Abstract
Controlling parasites in zoo animals is a significant challenge, making practical infection prevention methods essential. This study evaluated a novel solution using parasitophagous fungi-treated forage seeds to reduce soil parasite persistence. We conducted two experiments to assess the soil distribution of the fungi Mucor circinelloides (ovicidal) and Duddingtonia flagrans (larvicidal). Forage seeds were immersed in a submerged culture containing 106 spores/mL and subsequently sown in plastic trays (trial 1) and demarcated ground plots (40 × 30 cm) framed with wood (trial 2). Fifteen days later, Ascaris suum and cyathostomin eggs were placed above the germinated vegetation. After a 25–30-day period, the viability of roundworm eggs reduced by 62%, and half of them remained undeveloped; a 64% reduction in the counts of L3 cyathostomins was recorded. In trial 2, viability decreased by 55% in roundworm eggs, with an infectivity rate of 30%, while L3 counts lessened by 57%. It has been concluded that the risk of infection by ascarids and cyathostomins can be reduced by at least half by sowing the soil with forage seeds pre-treated with fungi, providing a practical solution for parasite control in zoos.
1. Introduction
The initial purpose of zoos was to exhibit animals for display as a kind of museum, preserving exotic specimens obtained on expeditions. Over time, there have been notable changes, and zoos now focus on conservation as wild habitats continue diminishing, and many animals are at greater risk of extinction [1]. In addition, activities are often organized to promote education among visitors and to participate in research projects, always following the highest standards of animal care and welfare [2].
Modern zoos strive to conserve a diverse array of animal species by maintaining them in habitats that closely resemble their natural ecosystems. For example, all enclosures for bears should contain a dry resting area, pool, and den [3]. Zones large enough to allow wolves to chase each other are suggested in zoological gardens, whereas small felids like lynxes or bobcats in captivity need perching platforms at or near the top of their enclosure, a place from which they can “hide” and peer out. They also require logs upon which they can “sharpen” their claws [4]. In constructing a shorebird habitat, a mixture of ground cover that includes sand, rocks, clay, grass mats, and saltwater is suggested. Driftwood and rocks—which they would find in their natural environment—serve as perfect “furniture” for them to perch on. There is also running water in their habitats, and the birds seem to enjoy wading and standing at the water’s edge. Regarding herbivores, significant efforts have been made in many zoos to prepare grassy plots in order to offer them the possibility to nourish, socialize, and interact with the environment [5].
Some of the measures introduced in recent years in zoos have improved animal welfare, but other problems remain unresolved and even seem to have worsened, such as the control of certain parasitic infections [6]. Providing herbivores with grassy enclosures replicates their natural habitats but also facilitates the lifecycle completion of strongylid nematodes, potentially leading to significant re-infections [7,8]. On the other hand, the presence of soil in the plots where carnivores are kept makes it easier for geohelminths such as ascarids or trichurids to finish their cycle until the respective infective stages are reached. Therefore, the situation becomes similar to that experienced by domestic animals, and deworming as the only measure reveals insufficient to obtain good results so that animals can reinfect often and quite rapidly [9], which reinforces the need to have complementary procedures to support the deworming.
Given recent efforts to restore natural environments, there is potential to decrease reliance on anthelmintics by focusing on soil-dwelling microorganisms that can disrupt or inhibit the development of parasitic stages pathogenic to animals [10,11]. In coincidence with this line, some experience acquired in the last decade underlines the possibility of using parasitophagous fungi for the control of parasitic forms affecting captive animals in zoos, focused mainly against parasites developing a direct life cycle as roundworms or strongylid nematodes [12]. Despite this information not being very abundant, different formulations involving the administration of saprophytic filamentous fungi such as Mucor circinelloides Van Thiegem (1875) and Trichoderma atrobrunneum F.B. Rocha, Chaverri & Jaklitsch (2015) (ovicidal), and Duddingtonia flagrans (Dudd.) R.C. Cooke (1969) (larvicidal) have been tried.
Given the satisfactory results obtained using various soil saprophytic filamentous fungi, a novel approach was developed to significantly reduce the prevalence of parasites in zoos. This strategy consists of soaking forage seeds in a submerged medium containing a mixture of chlamydospores of Mucor circinelloides and Duddingtonia flagrans, which are thick-walled structures that help fungi endure harsh conditions prior to sowing. The main objective is to restore natural soil conditions for wildlife by re-establishing the balance between hosts, pathogens, and antagonists. This method represents an innovative advance in the possibilities of biological parasite control, providing a practical and sustainable solution to improve the health and welfare of animals in zoological environments.
2. Materials and Methods
2.1. Parasitophagous Fungi
In the present investigation, a blend of two filamentous soil fungi (M. circinelloides CECT 20824 and D. flagrans CECT 20823) were co-cultured in the COPFr submerged medium to concentrations around 106 chlamydospores each per mL (Figure 1) [13].
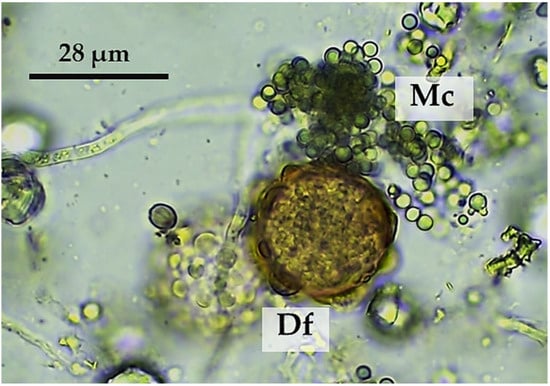
Figure 1.
Chlamydospores of M. circinelloides (Mc) and D. flagrans (Df) were jointly grown in the COPFr submerged medium.
These fungi were isolated by the COPAR Research Group (GI-2120; University of Santiago de Compostela, Spain) and deposited in the Spanish Type Culture Collection (CECT, Valencia, Spain) [13].
2.2. Preparation of Forage Seeds for Sowing
A commercial formulation containing forage seeds was utilized in the present research, composed of 40% Lolium perenne solen, 10% Lolium multiflorum locobelo, 40% Lolium x boucheanum Kunth, 5% Trifolium repens huia, and 5% Trifolium pratense rozeta.
In particular, pre-treatment of the seeds comprised placing them in trays at a 1:2 (mass/volume) ratio in a liquid culture medium containing the parasiticidal fungi at a proportion of 200 mL of medium per 100 g of seeds (Figure 2). A period of 2–5 h was allowed for the seeds to soak properly, and then they were stored in plastic bags until use, which was always performed within 7 days.

Figure 2.
Forage seeds were soaked in a submerged culture containing chlamydospores of M. circinelloides (Mc) and D. flagrans (Df) and then kept in plastic bags until use in trials 1 (trays) and 2 (ground).
In trial 1, the preparation of the trays consisted of placing 200 g of compost autoclaved in each and then planting 50 g seeds. Accordingly, a total of 32 trays were prepared and maintained in the lab at RT (17–22 °C). Twelve trays were assigned untreated seeds (controls). Six of these trays received 12 g of feces from piglets, shedding 3450 eggs of Ascaris suum per gram of feces (EPG). The other six trays received 12 g of feces from horses, shedding 685 cyathostomin EPG. Twenty trays were sown with pre-treated seeds (Figure 3) and then sorted into two groups of 10 each. The group of G-TAs received 12 g of feces from piglets, shedding 3450 A. suum EPG, and the G-TCy group received 12 g of feces from horses, shedding 685 cyathostomin EPG. In each group, half of the trays were analyzed 15 days later, and the remainder was analyzed at the end of the assay (day 30).
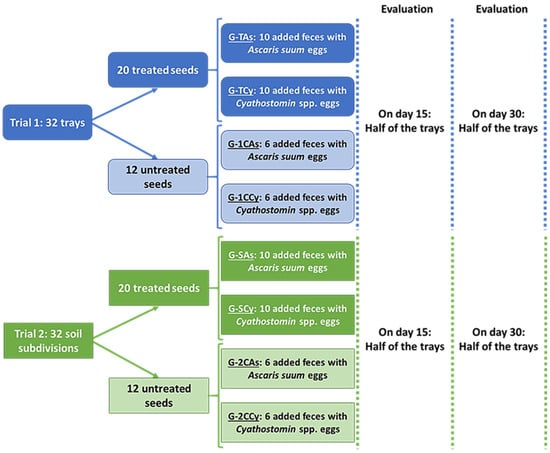
Figure 3.
Study design conducted with forage seeds soaked in submerged culture containing chlamydospores of M. circinelloides (Mc) and D. flagrans (Df).
In trial 2, a total of 32 wooden frames (15 × 40 × 30 cm) were placed on the ground to delimit the test areas (Figure 2). The controls consisted of 12 sections sown with untreated seeds: six received 50 g of feces from piglets, shedding 3450 EPG Ascaris suum, and the other received six 50 g of feces from horses, shedding 685 cyathostomin EPG.
Twenty soil areas were sown with 100 g seeds pre-treated with fungi and then divided into two lots; G-SAs composed of 10 areas received 50 g of feces from piglets, shedding 3450 EPG A. suum, and another 10 received 50 g of feces from horses, shedding 685 cyathostomin EPG. Fifteen days later, half of the areas in each group were taken and analyzed, and the remaining were analyzed at the end of the assay (day 30).
2.3. Evaluation of the Antiparasitic Effect
In order to test the influence of the parasiticidal strategy, variations in the numbers of viable eggs of A. suum and of cyathostomin third-stage larvae (L3) were analyzed. This evaluation consisted of the observation of a minimum of 200 parasites (eggs or larvae, respectively) in each of the prepared trays and soil areas. Consequently, more than 10,000 observations of parasitic forms were performed throughout the study period using coprological probes.
2.3.1. Roundworms
With the aim of analyzing the antagonistic effect against roundworm eggs, a modified sedimentation technique [14] was used in trial 1, consisting of directly taking the content of each tray and emulsifying it into 1 L water. After completely mixing, the contents were sequentially passed through sieves with different pore diameters (4, 0.52, 0.15, and 0.050 mm) to reduce the presence of coarse particles. The resulting liquid was deposited in sedimentation cups for 24 h. Finally, the sediment obtained was analyzed under a microscope by placing 50 μL aliquots between glass slides and coverslips [15]. A similar procedure was applied to trial 2 by taking half of each soil sample delimited by the wooden frames (ca. 30 × 20 cm and 5 cm deep) and sinking it into 2 L water until entirely submerged. The blend was filtered along different sieves, as mentioned above, and the filtrate was placed into sedimentation cups for 24 h. After discarding the supernatant until 100 mL was obtained, the final volume was observed under a microscope, as mentioned before. Based on previous investigations, the eggs were sorted into non-viable and viable by considering the presence of eggshell damage or disruption [16] (Figure 4).
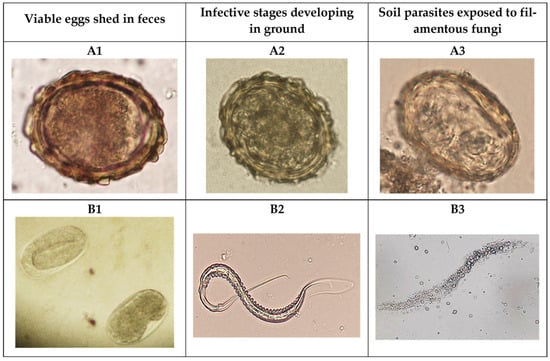
Figure 4.
Eggs of certain helminths like Ascaris sum (A1) or strongylid nematodes (B1) are shed in the feces of infected animals and, once in the ground, evolve to reach the infective stage; eggs containing an L2 inside (A2) or third-stage larvae (B2). The antagonistic activity of the fungi causes the non-viability of eggs (A3) and L3 larvae (B3).
The percentage of reduction (PR) in eggs of A. suum was calculated according to the counts of viable eggs per gram of feces (EPG) on days 15 and 30, as follows:
PR15 (%) = [1 − (EPGday15/EPGday0)] × 100
PR30 (%) = [1 − (EPGday30/EPGday0)] × 100
Nevertheless, by considering that a percentage of eggs of A. suum becomes naturally non-viable [11], the possible effect attributed to the antiparasitic fungi was adjusted by taking into account the difference between PR in the exposed and in the controls; then, the Adjusted percentage of reduction (%) was calculated as
APR (%) = PRtreated − PRcontrols
2.3.2. Cyathostomins
In trial 1, the action on cyathostomin third-stage larvae (L3s) was evaluated by placing half of the content of each tray into 1 L of water and mixing for 1 h. Then, the contents were successively filtered through sieves with different pore diameters (4, 0.52, 0.15, and 0.050 mm), and the final solution was analyzed by means of the Baermann test for the collection of L3s: the infective stages [17] (Figure 4). The same protocol was applied to trial 2 on half of the content of each ground area. The identification of L3 cyathostomins was conducted using morphological keys [15]. Finally, the reduction in cyathostomin L3 larvae (PRL3) was estimated as follows:
PRL3 (%) = [1 − (LPGtreated/PGcontrols)] × 100
2.4. Statistical Analysis
The Kolmogorov–Smirnov probe showed that the data collected for the fecal egg counts of A. suum were normally distributed (Z = 1.155, p = 0.138), and the Levene test revealed that the variances were homogeneous (Statistic = 4.792, p = 0.053). The analysis of the numbers of cyathostomin third-stage larvae (L3s) revealed that these data were also normal (Z = 0.866, p = 0.441), and the variances were homogeneous (Statistic = 4.792, p = 0.053). Accordingly, an ANOVA with repeated measures was conducted at a significance level of p < 0.05. All the probes were employed using the statistical software SPSS, version 22 (IBM SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Effect on Eggs of A. suum
As given in Figure 5, the number of viable A. suum eggs maintained in trays with herbage (G-TAs) for 30 days showed slight variations in the controls (ca. 6–10%). The number of viable eggs placed in those trays sown with seeds previously soaked in a liquid medium containing spores belonging to the two fungal species (G-1CAs) decreased by 19.3% during the first 15 days with respect to the controls (not exposed to the antiparasitic fungi) (F = 42.148, p = 0.023). At the end of the study (day 30), the number of viable eggs was reduced by 68%, and these differences were also significant (F = 37.130, p = 0.026).
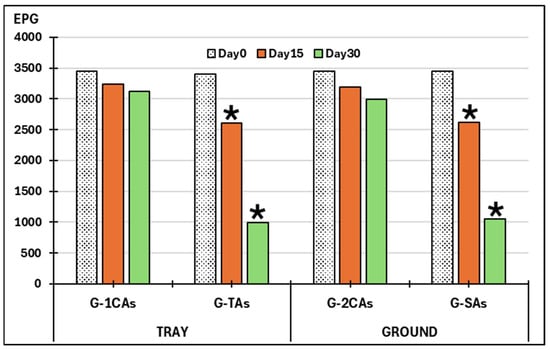
Figure 5.
Variations in the viability of eggs of Ascaris suum. G-1CAs: control group trays (seeds without fungi); G-TAs: trays sown with seeds soaked in submerged culture containing M. circinelloides and D. flagrans; G-2CAs: control soil parcels (without fungi). G-SAs: soil parcels sown with seeds pre-soaked in submerged culture containing M. circinelloides and D. flagrans. EPG: eggs of A. suum per gram of feces; (*): significant differences.
Regarding data collected in control small ground areas, the viability of A. suum eggs in the controls (G-SAs) was reduced by 7–13%. When eggs were placed in areas containing antiparasitic fungi (G-2CAs), there was a decrease in the viable eggs of the nematode by 17%, as recorded after 15 days (F = 23.125, p = 0.037), and 64.9% on day 30 (F = 20.892, p = 0.036).
By considering data acquired in the respective control and treated groups, the percentages of reduction were normalized (Table 1).

Table 1.
Adjusted reduction (%) of viable A. suum eggs exposed to the antiparasitic fungi M. circinelloides and D. flagrans.
3.2. Effect on Cyathostomin Third-Stage Larvae (L3s)
As given in Figure 6, no larvae were detected at the beginning of the study. The percentages of L3 developed in the control trays were 20% on day 15 and 60.4% on day 30. In the trays with fungi, the percentages of larvae recovered were 10.7% and 16.5%, respectively.
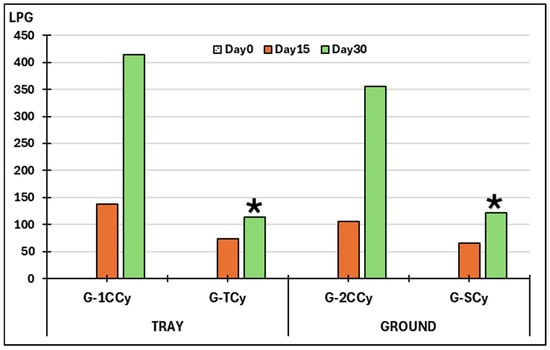
Figure 6.
Variations on the viability of L3 larvae of cyathostomins. G-1CCy: control group trays (seeds without fungi); G-TCy: trays sown with seeds soaked in submerged culture containing M. circinelloides and D. flagrans: G-2CCy: control soil parcels (without fungi); G-SCy: soil parcels sown with seeds soaked in submerged culture containing M. circinelloides and D. flagrans. LPG: L3 larvae of cyathostomins per gram of feces; (*): significant differences.
Regarding the small ground areas serving as controls, a percentage of 15.3% and 52% L3 larvae evolved from cyathostomin eggs on days 15 and 30, respectively. Concerning the exposure to antiparasitic fungi, the percentages recorded were 9.5% and 17.7% on days 15 and 30. Significant differences were recorded at the end of the study in the trays (F = 22.381, p = 0.031) and in the ground (F = 18.231, p = 0.042).
Table 2 shows that the PRL3 in the presence of fungi was higher in the trays with herbage than in small ground areas, but these differences were not significant (p > 0.05).

Table 2.
Reduction (%) of cyathostomin L3 larvae exposed to the antiparasitic fungi M. circinelloides and D. flagrans.
4. Discussion
Captive wild animals in zoos are at high risk of infection by parasites that develop some stages in the soil, and the problem increases when the animals are kept in plots with vegetation favoring certain parasites with a direct life cycle (as is the case with numerous helminths) survive and complete their evolvement in the soil [17,18]. Consequently, an investigation was designed to try to reduce the numbers and survival of infective parasitic stages in the soil by ensuring the presence of two antiparasitic fungi. For this purpose, forage seeds were soaked in a mixture of saprophytic filamentous fungi (M. circinelloides and D. flagrans) before sowing in plastic trays. Once the seeds germinated, feces of piglets shedding eggs of the gastrointestinal nematode Ascaris suum and feces of horses passing eggs of cyathostomins were placed. After a 30-day period, the counts of A. suum viable eggs reduced significantly by two-thirds, and those of cyathostomin third-stage (L3) larvae by three-quarters. These results are partially in agreement with previous studies involving fungal species such as M. circinelloides [19], Trichoderma atrobrunneum F.B. Rocha, Chaverri & Jaklitsch [20], or Clonostachys rosea Schroers, Samuels, Seifert & W. Gams [21] directly sprayed on the feces of pigs [22,23], where similar reduction percentages of two-thirds for Ascaris suum and one-third for strongylid nematodes were found.
Parasite control among wild animals kept in a zoo basically consists of administering drugs designed for livestock because neither the proper dosage nor the possible adverse effects are well known [5,7,12], and specific indications cannot be found in the commercial leaflets. Besides this, deworming does not affect the infective stages in the soil, and the risk of infection maintains and even increases as the pasture rotation practices suggested for livestock are not applicable in most zoos [24]. Because many infections provoked by gastrointestinal parasites originate when infective stages developing in the soil are ingested [25], i.e., while grazing, the need to have a realistic procedure to limit the numbers of these infective stages seems essential. Proper spreading of spores of soil filamentous fungi has been extensively tried, encompassing mostly their oral administration through different edible formulations, with the aim of the fungal spores are passed in the feces together with some parasitic stages, and once in the soil, develop their mycelia and act against the pathogens [26]. By means of water solutions and milled cereals [13], the spores of several antiparasitic fungi were distributed among captive equids in a zoological garden. Later, and based on the results achieved in horses maintained under continuous pasturing, nutritional pellets elaborated with chlamydospores of M. circinelloides and D. flagrans in an industrial factory provided highly successful results among different wild herbivores infected by strongylid nematodes [27]. In the current investigation, the usefulness of soaking forage seeds with a submerged culture containing a blend of M. circinelloides and D. flagrans before sowing them in the ground has been demonstrated by observing that the counts of the pig roundworm A. suum eggs and horse cyathostomins were reduced by 51.7% and 66%, respectively, after a period of 30 days. So far, this is the first and only contribution to prevent infection among captive animals and to limit the application of chemical dewormers without oral administration because the risk of infection can be significantly reduced with this strategy (by half and two-thirds, respectively). Another interesting point lies in the fact that no additional tasks are required for animal keepers, and the antagonistic and beneficial effect is achieved without depending on animal intake, palatability, etc. In a recent investigation, the administration of edible gelatins made with chlamydospores of M. circinelloides and D. flagrans reduced the viability of the gastrointestinal nematode Trichuris sp. in the feces of captive baboons by 44% [11], no evidence regarding the influence of soil types or extreme weather conditions on the development of filamentous fungi are available. It is well known that under adverse conditions, fungi remain latent as spores.
During the last two decades, many investigations have analyzed the practical possibilities of using parasitophagous fungi for biological control among animals and even people [17], with an important stress on the different and practical ways which ensure their proper distribution. Although the oral administration of parasiticidal fungal spores has provided promising results, its practical application entails certain difficulties, mainly consisting of the fact that wild animals might refuse to take feed containing the fungi. In the current assay, the usefulness of a totally novel application has been tested. The novelty consisted of soaking seeds of forage species in a submerged culture containing M. circinelloides and D. flagrans prior to seeding a small land area, with the aim of contributing to the spreading of that fungus along the land. Accordingly, the results obtained in the present investigation could be directly extrapolated to livestock reared on farms. Although it appears very interesting, the possibility of applying the proposed strategy on wildlife reserves appears very difficult due to the problems associated with spreading the fungal spores.
Ascarids and strongylid nematodes (such as cyathostomins) are parasites frequently identified mainly in the feces of animals that enjoy plots with soil and/or vegetation, and their presence persists despite the regular administration of effective anthelmintics [5,8,9,18,24]. A remarkable result in the present investigation was the observation of a reduction in the number of viable A. suum eggs by 10.1% at fifteen days, in contrast to 38.1% with respect to L3 cyathostomins. It should be taken into account that the roundworm eggshell provides protection against adverse environmental conditions (freezing, dryness, UV radiation...), which would explain the need for a certain amount of time to elapse before fungi are able to damage it, break it, and penetrate inside it [10,22,23,28].
Finally, the tests carried out in trays proved to be a very suitable procedure for the germination of forage species and for the distribution of chlamydospores and the development of mycelia belonging to the parasiticidal fungi M. circinelloides and D. flagrans, which is concluded considering the reduction percentages recorded in the numbers of viable eggs of A. suum, as well as in the counts of L3 cyathostomins.
5. Conclusions
Controlling parasites that are developing their infective stages in areas with land and vegetation requires preventive measures to reduce the risk of infection. Sowing the ground with seeds previously soaked in a submerged medium for culturing the parasitophagous fungi Mucor circinelloides and Duddingtonia flagrans offers an innovative and effective strategy to lessen the numbers of viable A. suum viable eggs and the counts of L3 cyathostomin. The performance of tests in trays for assessing antiparasitic offers a model that can be extrapolated to those developed in soil, so they are highly recommended.
Author Contributions
Conceptualization, J.Á.H., C.C.-M., A.P.-S., R.M., M.S.A. and M.C.; methodology, C.V., J.L., I.Z., M.B., I.A.-R., R.S., E.V. and G.P.-A.; software, J.Á.H. and M.C.; validation, C.C.-M., R.M. and M.S.A.; formal analysis, J.L., R.S., G.P.-A., M.S.A. and M.C.; investigation, C.V., M.B. and I.A.-R.; resources, E.V. and C.C.-M.; data curation, J.L., I.A.-R., G.P.-A. and R.M.; writing—original draft preparation, J.Á.H., C.V., I.A.-R. and R.S.; writing—review and editing, J.Á.H., A.P.-S., R.M., J.L. and M.S.A.; visualization, M.B., I.A.-R. and R.S.; supervision, J.Á.H., R.M. and G.P.-A.; project administration, I.Z., C.C.-M. and M.S.A.; funding acquisition, J.Á.H., A.P.-S., M.S.A. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This trial was partly supported by the Ministerio de Ciencia e Innovación (Spain) and the European Regional Development Fund (ERDF) (Research Project PID2020-120208RB-I00), the Consellería de Cultura, Educación e Universidades (Xunta de Galicia, Spain) (Research Project ED431B2021/07) and the Consellería do Medio Rural (Xunta de Galicia, Spain) (Research Project AEI 2022 Operational Groups).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors express their sincere gratitude to the Head of the “Granja Gayoso Castro” (Deputación Provincial de Lugo, Spain) and to “Marcelle Natureza Zoological Park” for their valuable collaboration.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rabb, G.B. The Evolution of Zoos from Menageries to Centers of Conservation and Caring. Curator. Mus. J. 2004, 47, 237–246. [Google Scholar] [CrossRef]
- Packer, J.; Ballantyne, R. The Role of Zoos and Aquariums in Education for a Sustainable Future. New Dir. Adult Contin. Educ. 2010, 2010, 25–34. [Google Scholar] [CrossRef]
- Conway, W.G. Buying Time for Wild Animals with Zoos. Zoo Biol. 2011, 30, 1–8. [Google Scholar] [CrossRef]
- Mellen, J.D. Zoo Standards for Keeping Small Felids in Captivity. Available online: https://nagonline.net/763/zoo-standards-keeping-small-felids-captivity/ (accessed on 13 February 2024).
- Gonzálvez, M.; Moreno, E.; Pérez-Cutillas, P.; Gilbert, T.; Ortiz, J.; Valera, F.; Espeso, G.; Benzal, J.; Ibáñez, B.; de Ybáñez, M.d.R.R. Zoological Institutions as Hotspots of Gastrointestinal Parasites That May Affect the Success of Ungulate Reintroduction Programmes. Vet. Rec. 2021, 189, e506. [Google Scholar] [CrossRef]
- Capasso, M.; Maurelli, M.P.; Ianniello, D.; Alves, L.C.; Amadesi, A.; Laricchiuta, P.; Silvestre, P.; Campolo, M.; Cringoli, G.; Rinaldi, L. Use of Mini-FLOTAC and Fill-FLOTAC for Rapidly Diagnosing Parasitic Infections in Zoo Mammals. Rev. Bras. Parasitol. Vet. 2019, 28, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Garijo, M.M.; Ortiz, J.M.; Ruiz de Ibáñez, M.R. Helminths in a Giraffe (Giraffa Camelopardalis Xgiraffa) from a Zoo in Spain. Onderstepoort J. Vet. Res. 2004, 71, 153–156. [Google Scholar] [CrossRef]
- Atanaskova, E.; Kochevski, Z.; Stefanovska, J.; Nikolovski, G. Endoparasites in Wild Animals at the Zoological Garden in Skopje, Macedonia. J. Threat. Taxa 2011, 3, 1955–1958. [Google Scholar] [CrossRef]
- Nath, T.C.; Eom, K.S.; Choe, S.; Hm, S.; Islam, S.; Ndosi, B.A.; Kang, Y.; Bia, M.M.; Kim, S.; Eamudomkarn, C.; et al. Insight into One Health Approach: Endoparasite Infections in Captive Wildlife in Bangladesh. Pathogens 2021, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Silva, M.I.; Palomero, A.M.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Biological Control of Soil Transmitted Helminths (STHs) in a Zoological Park by Using Saprophytic Fungi. Biol. Control 2018, 122, 24–30. [Google Scholar] [CrossRef]
- Paz-Silva, A.; Salmo, R.; Viña, C.; Miguel Palomero, A.; Ángel Hernández, J.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.; Sol Arias, M. Gelatin Treats Containing Filamentous Fungi to Promote Sustainable Control of Helminths among Pets and Zoo Animals. Biol. Control 2023, 179, 105184. [Google Scholar] [CrossRef]
- Salmo, R.; Viña, C.; Lozano, J.; Palomero, A.M.; Hernández, J.Á.; Bonilla, R.; Sánchez-Andrade, R.; Paz-Silva, A.; Madeira de Carvalho, L.M.; Arias, M.S.; et al. Saprophytic Filamentous Fungi against Helminths Affecting Captive Wild Animals. Encyclopedia 2024, 4, 91–100. [Google Scholar] [CrossRef]
- Arias, M.S.; Cazapal-Monteiro, C.F.; Suárez, J.; Miguélez, S.; Francisco, I.; Arroyo, F.L.; Suárez, J.L.; Paz-Silva, A.; Sánchez-Andrade, R.; Mendoza de Gives, P. Mixed Production of Filamentous Fungal Spores for Preventing Soil-Transmitted Helminth Zoonoses: A Preliminary Analysis. BioMed Res. Int. 2013, 2013, 567876. [Google Scholar] [CrossRef]
- Hernández, J.A.; Vázquez-Ruiz, R.A.; Cazapal-Monteiro, C.F.; Valderrábano, E.; Arroyo, F.L.; Francisco, I.; Miguélez, S.; Sánchez-Andrade, R.; Paz-Silva, A.; Arias, M.S. Isolation of Ovicidal Fungi from Fecal Samples of Captive Animals Maintained in a Zoological Park. J. Fungi 2017, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Cernea, M.; Madeira de Carvalho, L.; Vasile, C. Atlas of Diagnosis of Equine Strongylidosis; Editura Academic Press: Târgovişte, Romania, 2008; ISBN 978-973-744-127-0. [Google Scholar]
- Araújo, J.V.d.; Fonseca, J.d.S.; Barbosa, B.B.; Valverde, H.A.; Santos, H.A.; Braga, F.R. The Role of Helminthophagous Fungi in the Biological Control of Human and Zoonotic Intestinal Helminths. Pathogens 2024, 13, 741. [Google Scholar] [CrossRef] [PubMed]
- VanHoy, G. Overview of Gastrointestinal Parasites of Ruminants. Available online: https://www.msdvetmanual.com/digestive-system/gastrointestinal-parasites-of-ruminants/overview-of-gastrointestinal-parasites-of-ruminants#Prevention_v81479076 (accessed on 18 August 2024).
- Esteban-Sánchez, L.; García-Rodríguez, J.J.; García-García, J.; Martínez-Nevado, E.; de la Riva-Fraga, M.A.; Ponce-Gordo, F. Wild Animals in Captivity: An Analysis of Parasite Biodiversity and Transmission among Animals at Two Zoological Institutions with Different Typologies. Animals 2024, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Van Tieghem, P. Nouvelles recherches sur les Mucorinées. Fortin Masson 1875, 6, 175. [Google Scholar]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma Harzianum Species Complex and the Re-Identification of Commercial Biocontrol Strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Schroers, H.-J.; Samuels, G.J.; Seifert, K.A.; Gams, W. Classification of the Mycoparasite Gliocladium Roseum in Clonostachys as C. Rosea, Its Relationship to Bionectria Ochroleuca, and Notes on Other Gliocladium-like Fungi. Mycologia 1999, 91, 365–385. [Google Scholar] [CrossRef]
- Cortiñas, F.J.; Cazapal-Monteiro, C.F.; Hernández, J.A.; Arroyo, F.L.; Miguélez, S.; Suárez, J.; López de Arellano, M.E.; Sánchez-Andrade, R.; Mendoza de Gives, P.; Paz-Silva, A.; et al. Potential Use of Mucor Circinelloides for the Biological Control of Certain Helminths Affecting Livestock Reared in a Care Farm. Biocontrol Sci. Technol. 2015, 25, 1443–1452. [Google Scholar] [CrossRef]
- Viña, C.; Silva, M.I.; Palomero, A.M.; Voinot, M.; Vilá, M.; Hernández, J.Á.; Paz-Silva, A.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.F.; Arias, M.S. The Control of Zoonotic Soil-Transmitted Helminthoses Using Saprophytic Fungi. Pathogens 2020, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Rapiya, M.; Hawkins, H.-J.; Muchenje, V.; Mupangwa, J.F.; Marufu, M.C.; Dzama, K.; Mapiye, C. Rotational Grazing Approaches Reduces External and Internal Parasite Loads in Cattle. Afr. J. Range Forage Sci. 2019, 36, 151–159. [Google Scholar]
- Mendoza-de Gives, P. Soil-Borne Nematodes: Impact in Agriculture and Livestock and Sustainable Strategies of Prevention and Control with Special Reference to the Use of Nematode Natural Enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef]
- Hernández Malagón, J.Á.; Filipa Cazapal-Monteiro, C.; Bonilla Quintero, R.; Miguel Palomero Salinero, A.; Isabel Silva Torres, M.; Voinot Messnier, M.; Vilá Pena, M.; Romasanta Blanco, Á.; Pedreira García, J.; Paz Silva, A.; et al. Advantageous Fungi against Parasites Transmitted through Soil. In Fungal Infection; Silva de Loreto, É., Simoni Moraes Tondolo, J., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-468-8. [Google Scholar]
- Palomero, A.M.; Cazapal-Monteiro, C.F.; Valderrábano, E.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Soil Fungi Enable the Control of Gastrointestinal Nematodes in Wild Bovidae Captive in a Zoological Park: A 4-Year Trial. Parasitology 2020, 147, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.M.; Allanson, M.; Kwa, B.; Azizan, A.; Izurieta, R. Morphological Changes of Ascaris Spp. Eggs During Their Development Outside the Host. J. Parasitol. 2012, 98, 63–68. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).