Abstract
Tropical forest hornbills display complex social and reproductive behaviours that make them hard to reliably and frequently breed in ex situ facilities. This study investigated potential behavioural indicators of pair compatibility for two wreathed hornbills (Rhyticeros undulatus) at ARTIS Amsterdam Royal Zoo. We focused on behaviours linked to reproductive investment, such as time spent in proximity, vocalisation, and courtship display. Through systematic observations using ZooMonitor, we recorded behavioural and enclosure use data, as well as visitor presence. Key findings revealed that the male hornbill was more active and exploratory than the female. Courtship behaviours were more frequently performed by the male bird and were positively associated with perching at elevated positions. However, this behaviour decreased when the birds were in close proximity or inactive. Visitor presence influenced enclosure use, with the hornbills preferring higher perches during peak visitor times. We conclude that behaviours such as proximity, vocalisations, and courtship displays could potentially serve as indicators of compatibility in hornbill pairs but more data across more birds are needed for validation. Finally, we advocate for enclosure designs that consider hornbills’ natural behaviours and social needs to help ex situ facilities promote an environment conducive to pair bonding.
1. Introduction
Hornbills (Bucerotidae) are highly intelligent, long-lived birds with a complex breeding strategy [1]. Although commonly kept in zoological collections, many hornbill species remain challenging to breed regularly [2,3,4]; in particular, the larger Asian species have limited breeding success but are generally the most threatened species and therefore in greatest need of successful and sustainable ex situ breeding programs [5]. When successful breeding has occurred, there is associated anecdotal evidence that pair compatibility is of paramount importance [2,6]. Hornbill keepers can look for certain behaviours that indicate pair compatibility, but it appears difficult to assess which specific behaviours are directly related to long-term pair bonding that supports successful reproduction.
The literature available at the time of writing suggests several behaviours that could indicate pair compatibility, the most frequently mentioned being courtship feeding. Most hornbill species display a unique method for incubation and the rearing of chicks, with the female sealing herself into the nest cavity with her eggs/chicks. Therefore, she is completely reliant on the male to provide food for her and any chicks [7]. This period of provisioning by the male to the female bird can be up to four months long in species such as the wreathed hornbill (Rhyticeros undulatus) [1]. Given the importance of this behaviour to the female bird’s survival (and that of any offspring), it is not surprising that courtship feeding is mentioned often in the literature as an important indicator of pair compatibility and social bonding [2,6,8].
Other indicators of pair compatibility are also noted; for example, Kozlowski et al. [4] found that vocalisations by male great hornbills (Buceros bicornis) were the most frequently observed courtship behaviours during the pre-breeding season. The authors found that the amount of time great hornbill pairs spent in proximity was greater during years in which successful reproduction occurred. This suggests that the amount of time pairs spend in proximity may help evaluate compatibility and the likelihood of successful reproduction in this (and, potentially, related) species. Calling and displaying are also described by Kemp [8] as amongst the first breeding behaviours to occur. Vocalisations can travel for long distances across the hornbill’s natural habitat [9] and therefore may be used by each bird to gain knowledge as to the location of their partner.
Although literature suggests that most Asian hornbills are monogamous [7], there are several species that are observed to flock together outside of the breeding season, sometimes in groups of over 1000 individuals at communal roosting sites [1,10,11,12,13]. Such flocking activity likely serves a social function by enabling maturing birds to identify and evaluate potential breeding partners or provides a mechanism for already successfully breeding adults to change breeding partner for subsequent nesting seasons. Whilst this concept has yet to be formally tested [7], social choice may be an important element of a hornbill’s life history regarding knowledge and experience of potential mates. This social phenomenon would be difficult to replicate in zoo management regimes, due to the logistics of bringing birds together at the right age for flocking to occur and because populations of hornbill species are relatively small, and current low breeding success has resulted in only a limited number of juvenile birds.
An example of an Asian hornbill of conservation importance found in multiple zoos—38 institutions, 143 individuals as of October 2024 according to Species360 [14]—is the wreathed hornbill. Wreathed hornbills are a large species of hornbill, approximately 75–85 cm tall, with a mass of approximately 3650 g [1], and currently listed as Vulnerable on the IUCN Red List [15]. This hornbill is sexually dimorphic; the male has yellow throat skin with a blue stripe on each side and a dark brown crown, whilst the female has blue throat skin with a darker blue stripe on either sides and a black crown [1]. Depending on geographic range, wild wreathed hornbills breed between January and March, and April and June (but sometimes extended to September if conditions are appropriate) with fledging occurring three to four months after nesting reported [7,10]. Nest holes are high (up to 28 m in height) [10] and the same nest cavity can be used for successive breeding attempts [15].
Wild wreathed hornbills inhabit primary evergreen forests extending into secondary forests [10]. This species is mainly frugivorous, and a substantial part of the diet consists of wild fig species, Ficus spp. [7]. However, they are also known to forage on invertebrates (e.g., insects and crustaceans), small birds, and small reptiles [10,16], that are mainly consumed during the breeding season [1,8,10]. Most foraging occurs in the tree canopy, but birds will occasionally descend to the ground to feed on fallen fruits or to hunt for prey [1]. Although the species is widespread across southeast Asia and does not migrate, they can move large distances in search of fruiting trees and have been observed crossing between islands in search of food [1].
Groot et al. [17] conducted a survey across North American (AZA) and European (EAZA) zoos to identify potential predictors of breeding success for wreathed hornbills. The research showed that two behaviours could be potential indicators of pair bonding: the time a pair spends near each other (close proximity on the same perch) and, pair vocalisations and frequency of courtship display (prior and during the breeding season). Therefore, to contextualise some of these potential behavioural cues, we conducted behavioural observations on a pair of wreathed hornbills at ARTIS Amsterdam Royal Zoo to determine the amount of time dedicated to calling, pair bonding behaviours, and time spent in proximity, to hopefully guide interpretation of wreathed hornbill behaviour for other holders that are attempting to maintain viable breeding pairs. The main aim of this research was to quantify time spent on behaviours that are noted as indicators of pair bonding as well as determine the impact of environmental and enclosure variables on hornbill social behaviour and activity.
Overall, we wished to investigate bird behaviour, enclosure usage, and visitor presence within the aviary. As an individual animal’s activity pattern can influence how they use their enclosure, and likewise enclosure usage will impact on the behaviour of the individual at a given time [18,19,20], it was important that both these elements were recorded. The presence of visitors can also influence hornbill behaviour in zoos [3], and therefore the number of visitors within the hornbill’s enclosure was also counted to enable further evaluation of time–activity patterns and use of the aviary.
Our objective was to collect behavioural data on potentially important socially and reproductively orientated behaviours that may provide inferences on pair compatibility (and thus be indicators of successful future breeding). We predicted that if birds were comfortable in each other’s presence, they would direct positive, affiliative behaviours towards each other and spend more time in proximity to each other rather than being apart (e.g., at different ends of their aviary). We also predicted that vocalisation and courtship behaviour would be more commonly performed when the birds were in closer proximity.
2. Materials and Methods
Ethical approval and permission for behavioural observations was provided by the Research Department and Bird Department at ARTIS Amsterdam Royal Zoo on 1st February 2024 (A. van Dijk and M. de Vries).
2.1. Study Population and Location
A pair of wreathed hornbills was observed at ARTIS Amsterdam Royal Zoo, the Netherlands from 22nd February to 2nd May 2024, excluding all weekends. The birds are housed in the tropical section of the zoo’s birdhouse. The indoor section is 160 m2 with a maximum height of 6 m. The enclosure is densely planted and includes a waterbody flowing through the middle of the enclosure. Visitors walk through the enclosure on one side via a covered walkway. The outdoor section is 31.5 m2, has a maximum height of 5 m, and is also densely planted. The hornbills share their enclosure with 1.1.0 Argus pheasants (Argusianus argus) and 2.2.0 Baer’s pochards (Aythya baeri). Permanent fixtures within the enclosure include five indoor beams (at a height of 4.5 m) on which the hornbills can perch, several types of natural perching on different heights, and two nest boxes, one situated inside and one outside (Figure 1). The indoor enclosure has skylights across the entire length of the enclosure to let in natural sunlight. The birds are serviced daily between approximately 08:00 and 09:00 with one bowl of food—containing a selection of fruit and H16 pellets, with the occasional addition of insects or “pinkie” mice. The hornbills’ diet remained the same throughout. All water bowls were refreshed daily during this husbandry period.
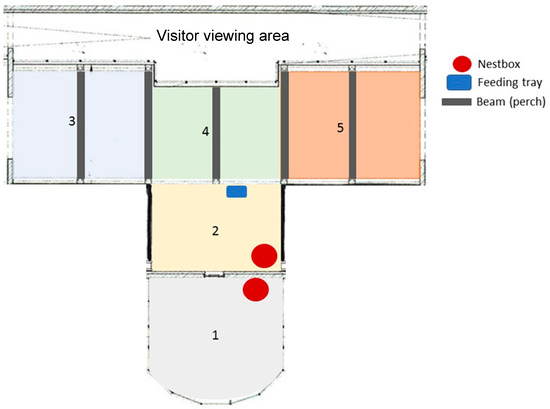
Figure 1.
Schematic overview of the hornbill’s indoor and outdoor enclosure. Visitors would usually pass through the indoor enclosure from right to left (5 to 3). Section 1 is outdoor (in grey), where visitors can walk around the enclosure in a U-shape.
The male wreathed hornbill was hatched on 4th June 2008 and has produced offspring in the past at ARTIS with a previous mate. The female wreathed hornbill was hatched 6th September 1996 and has also produced offspring in the past with a previous mate. The female was transferred to ARTIS on 14th June 2022. After an extensive period of acclimatisation and introduction, the pair has been permanently housed together since 28th September 2022.
2.2. Data Collection
Table 1 provides information on how all observational data were categorised, described, and recorded. Data on behavioural states (i.e., long duration activities) for each individual bird were collected by a single observer (KG) using instantaneous focal sampling [21] with 1 min intervals for a 30 min observation period. Observations took place once per day between 12:45 and 13:30 and, in total, 15 h of data per individual were recorded. Behavioural data were collected using ZooMonitor v.4.1 [22]. Prior observation of the birds and published behavioural recording methods [3,23] were used to determine an appropriate sampling interval (of 1 min). Instantaneous focal sampling provides a reliable method of inferring duration of state behaviours [24] and thus we employed this method to estimate each bird’s time–activity budget.

Table 1.
Description and categorisation of observational data collection.
During the 30 min observation period, each occurrence of specific event behaviours (i.e., short duration actions) were recorded. These behaviours, chosen based upon on how they might reveal the motivational state of each hornbill to be keen to socialise or engage with their conspecific, provide useful actions for others to look out for when attempting to understand pair compatibility in zoo-housed hornbills.
Birds were also observed to determine how they used their enclosure and if they were on the same perch as a partner. The location of each individual within their enclosure was recorded using the heatmap function in ZooMonitor using the same instantaneous focal sampling method with one-minute intervals. Alongside of this, the number of instances of birds perching on the same perch was counted during each 30 min observation period.
Finally, the total visitor count seen during each 30 min observation period was recorded.
During data collection, the observer did not wear ARTIS Zoo uniform so as to minimise any influence of observer presence on hornbill behaviour and aviary use.
2.3. Data Analysis
Data were analysed in RStudio v.2024.09.0 [25] run in R v.4.4.1 [26] and in Minitab v. 22.1 [27]. Using the “basic statistics” function in Minitab and the graph building feature, a box plot of the time spent on state behaviours for each bird was constructed. A combination of parametric and non-parametric analysis was applied to these data. Mann–Whitney tests were used to explore any difference between the sexes for inactive and exploratory behaviours. “Other” was normally distributed so a two-sample t-test was used to determine any difference between the male and female birds.
Event behaviours (display and calls) were normally distributed and so a two-sample t-test was used to compare male and female, whereas for all other event behaviours (aggression, interactions, preening) Mann–Whitney tests were used for investigating any significant difference in performance.
Regression analysis was used to understand potential predictors of calls and reproductive display performed by the hornbills. Due to the small number of records of other behaviours potentially indicative of a desire to breed, only calls and displays were analysed further. Firstly, a Poisson regression using a count of the number of displays and calls that may be predicted by whether birds were on the same perch, whether birds were within one bill length, if the birds were perched high up, and whether visitor number had an impact on displaying was run in RStudio. Non-significant predictors of visitor number and being on the same perch as a partner were removed from the model. Further review of variance inflation factors, calculated using the “car” package in RStudio [28], revealed some likely collinearity, and so the model was checked for overdispersion using the “AER” package [29]. The model was found to be highly overdispersed, and so a negative binomial regression was run instead using the “MASS” package [30]. This model had a lower AIC value and much reduced VIF (all 2.5 or below). The final model run was thus as follows: displays and calls ~ sex + inactivity + within one beak length of partner + perching up high.
To understand any impact of visitor presence on hornbill enclosure usage, Spearman’s rank correlation was run in Minitab using data on the total visitor count per observation and the time the birds were seen at the highest point of their enclosure. Enclosure usage was considered descriptively from the “heatmap” output generated by ZooMonitor [22].
All raw data used in these analyses are available at 10.6084/m9.figshare.28098107.
3. Results
3.1. State Behaviours
Figure 2 illustrates the time spent on state behaviours for the two hornbills during the period of observations. A Mann–Whitney test shows a significant difference in inactivity between the two hornbills (W = 568.00; n = 30; p < 0.001). The same test also reveals a significant difference in exploration (W = 1177; n = 30; p < 0.001). Data for “Other” was normally distributed, and a two-sample t-test reveals a significant difference between the male and female bird (t = 4.36; df = 57; p < 0.001). When hornbills were socialising together within one-bill length, the birds spent an equal amount of time exhibiting this behaviour.
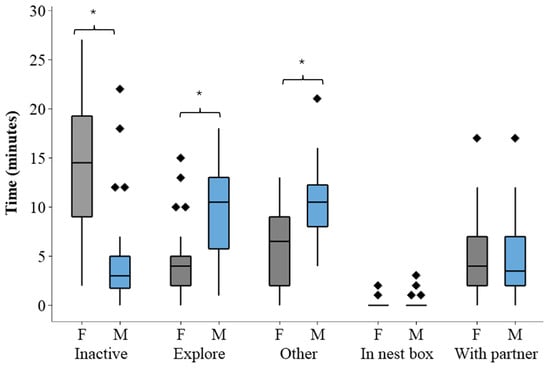
Figure 2.
The time–activity budget for the pair of male and female hornbills from February to May 2024. The box plot shows the median time spent on each behaviour and the interquartile ranges. The male bird was markedly more exploratory than the female and the female bird was more sedentary than the male. Time spent in the nest box during this period was negligible for both individuals. Outlier data points are shown by rhomboid markers. Significant differences are marked with asterisks.
3.2. Event (Courtship) Behaviours
Figure 3 shows the differences in performance of event behaviours during the period of observation. Although displays and calls were the most frequent courtship behaviour displayed by these two birds, there was a significant difference between the male and female (t = 7.11; df = 50; p < 0.001). The male displayed and called more than the female and instigated the majority of allofeeding interactions. Allopreening of the female by the male was initiated 33 times and was never initiated by the female to the male.
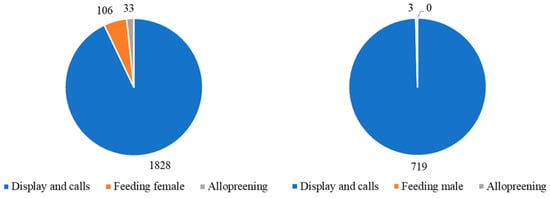
Figure 3.
Total number of occurrences of key event behaviours performed by the male bird (left) and the female bird (right). Displays and calls predominated in both species. Only the male bird performed allopreening and undertook most of the intra-pair feeding.
3.3. Event (Maintenance and Interaction) Behaviours
Aggression between the two hornbills was very low, with only 1 record of intra-pair aggression directed from the female bird to the male and 24 occurrences from the male bird to the female on five observation days (comprising 0.16 of the observation period). Similarly, interactions with the other species in the enclosure were also very low to non-existent with one occurrence of the male hornbill interacting with a non-hornbill species in the enclosure. Although the observed count of preening was higher for the male bird (151) compared to the female bird (87), this difference was not statistically significant (W = 1033; n = 30; p = 0.082). This result may, however, show a general trend that is worthy of future investigation (Figure 4A). As an indicator of social space use (Figure 4B), the maximum number of occurrences that the two hornbills were seen together on the same perch within a 30 min observation period was 25.
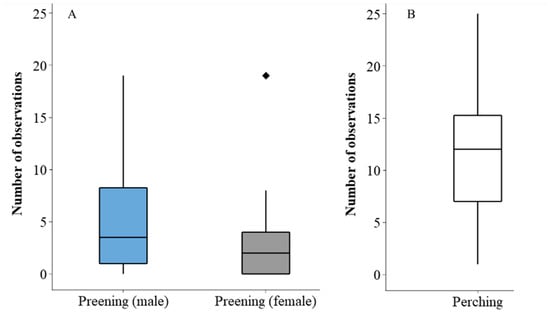
Figure 4.
Examples of event behaviours showing maintenance and social space use. Graph (A) illustrates the median number of observations of preening for the male and female hornbill. Graph (B) shows the median number of times the birds shared the same perch but outside of one beak length. Outliers shown by the rhomboid marker.
There may be some seasonality in performance of state behaviours (social association) and event behaviours (mean counts of displaying and sitting on the same perch), as noted in Figure 5, with increases in display rate as months progress and a decline in time spent together. This supports information presented in Table 2 that shows less courtship display is performed when individuals are closer together, and Figure 5 shows that this behaviour increases in May when individuals were less often seen in close proximity.
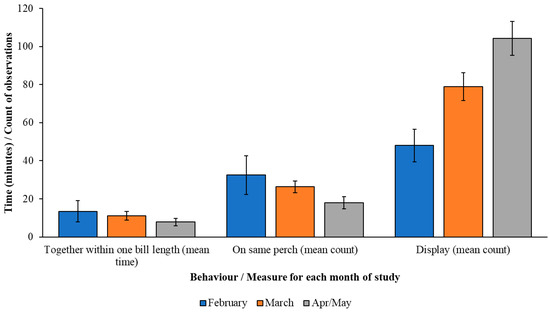
Figure 5.
Mean (+/− standard error) variation (by month) in the amount of time the birds were together within one bill length (mean number of minutes) and the mean count of observations of courtship display/vocalisation and being seen on the same perch.

Table 2.
Model output for a negative binomial regression for significant predictors of hornbill courtship display and courtship vocalisation.
3.4. Behavioural and Environmental Impacts on Courtship Display
The output from a negative binomial regression is displayed in Table 2. The male bird was more likely to display courtship and vocalisation compared to the female bird (p = 0.026), birds displayed more when perching higher up (p = 0.004), and there was significantly less display and vocalisation when the hornbills were perched within one-beak length (p < 0.001) or were inactive (p < 0.001). Figure 6 illustrates the spread of data points for occurrences of calling and displaying compared to where the bird was seen (high up) or what the bird’s state behaviour was (exploration, inactivity, or social association).
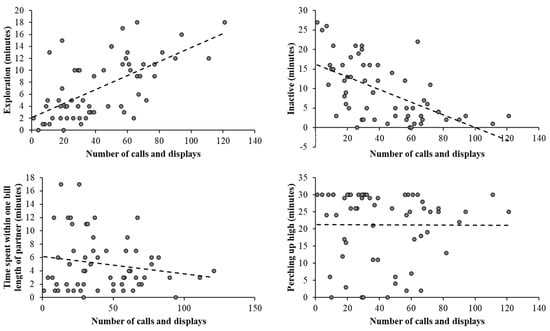
Figure 6.
Output from correlation analysis between exploratory behaviour, time spent inactive, time spent in close proximity, and time spent perching up high against the number of calls and courtship displays observed for the pair of hornbills. The direction of the correlation is shown by the dashed line. As correlation is descriptive and does not include all factors that potentially influence hornbill behaviour and space use, it is important to consider the multifactorial nature of behavioural responses and what further analyses are required.
Although there appears to be a relationship between exploration and display/calling (Figure 6 top left), the small sample size and the nature of these data being overdispersed (for many data points, the variance is greater than the mean) suggest this relationship may not be true and thus requires further exploration across hornbill pairs in other zoos. This is supported by the non-significant result when exploration was included as a potential predictor of courtship in one of the earlier models run (estimate = 0.006; SE = 0.022; Z value = 0.264; p = 0.792).
3.5. Enclosure Usage
Figure 7 shows that the birds spend most of their time in Sections 3 and 4 (indicated by the red areas on the heatmap). It also shows that the birds prefer to perch on the aviary beams within in the indoor section. Section 5 is the least used area within the enclosure, with only a few data points recorded, and this may relate to visitor presence or the quality of the habitat provided in this section. Data points in Section 1 (outdoor) are more scattered than indoor data points, potentially due to the more complex perching options in the outdoor enclosure. There is a strong positive correlation (Figure 8) between the hornbills spending more time in the highest parts of their aviary and increased number of visitors in the enclosure (r = 0.321; n = 60; p = 0.012).
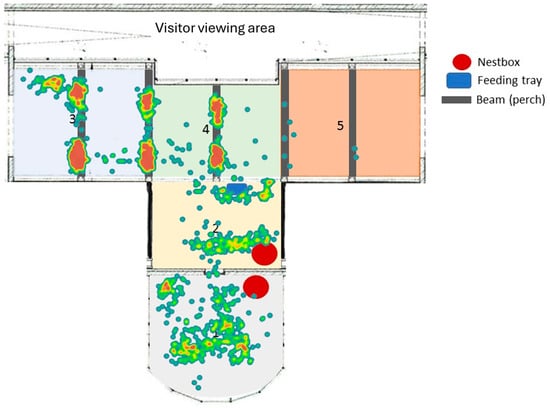
Figure 7.
The heatmap taken from ZooMonitor. Visitors would usually pass through the indoor enclosure from right to left (5 to 3). Areas most frequently used are shown in red; areas less frequently used are shown in green and dark blue, respectively.
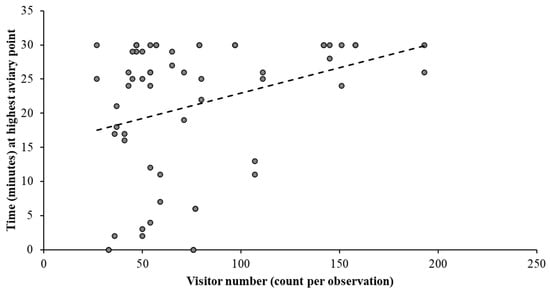
Figure 8.
Correlation between visitor presence and location of the hornbills in their aviary suggesting a potential relationship between increased visitor presence and time spent on highest perching.
4. Discussion
This research identified several differences in the time–activity budget of a male and female wreathed hornbill at ARTIS Amsterdam Royal Zoo. Our results show that the female bird was significantly less active than the male and that she spent less time on exploration compared to the male. Data on the performance of behaviour in the “other” category was also significantly different between the two birds, and this provides useful evidence for further research investigation. We show complex relationships between performance of vocalisation/courtship displays and other behavioural or environmental variables, and these also lend themselves to further research extension. Inactivity and spending time in close proximity were significantly associated with less courtship and vocalisation. Our results also show that hornbill activity and enclosure usage may be influenced by visitor presence, with increased use of higher perches with increasing visitor number.
4.1. Behavioural Outcomes
Inactivity is noted as a prevalent behaviour of zoo-housed hornbills [3,31], and the degree of inactivity in this pair of birds could be explained by pair incompatibility, a lack of environmental stimulation, health reasons, and visitor presence. The amount of time spent inactive by the female bird is particularly noteworthy. Post-data collection, a health check conducted by ARTIS revealed that the female had developed cataract, and the potential impact of this on the animal’s ability to navigate through the available space and initiate social interactions cannot be discounted. However, sex differences in activity are noted in other studies as well. Kozlowski et al. (2015) show that female great hornbills perform the same repertoire of behaviours as males, just at a lower frequency of occurrence [4]. This difference in time–activity budget was also identified in a pair of black-casqued hornbills (Ceratogymna atrata), with the female spending more time inactive [3].
The total time a hornbill pair spend near each other could be a potential indicator of bonding [17]. The pair was observed socialising together within one-bill length, for an equivalent amount of time, which would indicate that these individuals are comfortable in each other’s presence. This behaviour could therefore be deemed an important indicator of pair compatibility in this species of hornbill; nonetheless, it should be examined within multiple pairs to see if this hypothesis holds up.
Proximity to a partner, and some outlet of aggressive behaviour, predicts successful nesting in other large, Asian hornbills [4]. However, external stressors (such as a change in husbandry routine and increased visitor presence) are also associated with increased social proximity [32]. Although data were collected on aggressive interactions in these wreathed hornbills, performance of aggression was minimal and not analysed due to the small number of data points. This lack of aggression, alongside of time spent in proximity, is worthy of further investigation across other pairs of wreathed hornbills. Such research extension would contextualise meaning in terms of different rates of social interaction and association and benchmark potential behavioural indicators of breeding compatibility. The frequency of aggressive interactions differs between pairs [6]. Frequent copulations by a successful pair of rhinoceros hornbills (Buceros rhinoceros) were “always preceded by pursuit of the female with intermediate periods of bill-fencing” [33]. Furthermore, Galama et al. [6] describes higher frequencies of partner nudging, i.e., using the bill for jabbing or pushing, as a good indicator of egg laying and chick production for both male and female great hornbills. Galama et al. [6] recommends adding flexible branches into a hornbill aviary that male birds can wrestle with and bounce on to build momentum for breeding-related social interactions. The use of higher perching and higher rates of exploration by the male bird in our study may be indicative of this need to locate, and then use, different perching structures to help direct specific behaviour patterns.
Control and initiation of socially mediated vocalisation may be very species-specific in hornbills. For example, whilst our study showed vocalisation to be more prevalent from the male bird, research on southern ground hornbills (Bucorvus leadbeateri) showed that female birds performed more calling [23]. Research on the great hornbill, however, mirrors our findings, as Jaroenwatee et al. [34] show elevated vocalisation by a male bird compared to the female. The female’s health status may have played a role in her lack of courtship display performance. As the birds displayed more when perched up high, investigation of aviary furnishing across more facilities would be useful. Our results may indicate that hornbills require elevated, long horizontal perches to be comfortable and thus initiate performance of courtship behaviour. Again, the increased rate of exploration performed by the male could suggest that this bird was motivated in trying to find such style of perching to vocalise and call from. As hornbills use contact calling to locate conspecifics within a habitat [35] and vocalisations are individual-specific, revealing information about them to other birds [36], further research is required to understand how hornbills in ex situ facilities both perform and respond to vocalisations in terms of their environmental and social context. As calling ceased when these two hornbills were close together (Table 2) but increased during periods of exploration, we may be seeing a mimicking of this natural social purpose of vocalisation.
Allofeeding (courtship feeding) is a commonly described activity that can indicate pair bonding and compatibility [2,8]. However, Groot et al. [17] did not find this behaviour to be indicative of breeding success in Rhyticeros hornbills. The pair observed in this study were only observed courtship feeding occasionally (Figure 3). Hornbills may perform some reproductive behaviours such as courtship feeding for multiple years without any reproduction attempts [6]. Some individuals are documented as feeding other bird species, their keepers, or the public [2,6], suggesting that the motivation to perform this behaviour is strong. The function of courtship feeding should therefore be investigated in more pairs of captive hornbills to further define how performance differs between successful and unsuccessful breeding attempts, in a multi-institutional study.
Mutual preening and allopreening are common social activities used to establish and reinforce social bonding and hierarchy, and allopreening frequency increases (directed by the male towards the female) as the breeding season approaches [1,8]. Increased rates of allopreening may encourage the female to crouch, which aids in copulation [6]. Predictably, allopreening was only observed from male towards female in this study and we recommend further study across a longer timeframe to contextualise the rate of allopreening, as well as social cues associated with its performance, and description of behaviours that may result from intense periods of allopreening.
In the wild, wreathed hornbills have large home ranges, and their population density increases with the abundance of their preferred dietary fruits [37]. The exploratory behaviour of the male bird in our study (Figure 2) may be linked to an intrinsic urge to travel widely in search of foraging opportunities and associated social contact. Poonswad and Tsuji [38] note the markedly large home range of wreathed hornbills, compared to similar species, and they suggest this is due to the species’ dietary and reproductive needs. Further study on providing spatial complexity and environmental heterogeneity may be useful for this species in zoos, to provide an outlet for such behaviours.
4.2. Enclosure and Space Use
Our research shows that these hornbills favour Sections 3 and 4 of their enclosure (Figure 7). Section 3 contains two larger trees, which could give the birds some more privacy from visitors; it is also one of the only places where natural perching options are available at this height. As the visitor flow is directed from Sections 5 to 3, (Figure 7), the hornbills may prefer enclosure areas that offer a good view of people arriving in their enclosure. Our research also found a strong positive correlation (Figure 8) between time spent at the highest parts of the aviary and higher visitor numbers. This may suggest that the hornbills like to perch high to feel more comfortable when visitor presence increases. The aviary provides the birds with opportunities to perch in multiple places, with choice of which elevation to perch at, affording these hornbills agency over the use of their immediate environment. As choice and control over enclosure usage is a key welfare indicator [39], this enclosure design may promote positive welfare states during periods of busy zoo visitation. Other research into any visitor effect on hornbill behaviour shows inconclusive relationships [3,40]; therefore, any influence of visitors on hornbill behaviour and space use is likely a factor of each individual bird’s characteristics. In other zoo species, responses to visitors are mediated by personality [41], and thus zoos should be aware of an animal’s personality, preferences, and temperament when designing housing and husbandry.
4.3. Research Extensions and Husbandry Developments
The potential behavioural indicators of pair bonding and compatibility suggested in this paper should be investigated across multiple pairs of birds at different zoos to validate their meaning and thus determine their application to population management for wreathed hornbills. As wreathed hornbill behaviour and enclosure use may be influenced by visitor presence, such study also needs to be repeated for longer periods of time, at multiple times per day, across high and low season, and across facilities. The complexity of social interactions, what they may mean at specific times, and how they are nuanced according to the bird’s motivational state reveals the importance of understanding hornbill natural history when trying to manage reproductively compatible pairs. The use of continuous recording of key state and event behaviours, via video recording and use of behavioural analysis software such as BORIS [42,43], could provide further detailed insight into captive wreathed hornbill time–activity budgets, social interactions, and space use.
Hornbills will flock socially [1,8], and whilst such social flocking would be challenging to replicate in zoos, the importance of this behaviour (for information transfer, mate choice, and as an outlet for sexually selected mechanisms of individual quality) should not be disregarded. As accurate data on hornbill longevity do not currently exist for any species [6], our understanding of how to improve pair compatibility would be strengthened by such basic biology information, as it could be used to create social opportunities at ages of dispersal or maturation, for example. The long-term survival and sustainability of ex situ hornbill populations are an important reason for their continued presence in zoos, and therefore these facilities should collaborate to provide opportunities for mixing and group socialising of their birds (especially for juvenile birds). Such efforts could yield improved reproductive outputs, as has already been shown in enclosure design considerations for cinereous vultures (Aegypius monachus), another long-lived bird species with complex pair compatibility that has benefited from the successful implementation of socialising aviaries [44].
Behavioural ecology information must be embedded in aviary design [45], and in this case, housing that provides a female with exposure to different males could promote compatible pair bonding. Just as “lekking aviaries” work for bird-of-paradise breeding [46,47], the use of such housing systems would be an excellent opportunity for zoos to promote an evidence-based approach to avicultural practices for threatened species [48]. Previous attempts at using “dating aviaries” for hornbills were inconclusive [6], but demonstrated that individuals easily “repaired” in such dating centres. However, more trials are required over a longer timeframe and on a larger scale to fully determine causation and positive influences over breeding success.
Increasing the scale of such aviaries and managing the number of choices a female hornbill has could be worth trying (Figure 9). Avian personality has an impact on fitness [49]. Therefore, considering the behavioural profiles of each hornbill, using a standardised scoring chart that categorises birds as shy, bold, confident, or relaxed, would help zoos match pairs based on matching personality traits. Such behavioural profiles could be shared with other zoos to help construct socially compatible pairs that may be more likely to nest successfully.
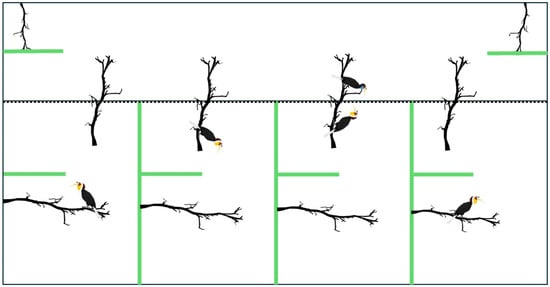
Figure 9.
An overview for a suggested dating aviary setup, with a long enclosure at the top for a female and four smaller enclosures for males. The wall between the female’s and male enclosure consists of mesh (dotted line), while the walls indicated by green lines are opaque, to allow individuals privacy when they choose. A continuous perch is installed in every male enclosure, extending into the female’s enclosure to allow for birds to socialise if they choose to do so.
Captive hornbills can display the same time–activity patterns as performed by wild birds [23]. We therefore suggest more behavioural studies be completed to enable longitudinal data comparison between zoos, and between wild populations and those in the zoo. Multi-individual, multi-zoo study provides important baselines for behavioural normality, validation of welfare indicators, and thus interpretation of individual animal time budgets [50]. Such an approach may help decipher some of the reproductive inactivity in zoo-housed large Asian hornbills. Although our study illustrates some aspects of courtship and reproductive behaviours in wreathed hornbills, future research should investigate the nuances of these behaviours in more detail. Our behavioural category of Calling and Displaying could be split up into more specific behavioural types to record time spent on each specific part of the courtship routine by an individual bird and when performed in synchrony with a partner. We encourage further research into when and where hornbills are vocalising, and to whom the vocalisation is directed (and whether a response is provided), as this could help unravel any link between calling, bird location, and partner response. Similarly, we encourage further research into measures of hornbill association (“within one beak length” in our paper) and social space use (“how many times was a perch shared” in our paper) to validate biological relevant measures of positive hornbill social behaviours that may be cues for partner compatibility.
Distinction between successful and unsuccessful allofeeding should be investigated to provide information on how such social interaction is being reinforced by the response of one individual to another. Further review of how to record aggression should also be considered, and preening could be redefined to measure this as a behavioural state within a social context (i.e., with and without partner present).
5. Conclusions
This study investigated behaviour and aviary use in one zoo-housed pair of wreathed hornbills, providing useful details on hornbill activity and social behaviour. Specifically, we note the importance of high perching to enable hornbills to feel comfortable when being viewed by zoo visitors. We have shown that individual hornbills differ in the amount of time spent on specific behaviours within their wider activity budget, and that such behaviours as proximity, vocalisations, and frequency of courtship display could potentially serve as indicators of pair compatibility for tropical Asian hornbills. To thoroughly understand the validity of socially and reproductively orientated behaviours as predictors of pair compatibility, this study design should evolve to cover multiple pairs, across a longer time period, and across facilities. Our research also shows the importance of interpretation of behavioural profiles alongside of individual animal health status. Whilst case study approaches benefit our understanding of specific individuals within a specific environment, generalisations across a species to inform better practice guidelines must arise from wider observational study. However, we do recommend that hornbills in zoos are provided with high perching opportunities, a good view of visitor presence in their enclosure, and opportunities to explore different heights of perching and branch sizes/structures to promote positive behavioural outputs. We recommend that future study focusses on the social context of behaviour, as well as further classification of aggression and other bird-to-bird interactions. We recommend that hornbill enclosure design includes ecological features that stimulate natural behaviour and cater for social needs (e.g., opportunities for exploration and perching that instigates display) to provide an environment that both promotes and facilitates pair bonding.
Author Contributions
Conceptualisation, P.R.; methodology, P.R. and K.G.; formal analysis, P.R.; data curation, K.G.; writing—original draft preparation, K.G. and P.R.; writing—review and editing, K.G. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the research policies and practices of ARTIS Amsterdam Royal Zoo. Ethical approval and permission by the Research Department and Bird Department at ARTIS Amsterdam Royal Zoo were provided on 1 February 2024 (A. van Dijk and M. de Vries).
Data Availability Statement
Data are available upon reasonable request to the corresponding authors.
Acknowledgments
With thanks to the bird department and research staff at ARTIS Amsterdam Royal Zoo for allowing observational data collection to be conducted on their birds. We would also like to thank M. Fredskov for the provision of the wreathed hornbill icons utilised in Figure 9.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kemp, A.C. Hornbills (Bucerotiformes). In Handbook of the Birds of the World; del Hoyo, J., Elliot, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 2001; Volume 6, pp. 436–523. [Google Scholar]
- Beilby, J. The behavioural biology of hornbills, toucans, and kingfishers. In The Behavioural Biology of Zoo Animals; Rose, P.E., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 185–196. [Google Scholar]
- Rose, P.E.; Scales, J.S.; Brereton, J.E. Why the “visitor effect” is complicated. Unraveling individual animal, visitor number, and climatic influences on behavior, space use and interactions with keepers- a case study on captive hornbills. Front. Vet. Sci. 2020, 7, 236. [Google Scholar] [CrossRef]
- Kozlowski, C.P.; Bauman, K.L.; Asa, C.S. Reproductive behavior of the great hornbill (Buceros bicornis). Zoo Biol. 2015, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, K.; Lammers, J.; Aparici Plaza, D.; Van Der Meer, R.; Hausen, N. EAZA Hornbill Taxon Advisory Group Regional Collection Plan; EAZA: Amsterdam, The Netherlands, 2020; p. 350. [Google Scholar]
- Galama, W.; King, C.E.; Brouwer, K. EAZA Hornbill Husbandry and Management Guidelines; EAZA: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Kinnaird, M.F.; O’Brien, T.G.; Laman, T. The Ecology and Conservation of Asian Hornbills: Farmers of the Forest; University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Kemp, A. The Hornbills: Bucerotiformes (Bird Families of the World); Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Haimoff, E.H. A spectrographic analysis of the loud calls of helmeted hornbills Rhinoplax vigil. Ibis 1987, 129, 319–326. [Google Scholar] [CrossRef]
- Kemp, A.C.; Boesman, P.F.D. Wreathed Hornbill—Rhyticeros undulatus. Available online: https://birdsoftheworld.org/bow/species/wrehor1/cur/introduction (accessed on 23 October 2024).
- Kemp, A.C.; Kirwan, G.M. Blyth’s Hornbill—Rhyticeros plicatus. Available online: https://birdsoftheworld.org/bow/species/blyhor1/cur/introduction (accessed on 23 October 2024).
- Naniwadekar, R.; Mishra, C.; Isvaran, K.; Datta, A. Gardeners of the forest: Hornbills govern the spatial distribution of large seeds. J. Avian Biol. 2021, 52. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Yang, Z.; Chan, B.P.L. Flocking of hornbills observed in Tongbiguan Nature Reserve, Yunnan, China. Hornbill Nat. Hist. Conserv. 2020, 1, 42–44. [Google Scholar]
- Species360. Zoological Information Management System. Available online: https://zims.species360.org/Main.aspx (accessed on 3 July 2024).
- BirdLife International. Rhyticeros undulatus. IUCN Red List of Threatened Species. 2018, p. e.T22682528A132400385. Available online: https://datazone.birdlife.org/species/factsheet/wreathed-hornbill-rhyticeros-undulatus (accessed on 23 October 2023).
- Hadiprakarsa, Y.-Y.; Kinnaird, M.F. Foraging characteristics of an assemblage of four Sumatran hornbill species. Bird Conserv. Int. 2004, 14, S53–S62. [Google Scholar] [CrossRef]
- Groot, K.; Brereton, J.E.; King, C.E.; Rose, P.E. A preliminary global investigation into potential impacts on successful captive breeding for two species of Rhyticeros hornbill. Zoo Biol. 2024, 43, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Mallapur, A.; Qureshi, Q.; Chellam, R. Enclosure design and space utilization by Indian leopards (Panthera pardus) in four zoos in southern India. J. Appl. Anim. Welf. Sci. 2002, 5, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Robert, R. Evaluating the activity patterns and enclosure usage of a little-studied zoo species, the sitatunga (Tragelaphus spekii). J. Zoo Aquar. Res. 2013, 1, 14–19. [Google Scholar]
- Forthman Quick, D.L.; Pappas, T.C. Enclosure utilization, activity budgets, and social behavior of captive chamois (Rupicapra rupicapra) during the rut. Zoo Biol. 1986, 5, 281–292. [Google Scholar] [CrossRef]
- Bateson, M.; Martin, P. Measuring Behaviour: An Introductory Guide, 4th ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMonitor app. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- Cooper, M.; Jordan, L. Random time-activity budgets in captive Southern Ground Hornbill Bucorvus leadbeateri: Commentary. S. Afr. J. Sci. 2013, 109, 1–2. [Google Scholar] [CrossRef]
- Lehner, P.N. Sampling methods in behavior research. Poult. Sci. 1992, 71, 643–649. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R. Available online: http://www.rstudio.com (accessed on 12 October 2024).
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Minitab 22.1. Minitab Statistical Software. Minitab: State College, PA, USA, 2024; Available online: www.minitab.com (accessed on 12 October 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Brereton, J.E.; Myhill, M.N.G.; Shora, J.A. Investigating the effect of enrichment on the behavior of zoo-housed southern ground hornbills. J. Zool. Bot. Gard. 2021, 2, 600–609. [Google Scholar] [CrossRef]
- Readyhough, T.S.; Joseph, S.L.; Vyas, K.; Schreier, A.L. The effects of Zoo Lights on animal welfare: A case study of great Indian hornbills at Denver Zoo. Zoo Biol. 2022, 41, 263–270. [Google Scholar] [CrossRef]
- Reilly, S.E. Breeding the rhinoceros hornbill buceros rhinoceros at the Audubon Park and Zoological Garden. Int. Zoo Yearb. 1988, 27, 263–269. [Google Scholar] [CrossRef]
- Jaroenwatee, W.; Pongsupath, N.; Wongyuen, N.; Suwanpugdee, A. A study on behavior in breeding season and develop nest box of great hornbill (Buceros bicornis linaeus) in Dusit Zoo. J. Agric. Res. Ext. 2018, 35, 963–971. [Google Scholar]
- Leighton, M. Hornbill social dispersion: Variations on a monogamous theme. In Ecological Aspects of Social Evolution; Rubenstein, D.I., Wrangham, R.W., Eds.; Princeton University Press: Princeton, NJ, USA, 1986; pp. 108–130. [Google Scholar]
- Policht, R.; Petrů, M.; Lastimoza, L.; Suarez, L. Potential for the use of vocal individuality as a conservation research tool in two threatened Philippine hornbill species, the Visayan hornbill and the rufous-headed hornbill. Bird Conserv. Int. 2009, 19, 83–97. [Google Scholar] [CrossRef]
- Sibarani, M.C.; Utoyo, L.; Pratama, R.D.; Danus, M.A.; Sudrajat, R.; Surahmat, F.; Marthy, W. Long-term monitoring of nesting behavior and nesting habitat of four sympatric hornbill species in a Sumatran lowland tropical rainforest of Bukit Barisan Selatan National Park. Hornbill Nat. Hist. Conserv. 2020, 1, 17–29. [Google Scholar]
- Poonswad, P.; Tsuji, A. Ranges of males of the great hornbill Buceros bicornis, brown hornbill Ptilolaemus tickelli and wreathed hornbill Rhyticeros undulatus in Khao Yai National Park, Thailand. Ibis 1994, 136, 79–86. [Google Scholar] [CrossRef]
- Ross, S.R. Issues of choice and control in the behaviour of a pair of captive polar bears (Ursus maritimus). Behav. Process. 2006, 73, 117–120. [Google Scholar] [CrossRef]
- Thicks, S.F. Is there a visitor effect on Abyssinian ground hornbills (Bucorvus abyssinicus), Papuan wreathed hornbills (Aceros plicatus), wrinkled hornbills (Aceros corrugatus) and toco toucans (Ramphastos toco) in a captive zoo environment? Plymouth Stud. Sci. 2008, 1, 30–55. [Google Scholar]
- Phillips, C.; Peck, D. The effects of personality of keepers and tigers (Panthera tigris tigris) on their behaviour in an interactive zoo exhibit. Appl. Anim. Behav. Sci. 2007, 106, 244–258. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Weber, W.D.; Fisher, H.S. An inexpensive wireless video recording system for continuous behavioral observations. BioRxiv 2019. [Google Scholar] [CrossRef]
- Huyghe, M. EAZA Best Practice Guidelines Aegypius Monachus—Cinereous Vulture; European Association of Zoos and Aquaria: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Rose, P.E.; Freeman, M.; Hickey, I.; Kelly, R.; Greenwell, P.J. Considering what animals “need to do” in enclosure design: Questions on bird flight and aviaries. Birds 2024, 5, 586–603. [Google Scholar] [CrossRef]
- Rimlinger, D.; Theule, J.; Bass, K. Breeding history and husbandry of the superb bird-of-paradise (Lophorina superba). Zoo Biol. 2021, 40, 485–490. [Google Scholar] [CrossRef]
- Theule, J.; Rimlinger, D. Breeding history and husbandry of the raggiana bird-of-paradise (Paradisaea raggiana). Zoo Biol. 2023, 42, 162–170. [Google Scholar] [CrossRef]
- Rose, P.E. Evidence for aviculture: Identifying research needs to advance the role of ex situ bird populations in conservation initiatives and collection planning. Birds 2021, 2, 77–95. [Google Scholar] [CrossRef]
- Both, C.; Dingemanse, N.J.; Drent, P.J.; Tinbergen, J.M. Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 2005, 74, 667–674. [Google Scholar] [CrossRef]
- Wark, J.D.; Cronin, K.A. The behavior patterns of giraffes (Giraffa camelopardalis) housed across 18 US zoos. PeerJ 2024, 12, e18164. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).