + | SAc–SAl | [27,29] | ||
| Epipactis palustris L. (Crantz) | + | SAc–SAl | [27] | ||
| Epipactis atrorubens (Hoffm.) Besser | + | N–Al | [31] | ||
| Cypripedium calcelous L. | + | N–Al | [32] | ||
| Neottia ovata (L.) Bluff & Fingerh. | + | SAc–SAl | [33] | ||
| Anacamptis pyramidalis (L.) Rich. | + | N–SAl | [27] | ||
| Neotinea tridentata (Scop.) R.M. Bateman, Pridgeon & M.W. Chase | + | N–SAl | [27] | ||
| Neotinea ustulata (L.) R.M. Bateman, Pridgeon & M.W. Chase | + | N–SAl | [27] | ||
| Gymnadenia conopsea (L.) R. Br. | + | N–SAl | [27] | ||
| Gymnadenia odoratissima (L.) Rich. | + | N–SAl | [27] | ||
| Himantoglossum jankae Somlyay, Kreutz & Ovary | + | + | N–SAl | [27] | |
| Spiranthes spiralis (L.) Chevall. | + | N–SAl | [27] | ||
| Dactylorhiza incarnata (L.) Soó | + | SAc–Al | [34] | ||
| Ophrys sphegodes Mill. | + | N–SAl | [27] | ||
| Ophrys scolopax Cav. | + | N–SAl | [27] | ||
| Zone | Trees | Shrubs | Low Vegetation, Climbers, and Ground Cover Plants | Orchid Species |
|---|---|---|---|---|
| Main Entry Zone | Tilia cordata Mill. | Ruscus aculeatus L. Aucuba japonica Thunb. Hydrangea arborescens L. ‘Annabelle’ | Lonicera caprifolium L. | Cephalanthera rubra (L.) Rcih. Cephalanthera longifolia (L.) Fritsch Orchis mascula (L.) L. ssp. signifera (Vest) Soó Limodorum abortivum (L.) Sw. Platanthera bifolia (L.) Rich. Orchis purpurea Huds. |
| Buffer Zone | Carpinus betulus L. ‘Fastigiata’ | Hydrangea paniculata Siebold Ruscus aculeatus L. Cornus alba L. ‘Elegantissima’ | Pteridium aquilinum (L.) Kuhn Veronica chamaedrys L. | Anacamptis laxiflora (Lam.) R.M. Bateman, Pridgeon & M.W. Chase Epipactis microphylla (Ehrh) Sw. Epipactis atrorubens (Hoffm.) Besser Neottia ovata (L.) R. Br. Bluff & Fingerh |
| Orchid Meadow Garden | / | / | / | Ophrys sphegodes Mill. Ophrys scolopax Cav. Gymnadenia conopsea (L.) R. Br. Anacamptis pyramidalis (L.) Rich. Neotinea tridentata (Scop.) R.M. Bateman, Pridgeon & M.W. Chase Neotinea ustulata (L.) R.M. Bateman, Pridgeon & M.W. Chase Gymnadenia odoratissima (L.) Rich. Spiranthes spiralis (L.) Chevall. Platanthera bifolia (L.) Rich. Orchis purpurea Huds. Orchis militaris L. Himantoglossum jankae Somlyay, Kreutz & Ovary Cephalanthera longifolia (L.) Fritsch. Orchis mascula (L.) L. ssp. signifera (Vest) Soó Epipactis helleborine (L.) Crantz Epipactis microphyilla (Ehrh) Sw. |
| Orchid Wetland Garden | Pinus nigra L. Picea glauca (Moench) Voss ‘Rainbow’s End’ | Cornus alba L. ‘Elegantissima’ Pinus mugo Turra Ligustrum vulgare L. Hibiscus syriacus L. | Carex riparia Curtis Equisetum arvense L. Lysimachia nummularia L. Calamagrostis epigejos (L.) Roth | Dactylorhiza incarnata (L.) Soó Epipactis palustris L. (Crantz) Anacamptis laxiflora (Lam.) R.M. Bateman, Pridgeon & M.W. Chase |
| Orchid Shadow Grove | Pinus nigra L. Tilia tomentosa Moench. | Pinus mugo Turra Juniperus communis L. Deutzia scabra Thunb. Aucuba japonica Thunb. Hydrangea arborescens L. ‘Annabelle’ Cornus alba L. ‘Variegata’ Ligustrum vulgare L. | Mahonia aquifolium (Pursh) Nutt. Hosta undulata L.H.Bailey Hosta ventricosa Stearn Pteridium aquilinum (L.) Kuhn Rhododendron impeditum Balf.f. & W.W.Sm. Rhododendron ponticum L. | Orchis mascula (L.) L. ssp. signifera (Vest) Soó Cephalanthera damasonium (Mill.) Druce Cephalanthera rubra (L.) Rich. Orchis simia Lam. Epipactis atrorubens (Hoffm.) Besser |
| Research and Learning Hub | Chamaecyparis lawsoniana (A. Murray bis) Parl. ‘Ellwood’s Empire’ | Pinus mugo Turra Ruscus aculeatus L. Hibiscus syriacus L. | Pteridium aquilinum (L.) Kuhn Veronica chamaedrys L. | Epipactis helleborine (L.) Crantz Epipactis atrorubens (Hoffm.) Besser Orchis simia Lam. Limodorum abortivum (L.) Sw. Platanthera bifolia (L.) Rich. Orchis purpurea Huds. Orchis militaris L. |
| Orchid Display Garden | Chamaecyparis lawsoniana (A.Murray bis) Parl. ‘Ellwood’s Empire’ Picea glauca (Moench) Voss ‘Rainbow’s End’ Cornus florida L. Prunus fruticosa Pall. ‘Globosa’ Robinia hispida L. | Juniperus communis L. Deutzia scabra Thunb. | Salvia pratensis L. Festuca sulcata L. Lysimachia nummularia L. Aegopodium podagraria L. Lavandula angustifolia Mill. | Ophrys sphegodes Mill. Ophrys scolopax Cav. Gymnadenia conopsea (L.) R. Br. Neotinea ustulata (L.) R.M. Bateman, Pridgeon & M.W. Chase Anacamptis pyramidalis (L.) Rich. Spiranthes spiralis (L.) Chevall. Platanthera bifolia (L.) Rich. Himantoglossum jankae Somlyay, Kreutz & Ovary Orchis purpurea Huds. Orchis militaris L. Epipactis helleborine (L.) Crantz |
| Garden Market Pavilion | Pinus nigra L. Prunus cerasifera Ehrh. ‘Pissardii Nigra’ | Juniperus communis L. Ligustrum vulgare L. | Lysimachia nummularia L. Potentilla cinerea Chaix ex Vill. | Anacamptis pyramidalis (L.) Rich. Neotinea ustulata (L.) R.M. Bateman, Pridgeon & M.W. Chase Spiranthes spiralis (L.) Chevall |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojić, J.; Narandžić, T.; Grubač, M.; Pavlović, L.; Ljubojević, M. Establishment of the First Orchidarium in Serbia: Strategy for Sustainable Management of Native Orchid Genetic Resources. J. Zool. Bot. Gard. 2025, 6, 37. https://doi.org/10.3390/jzbg6030037
Ostojić J, Narandžić T, Grubač M, Pavlović L, Ljubojević M. Establishment of the First Orchidarium in Serbia: Strategy for Sustainable Management of Native Orchid Genetic Resources. Journal of Zoological and Botanical Gardens. 2025; 6(3):37. https://doi.org/10.3390/jzbg6030037
Chicago/Turabian StyleOstojić, Jovana, Tijana Narandžić, Milica Grubač, Lazar Pavlović, and Mirjana Ljubojević. 2025. "Establishment of the First Orchidarium in Serbia: Strategy for Sustainable Management of Native Orchid Genetic Resources" Journal of Zoological and Botanical Gardens 6, no. 3: 37. https://doi.org/10.3390/jzbg6030037
APA StyleOstojić, J., Narandžić, T., Grubač, M., Pavlović, L., & Ljubojević, M. (2025). Establishment of the First Orchidarium in Serbia: Strategy for Sustainable Management of Native Orchid Genetic Resources. Journal of Zoological and Botanical Gardens, 6(3), 37. https://doi.org/10.3390/jzbg6030037









