Long-Lasting Bisexual Lures for Assessing Moth Biodiversity and Monitoring Alien Species in Zoos and Botanical Gardens: Case Study in Zoo of Debrecen (NE Hungary)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Tests
2.2. Traps
2.3. Baits
2.4. Data Analysis
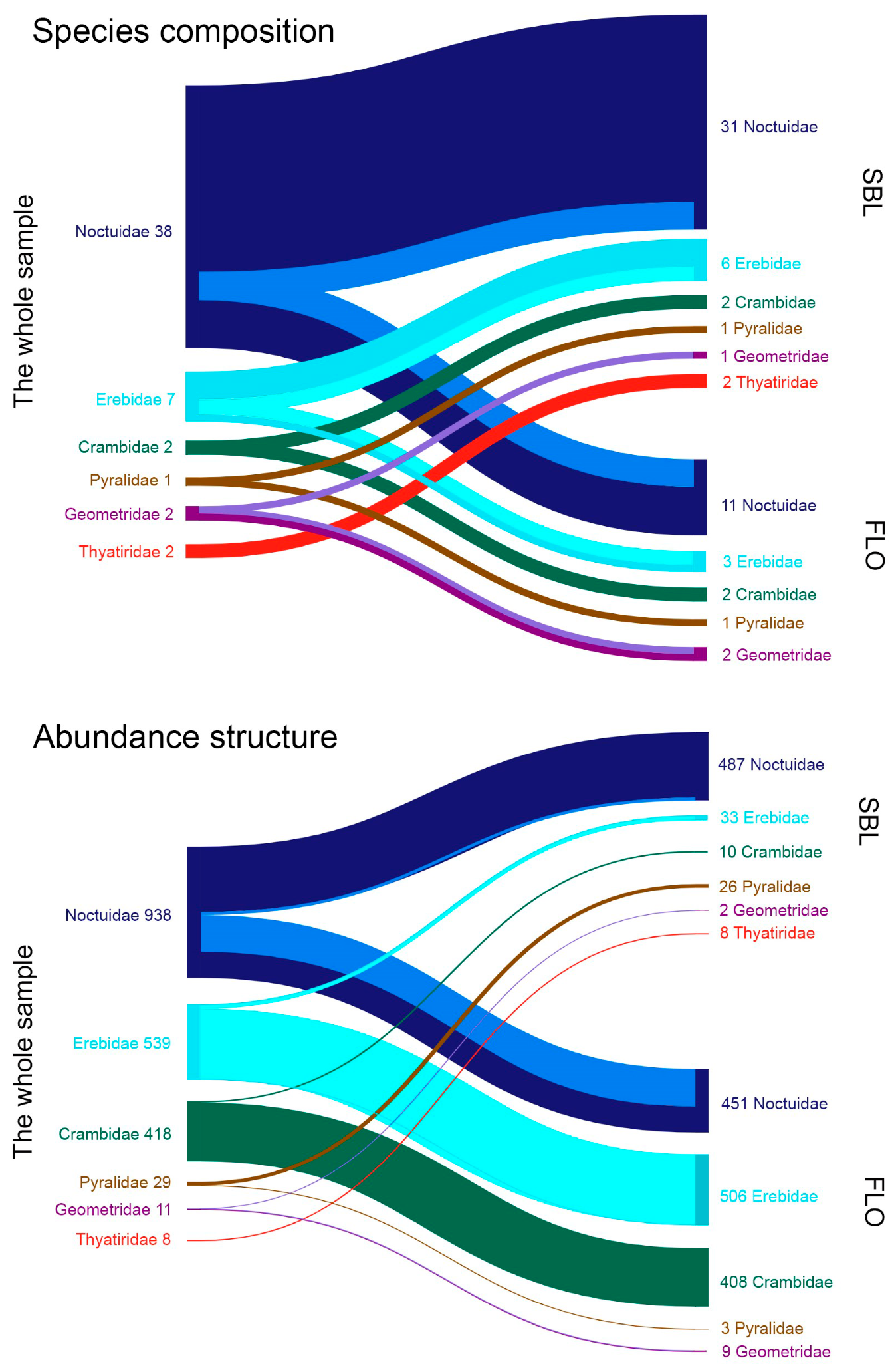
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lintott, P.R.; Bunnefeld, N.; Fuentes-Montemayor, E.; Minderman, J.; Blackmore, L.M.; Goulson, D.; Park, K.J. Moth species richness, abundance and diversity in fragmented urban woodlands: Implications for conservation and management strategies. Biodivers. Conserv. 2014, 23, 2875–2901. [Google Scholar] [CrossRef]
- Ellis, E.E.; Wilkinson, T.L. Moth assemblages within urban domestic gardens respond positively to habitat complexity, but only at a scale that extends beyond the garden boundary. Urban Ecosyst. 2020, 24, 469–479. [Google Scholar] [CrossRef]
- Paul, M. Impact of urbanization on moth (Insecta: Lepidoptera: Heterocera) diversity across different urban landscapes of Delhi, India. Acta Ecol. Sin. 2021, 41, 204–209. [Google Scholar] [CrossRef]
- Szanyi, S.; Molnár, A.; Szanyi, K.; Tóth, M.; Jósvai, J.; Varga, Z.; Nagy, A. Traps with synthetic, bisexual generic attractants as a new method supplementing light traps for assessing composition and diversity of Macroheterocera assemblages. Sci. Rep. 2024, 14, 20212. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Jacobson, M. Phenylacetaldehyde attracts moths to bladder flower and to blacklight traps. Environ. Entomol. 1979, 8, 444–447. [Google Scholar] [CrossRef]
- Landolt, P.J. New chemical attractants for trapping Lacanobia subjuncta, Mamestra configurata, and Xestia c-nigrum (Lepidoptera: Noctuidae). J. Econ. Entomol. 2000, 93, 101–106. [Google Scholar] [CrossRef]
- Landolt, P.J.; Alfaro, J.F. Trapping Lacanobia subjuncta, Xestia c-nigrum and Mamestra configurata (Lepidoptera: Noctuidae) with acetic acid and 3-methyl-1-butanol in controlled relesase dispensers. Environ. Entomol. 2001, 30, 656–662. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Dorogi, B.; Gulyás, A.; Nagy, P.; Rozgonyi, Z. Male and female noctuid moths attracted to synthetic lures in Europe. J. Chem. Ecol. 2010, 36, 592–598. [Google Scholar] [CrossRef]
- Nagy, A.; Szarukán, I.; Gém, F.; Nyitrai, R.; Füsti-Molnár, B.; Némerth, A.; Kozák, L.; Molnár, A.; Katona, K.; Szanyi, S.; et al. Preliminary data on the effect of semi-synthetic baits for Noctuidae (Lepidoptera) on the non-target Lepidoptera species. Acta Agrar. Debreceniensis 2015, 66, 71–80. [Google Scholar] [CrossRef]
- Szanyi, S.; Nagy, A.; Molnár, A.; Katona, K.; Tóth, M.; Varga, Z. Night-active Macroheterocera species in traps with synthetic attractants in the Velyka Dobron’ Game Reserve (Ukraine, Transcarpathia). Acta Zool. Acad. Sci. Hung. 2017, 63, 97–114. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Nagy, A.; Gém, F.; Nyitrai, R.; Kecskés, Z.; Krakkó, L.; Jósvai, J.K.; Bélai, I. Félszintetikus “biszex” csalétkek kártevő rovarok nőstényeinek és hímjeinek fogására [Semi-synthetic “bisex” baits for catching of males and females of pest species]. Növényvédelem 2015, 51, 197–205. [Google Scholar]
- Szanyi, S.; Molnár, A.; Kozák, L.; Szalárdi, T.; Varga, Z.; Tóth, M.; Nagy, A. Nyírségi Macroheterocera együttesek vizsgálata illatanyagcsapdák alkalmazásával—[Study on the Macroheterocera assemblages of the Nyírség (Northeast Hungary) using volatile traps—In Hungarian]. Erdészettudományi Közlemények 2019, 9, 51–68. [Google Scholar] [CrossRef]
- Szanyi, S.; Szarukán, I.; Nagy, A.; Jósvai, J.; Imrei, Z.; Varga, Z.; Tóth, M. Comparing performance of synthetic sex attractants and a semisynthetic bisexual lure in Orthosia and Conistra species (Lepidoptera: Noctuidae). Acta Phytopathol. Entomol. Hung. 2020, 55, 115–122. [Google Scholar] [CrossRef]
- Szanyi, S.; Varga, Z.; Nagy, A.; Jósvai, J.K.; Imrei, Z.; Tóth, M. Bisexual lures and their comparison with synthetic sex attractants for trapping Orthosia species (Lepidoptera: Noctuidae). J. Appl. Entomol. 2022, 146, 1109–1115. [Google Scholar] [CrossRef]
- Szalárdi, T.; Szanyi, S.; Szarukán, I.; Tóth, M.; Nagy, A. Semiochemical baited traps of lepidopteran pests of economic importance can deliver reliable data also on wide range of non-target species: Case study in the Hajdúság Region of East Pannonian Lowland (East Hungary). Biodivers. Data J. 2021, 9, e72305. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.; Nagy, A.; Szarukán, I.; Ary, K.; Cserenyec, A.; Fenyődi, B.; Gombás, D.; Lajkó, T.; Merva, L.; Szabó, J.; et al. One Decade’s Research Efforts in Hungary to Develop a Bisexual Lure for the Cotton Bollworm Helicoverpa armigera Hübner. Acta Phytopathol. Entomol. Hung. 2020, 55, 53–62. [Google Scholar] [CrossRef]
- Chowdary, L.R.; Kumar, G.V.S.; Bharathi, S.; Sarada, O.; Nagaraju, Y.; Chandrashekara, K.M.; Harish, G.N. Off-season survival and life history of beet armyworm, Spodoptera exigua (Hubner) on various host plants. Sci. Rep. 2024, 14, 13721. [Google Scholar] [CrossRef]
- Molnár, B.P.; Kárpáti, Z.; Nagy, A.; Szarukán, I.; Csabai, J.; Koczor, S.; Tóth, M. Development of a Female-Targeted Lure for the Box Tree Moth Cydalima perspectalis (Lepidoptera: Crambidae): A Preliminary Report. J. Chem. Ecol. 2019, 45, 657–666. [Google Scholar] [CrossRef]
- Nagy, A.; Szarukán, I.; Csabai, J.; Molnár, A.; Molnár, B.P.; Kárpáti, Z.; Szanyi, S.; Tóth, M. Distribution of the box tree moth (Cydalima perspectalis Walker 1859) in the north-eastern part of the Carpathian Basin with a new Ukrainian record and Hungarian data. EPPO Bull. 2017, 47, 279–282. [Google Scholar] [CrossRef]
- Tóth, M.; Répási, V.; Szocs, G. Chemical attractants for females of pest Pyralids and Phycitids (Lepidoptera: Pyralidae, Phycitidae). Acta Phytopathol. Entomol. Hung. 2002, 37, 375–384. [Google Scholar] [CrossRef]
- Varga, Z. Magyarország Nagylepkéi—Macrolepidoptera of Hungary; Heterocera Press: Budapest, Hungary, 2012; p. 354. [Google Scholar]
- Lafontaine, D.; Schmidt, C. Annotated check list of the Noctuoidea (Insecta, Lepidoptera) of North America north of Mexico. ZooKeys 2010, 40, 1–239. [Google Scholar] [CrossRef]
- Zahiri, R.; Holloway, J.D.; Kitching, I.J.; Lafontaine, J.D.; Mutanen, M.; Wahlberg, N. Molecular phylogenetics of Erebidae (Lepidoptera, Noctuoidea). Syst. Entomol. 2011, 37, 102–124. [Google Scholar] [CrossRef]
- Mészáros, Z.; Szabóky, C. A Magyarországi Molylepkék Gyakorlati Albuma; Balázs, K., Ed.; Agroinform: Budapest, Hungary, 2005; pp. 1–178. [Google Scholar]
- Mészáros, Z.; Szabóky, C. A Magyarországi Nagylepkék Gyakorlati Albuma; Szalkay József Magyar Lepkészeti Egyesület: Budapest, Hungary, 2012; p. 185. [Google Scholar]
- Zúbrik, M.; Kunca, A.; Csóka, G. Insects and Diseases Damaging Trees and Shrubs of Europe; Zúbrik, M., Kunca, A., Csóka, G.N.A.P., Eds.; NAP: Ann Arbor, MI, USA, 2013; 535p, Available online: www.napeditions.com (accessed on 12 November 2024).
- Varga, Z.; Ronkay, L.; Bálint, Z.; László, M.G.; Peregovits, L. Checklist of the Fauna of Hungary; Macrolepidoptera; Hungarian Natural History Museum: Budapest, Hungary, 2004; Volume 3, p. 106. [Google Scholar]
- Varga, Z. Zoogeographic division of Hungary based on components of the Macrolepidoptera-fauna (in Hung. with German summary). Folia Entomol. Hung. 1964, 17, 119–167. [Google Scholar]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Di Mauro, D.; Dietz, T.; Rockwood, L. Determining the effect of urbanization on generalist butterfly species diversity in butterfly gardens. Urban Ecosyst. 2007, 10, 427–439. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Rickman, J.K.; Connor, E.F. The effect of urbanization on the quality of remnant habitats for leaf-mining Lepidoptera on Quercus agrifolia. Ecography 2003, 26, 777–787. [Google Scholar] [CrossRef]
- Merckx, T.; Marini, L.; Feber, R.E.; Macdonald, D.W. Hedgerow trees and extended-width field margins enhance macro-moth diversity: Implications for management. J. Appl. Ecol. 2012, 49, 1396–1404. [Google Scholar] [CrossRef]
- Arnóczkyné Jakab, D.A.; Tóth, M.; Szarukán, I.; Szanyi, S.; Józan, Z.; Sárospataki, M.; Nagy, A. Long-term changes in the composition and distribution of the Hungarian bumble bee fauna (Hymenoptera, Apidae, Bombus). J. Hymenopt. Res. 2023, 96, 207–237. [Google Scholar] [CrossRef]
- Nagy, A.; Katona, P.; Molnár, A.; Rádai, Z.; Tóth, M.; Szanyi, K.; Szanyi, S. Wide range of brachyceran fly taxa attracted to synthetic and Semi-Synthetic generic noctuid lures and the description of new attractants for sciomyzidae and heleomyzidae families. Insects 2023, 14, 705. [Google Scholar] [CrossRef]
- Nagy, A.; Ősz, A.; Tóth, M.; Rácz, I.A.; Kovács, S.; Szanyi, S. Nontarget catches of traps with chemical lures may refer to the flower-visitation, probable pollination, and feeding of bush crickets (Ensifera: Tettigoniidae). Ecol. Evol. 2023, 13, e10249. [Google Scholar] [CrossRef] [PubMed]

| Family | Subfamily | Species | FLO | SBL |
|---|---|---|---|---|
| Crambidae | Spilomelinae | Cydalima perspectalis (Walker, 1859) | + | + |
| Crambidae | Patania ruralis (Scopoli, 1763) | + | + | |
| Pyralidae | Phycitinae | Plodia interpunctella (Hübner, [1813]) | + | + |
| Thyatiridae | Thyatirinae | Habrosyne pyritoides (Hufnagel, 1766) | + | |
| Thyatiridae | Thyatirinae | Tethea or ([Denis & Schiffermüller], 1775) | + | |
| Geometridae | Ennominae | Ligdia adustata ([Denis & Schiffermüller], 1775) | + | + |
| Geometridae | Sterrhinae | Idaea aversata (Linnaeus, 1758) | + | |
| Erebidae | Hypeninae | Hypena rostralis (Linnaeus, 1758) | + | |
| Erebidae | Scoliopteryginae | Scoliopteryx libatrix (Linnaeus, 1758) | + | |
| Erebidae | Catocalinae | Catocala promissa ([Denis & Schiffermüller], 1775) | + | + |
| Erebidae | Catocalinae | Catocala nupta (Linnaeus, 1767) | + | |
| Erebidae | Catocalinae | Lygephila pastinum (Treitschke, 1826) | + | |
| Erebidae | Ctenuchinae | Amata phegea (Linnaeus, 1758) | + | + |
| Erebidae | Ctenuchinae | Dysauxes ancilla (Linnaeus, 1767) | + | |
| Noctuidae | Acronictinae | Acronicta rumicis (Linnaeus, 1758) | + | |
| Noctuidae | Amphipyrinae | Amphipyra pyramidea (Linnaeus, 1758) | + | |
| Noctuidae | Plusiinae | Autographa gamma (Linnaeus, 1758) | + | |
| Noctuidae | Plusiinae | Trichoplusia ni (Hübner, [1803]) | + | |
| Noctuidae | Plusiinae | Macdunnoughia confusa Stephens, 1850 | + | + |
| Noctuidae | Plusiinae | Abrostola triplasia (Linnaeus, 1758) | + | + |
| Noctuidae | Plusiinae | Abrostola tripartita (Hufnagel, 1766) | + | |
| Noctuidae | Plusiinae | Diachrysia chrysitis (Linnaeus, 1758) | + | |
| Noctuidae | Plusiinae | Diachrysia stenochrysis (Warren, 1913) | + | |
| Noctuidae | Heliothinae | Helicoverpa armigera (Hübner, [1808]) | + | |
| Noctuidae | Hadeninae | Lacanobia oleracea (Linnaeus, 1758) | + | |
| Noctuidae | Hadeninae | Mamestra brassicae (Linnaeus, 1758) | + | |
| Noctuidae | Hadeninae | Hadena bicruris (Hufnagel, 1766) | + | |
| Noctuidae | Hadeninae | Mythimna turca (Linnaeus, 1761) | + | + |
| Noctuidae | Hadeninae | Mythimna vitellina (Hübner, [1808]) | + | |
| Noctuidae | Hadeninae | Mythimna albipuncta ([Denis & Schiffermüller], 1775) | + | + |
| Noctuidae | Noctuinae | Noctua pronuba (Linnaeus, 1758) | + | |
| Noctuidae | Noctuinae | Noctua fimbriata (Schreber, 1759) | + | |
| Noctuidae | Noctuinae | Noctua orbona (Hufnagel, 1766) | + | |
| Noctuidae | Noctuinae | Noctua janthe (Borkhausen, 1792) | + | |
| Noctuidae | Noctuinae | Agrotis segetum ([Denis & Schiffermüller], 1775) | + | |
| Noctuidae | Noctuinae | Agrotis exclamationis (Linnaeus, 1758) | + | |
| Noctuidae | Noctuinae | Agrotis bigramma (Esper, 1790) | + | |
| Noctuidae | Xyleninae | Charanyca trigrammica (Hufnagel, 1766) | + | |
| Noctuidae | Xyleninae | Dypterygia scabriuscula (Linnaeus, 1758) | + | |
| Noctuidae | Xyleninae | Trachea atriplicis (Linnaeus, 1758) | + | |
| Noctuidae | Xyleninae | Athetis gluteosa (Treitschke, 1835) | + | |
| Noctuidae | Xyleninae | Oligia latruncula ([Denis & Schiffermüller], 1775) | + | |
| Noctuidae | Xyleninae | Oligia strigilis (Linnaeus, 1758) | + | |
| Noctuidae | Xyleninae | Phlogophora meticulosa (Linnaeus, 1758) | + | |
| Noctuidae | Xyleninae | Cosmia affinis (Linnaeus, 1767) | + | |
| Noctuidae | Xyleninae | Thalpophila matura (Hufnagel, 1766) | + | |
| Noctuidae | Xyleninae | Apterogenum ypsillon ([Denis & Schiffermüller], 1775) | + | |
| Noctuidae | Xyleninae | Agrochola litura (Linnaeus, 1761) | + | |
| Noctuidae | Xyleninae | Tiliacea aurago ([Denis & Schiffermüller], 1775) | + | |
| Noctuidae | Xyleninae | Conistra vaccinii (Linnaeus, 1761) | + | |
| Noctuidae | Xyleninae | Conistra erythrocephala ([Denis & Schiffermüller], 1775) | + | |
| Noctuidae | Noctuinae | Xestia xanthographa ([Denis & Schiffermüller], 1775) | + |
| Species | Individuals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBL | FLO | Sum | SBL | FLO | Sum | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Invasives | 1 | 2.3 | 3 | 15.8 | 3 | 5.8 | 1 | 0.2 | 155 | 11.3 | 156 | 8.0 |
| Pests | 2 | 4.7 | 9 | 47.4 | 9 | 17.3 | 88 | 15.5 | 144 | 10.5 | 232 | 11.9 |
| S | 43 | 19 | 52 | 566 | 1377 | 1943 | ||||||
| Life forms (macro-moths: 49 species, 1496 individuals) | ||||||||||||
| Generalist | 13 | 32.5 | 2 | 12.5 | 13 | 26.5 | 247 | 46.6 | 87 | 9.0 | 334 | 22.3 |
| Migratory | 0 | 0 | 3 | 18.75 | 3 | 6.1 | 0 | 0.0 | 150 | 15.5 | 150 | 10.0 |
| Steppic | 3 | 7.5 | 1 | 6.25 | 4 | 8.2 | 5 | 0.9 | 7 | 0.7 | 12 | 0.8 |
| Mesophilous | 3 | 7.5 | 2 | 12.5 | 4 | 8.2 | 7 | 1.3 | 14 | 1.4 | 21 | 1.4 |
| Altoherbosa | 1 | 2.5 | 4 | 25 | 4 | 8.2 | 9 | 1.7 | 200 | 20.7 | 209 | 14.0 |
| Moor–marsh | 2 | 5 | 0 | 0 | 2 | 4.1 | 2 | 0.4 | 0 | 0.0 | 2 | 0.1 |
| Silvicolous | 11 | 27.5 | 3 | 18.75 | 12 | 24.5 | 236 | 44.5 | 10 | 1.0 | 246 | 16.4 |
| Birch–alder | 1 | 2.5 | 0 | 0 | 1 | 2.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.1 |
| Oakwood | 1 | 2.5 | 0 | 0 | 1 | 2.0 | 3 | 0.6 | 0 | 0.0 | 3 | 0.2 |
| Willow–poplar | 4 | 10 | 0 | 0 | 4 | 8.2 | 13 | 2.5 | 0 | 0.0 | 13 | 0.9 |
| Lichenophagous | 1 | 2.5 | 1 | 6.25 | 1 | 2.0 | 7 | 1.3 | 498 | 51.6 | 505 | 33.8 |
| Sum | 40 | 16 | 49 | 530 | 966 | 1496 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szanyi, S.; Kovács, C.L.; Tóth, M.; Lincz, O.; Nagy, G.S.; Varga, Z.; Nagy, A. Long-Lasting Bisexual Lures for Assessing Moth Biodiversity and Monitoring Alien Species in Zoos and Botanical Gardens: Case Study in Zoo of Debrecen (NE Hungary). J. Zool. Bot. Gard. 2025, 6, 11. https://doi.org/10.3390/jzbg6010011
Szanyi S, Kovács CL, Tóth M, Lincz O, Nagy GS, Varga Z, Nagy A. Long-Lasting Bisexual Lures for Assessing Moth Biodiversity and Monitoring Alien Species in Zoos and Botanical Gardens: Case Study in Zoo of Debrecen (NE Hungary). Journal of Zoological and Botanical Gardens. 2025; 6(1):11. https://doi.org/10.3390/jzbg6010011
Chicago/Turabian StyleSzanyi, Szabolcs, Csenge Lelle Kovács, Miklós Tóth, Ottó Lincz, Gergely Sándor Nagy, Zoltán Varga, and Antal Nagy. 2025. "Long-Lasting Bisexual Lures for Assessing Moth Biodiversity and Monitoring Alien Species in Zoos and Botanical Gardens: Case Study in Zoo of Debrecen (NE Hungary)" Journal of Zoological and Botanical Gardens 6, no. 1: 11. https://doi.org/10.3390/jzbg6010011
APA StyleSzanyi, S., Kovács, C. L., Tóth, M., Lincz, O., Nagy, G. S., Varga, Z., & Nagy, A. (2025). Long-Lasting Bisexual Lures for Assessing Moth Biodiversity and Monitoring Alien Species in Zoos and Botanical Gardens: Case Study in Zoo of Debrecen (NE Hungary). Journal of Zoological and Botanical Gardens, 6(1), 11. https://doi.org/10.3390/jzbg6010011






