Abstract
The long-term sustainability of ex situ animal populations requires coordination across facilities through cooperative breeding programs. Here, we investigate the reasons for inconsistent reproductive success in the zoo-based North American Asian small-clawed otter (ASCO; Aonyx cinereus) population. Reproductive viability analysis (RVA) was used to identify which characteristics of ASCOs in breeding pairs were most predictive of reproductive success. The RVA identified pair type, contraception history, and age as the most significant predictors of offspring production. The use of deslorelin in males and long-term deslorelin use in females hinder future reproductive potential and should, therefore, be considered carefully in genetically valuable individuals and potential breeders. Moreover, genetically valuable animals should be paired with younger mates, as advancing male and female age decreases the likelihood of success. The lack of reproductive success observed after 1 year of attempted breeding among new pairs provides evidence of potential mate incompatibility, therefore, population managers should consider splitting up pairs that remain unsuccessful over time, because the likelihood of offspring production is low. Lastly, the inclusion of dens and/or caves and pools designed with ample shallow water areas in ASCO habitats may improve breeding success.
1. Introduction
In order for facilities accredited by the Association of Zoos and Aquariums (AZA) to support their mission-oriented goals, it is critically important to manage the animal populations in their care for long-term sustainability [1,2]. This requires cooperation and coordination across holding institutions to facilitate the genetic and demographic management of these populations through breeding and transfer recommendations. Unfortunately, despite ongoing efforts, some zoo-based animal populations are not considered sustainable, i.e., they are not currently meeting their target genetic and demographic goals [3]. In some cases, this is due to as-yet-unidentified factors leading to low and/or variable rates of reproduction [4,5]. Identifying these factors has become a priority for many animal programs in order to improve the reproductive outcomes from future breeding and transfer recommendations. This study investigates the reasons for inconsistent reproductive success in the Asian small-clawed otter (Aonyx cinereus) Species Survival Plan® (SSP) population, managed among AZA facilities.
The Asian small-clawed otter (ASCO) is one of five otter species currently cared for in AZA facilities and is native to the coastal swamps and wetlands of Southeast Asia, China, Indonesia, and the Philippines [6,7,8,9,10,11,12]. The main physical traits of otters are their dense and waterproof fur, webbed feet, and long and sinuous bodies, which are adaptations to living in rivers, lakes, wetlands, and/or marine environments. ASCOs are the smallest otter species and are distinct from other otters in several ways, including their very short claws, as well as dexterous fingers, which are beneficial when searching and foraging for food [8].
Reproductive strategies also differ greatly across otter species. For example, North American river otters (Lontra canadensis) are monestrous, seasonal breeders, are primarily induced ovulators (yet capable of spontaneously ovulating), and experience delayed implantation [13,14]. In contrast, ASCOs are polyestrous, non-seasonal breeders, and are spontaneous ovulators, without delayed implantation [13,15]. There are few published studies on wild ASCOs; thus, most of what is known about their reproduction comes from studying otters in human care, as has been described in Reed-Smith et al., 2009. Briefly, the estrous cycle in ASCO lasts approximately 30–37 days, with an estrus length of anywhere from 1 to 13 days [15]. While the onset of estrus does not appear to be stimulated by any significant environmental cues, it may be associated with increased rubbing and marking behaviors [12,15]. ASCOs form monogamous breeding pairs, and a bond must be established for successful reproduction [15]. The male will pursue the female and court her for 1–3 days [15]. The pair will often copulate in shallow water, but will occasionally do so on land as well, and a single copulation generally lasts 5–25 min [12,15]. The gestation period for ASCO is 60–74 days and, as the end of gestation approaches, both the male and female will start building a nest [12,15]. In situ, these nests are often built by digging burrows that end in a cave-like den in the muddy banks of the rivers where they live, with the otters gathering dry grass, reeds and/or leaves for bedding materials [8]. In zoos, otters will similarly gather available substrates to create their nests. The female will then give birth to a litter of generally 1–7 pups [12,15]. Both the male and female take part in rearing their offspring [15]. Older siblings also assist in raising pups; therefore, previous offspring should be housed with their parents, though the presence of offspring can sometimes interfere with copulation attempts [15]. Additionally, as the group size becomes too large (relative to habitat space), aggression may increase as a result of over-crowding, at which point it may be more beneficial to remove older offspring [15].
A growing ASCO population has been managed in North America since AZA facilities began a zoo-based breeding program in the 1970s [16]. The population went through a period of particularly rapid growth between 2004 and 2009, increasing from 125 to 206 animals. Without a similar increase in SSP program participation by new facilities, the program began using the contraceptive implant Suprelorin® (deslorelin acetate) to manage reproduction, as well as to control aggression, in both sexes. Suprelorin® is a GnRH agonist with a short-term stimulatory phase, followed by the down-regulation of the hypothalamic–pituitary–gonadal (HPG) axis, which results in reproductive suppression [17,18,19], but the duration of efficacy can be highly variable. Given that reversal from deslorelin treatment has been unpredictable, the complex social dynamics of ASCOs [15], and the reported lack of institutional interest in holding this species [16], the demographic management of this population has been more challenging in recent years.
The AZA Reproductive Management Center (RMC) developed reproductive viability analysis (RVA) as a tool to identify factors that correlate with successful reproduction among breeding pairs, with a focus on the inherent biological and reproductive characteristics of each individual in a breeding pair, as well as the attributes of the pairs themselves [20]. The RVA process identifies predictors of reproductive success based on the past performance of breeding pairs in the population, using statistical modeling techniques that consider multiple individual and breeding pair attributes simultaneously, in order to identify which characteristics of animals in breeding pairs are most predictive of reproductive success. Identifying these factors can inform population managers on best practices for population management [20,21,22], leading to an improvement in genetic diversity and more stable demographics over time [23].
This study presents the results of an RVA for the ASCO population managed within the AZA, identifying the factors that are driving reproductive success in this population and are thus the most important to consider when making future breeding recommendations. We hypothesized that offspring production would decrease with advancing female age, as has been previously documented for tigers (Panthera tigris) [22], red wolves (Canis rufus) [24,25], fennec foxes (Vulpes zerda) [20,23], and, more recently, Tasmanian devils (Sarcophilus harrisii) [21]. Given the need for the male and female to form a strong bond prior to copulation, we also anticipated that established breeding pairs would have greater reproductive success than new pairs. In addition, we hypothesized that environmental factors may influence breeding success, as has been previously indicated for giant river otters (Pteronura brasiliensis) [26]. Information on animal habitats is not traditionally included in an RVA [20], but for the ASCO, information about habitats was also collected and incorporated into a modified RVA in order to make recommendations for habitat design that may improve breeding success. Lastly, as contraception has been commonly used in this population, but reversal from treatment has reportedly been unpredictable, the time to breeding success post-contraceptive treatment was also investigated.
2. Materials and Methods
2.1. Reproductive Viability Analysis (RVA)
A breeding and transfer plan (BTP) for the ASCO SSP has been published every 1–4 years since 2001, outlining which individuals are to breed, who they are to breed with, and which individuals should not breed. The RVA utilized data from ten BTP periods (2001–2019), across which there were 195 breeding attempts made among 32 facilities within the AZA-managed ASCO population. Among the 195 breeding attempts, there were 88 unique pairings, made up of 61 male and 72 female individuals (Table 1). It should be noted that some of these attempts may have been the result of interim recommendations that were not captured in the BTPs, some pairs may not have been recommended to breed, and some pairs were bred multiple times within a BTP period. Sarah Duncan is the current program coordinator and studbook keeper for the ASCO SSP and was the primary contact with AZA facilities to make interim breeding recommendations and collect information on which breeding recommendations outlined in the BTPs were actually attempted.

Table 1.
Sample sizes for the RVA and contraception reversal analyses, specifically for pair type, inbreeding, and contraception variables.
Table 2 (attributes of pairs) and Table 3 (attributes of individuals) present the variables used in the RVA, along with their definitions and data sources. The binary response variable (“Success”) for the RVA models was the reproductive success (or failure) for each breeding pair, based on whether offspring were produced (including stillborn offspring) prior to the end of the breeding attempt. The length of the breeding attempt was calculated with the best information available for a pair. For some pairs, mate access date information was taken from the RMC’s contraception database or other animal records (e.g., SSP program leader notes or a facility’s husbandry notes). However, in some cases, assumptions were made regarding the start and end dates of breeding attempts. For example, a breeding attempt could not start until both otters were at the breeding location, and a breeding attempt ended if one of the otters was transferred away or died. In cases where the two otters were already at the same location without a prior breeding recommendation, the date of the first published BTP that included a recommendation for the pair to breed was assigned as the start date if no other mate access information was available. For experienced pairs (see “PairType”, Table 2) for which there was no indication of separation or contraception used between litters, the previous parturition date was used for the start date of the next breeding attempt. The start dates for breeding attempts were also never assigned earlier than the anticipated expiration dates of contraceptives. In those cases, a breeding attempt “started” when the contraceptive was either discontinued (daily oral treatments) or removed (surgical removal of non-expired implants), or it was on the anticipated expiration date based on the RMC’s recommended retreatment intervals (implants: melengestrol acetate = 2 years, deslorelin acetate = 6 months (4.7 mg) or 12 months (9.4 mg), and injections: medroxyprogesterone acetate = 2 months). Breeding attempts that were still ongoing at the end of the study, which was determined as the date the 2020 BTP was published, were assigned 23 September 2020 as their end date. The elapsed time between the assumed start and end dates of the breeding attempts (or birth of a litter) was the variable “AttemptTime”. The distributions of individual levels of inbreeding for both males and females were zero-inflated: only 16 of 61 males (26.2%) and 26 of 72 females (36.1%) had non-zero inbreeding coefficients; therefore inbreeding was treated as a binary variable (see “MHasInbreeding” and “FHasInbreeding”, Table 3).

Table 2.
Description of variables that were attributes of pairs used in the RVA with definition and data source information.

Table 3.
Description of variables that are attributes of individuals used in the RVA with definitions and data source information.
Survival analysis was performed to investigate which variables may have affected the probability of reproductive success over time. This statistical method was chosen in order to control for the length of time of the breeding attempt. The analysis was performed in SAS® Studio (Release: 3.8 (Enterprise Edition), Copyright © 2012–2018, SAS Institute Inc., Cary, NC, USA). Survival analysis involved the modeling of “time to event” data. In the case of RVA, reproductive success is considered the “event”. For breeding pairs that are not reproductively successful, the data become “censored” at the time the breeding attempt ends. The time to reproductive success (i.e., the survival function) was modeled using the Kaplan–Meier method (PROC LIFETEST). A separate survivor function was estimated for each pair type, “New” and “Experienced” (see “PairType”, Table 2), and a chi-square test was performed to test for the homogeneity of those survivor functions. “Carryover” (N = 1) and “Unknown” (N = 4) pair types were excluded from the survival analyses.
Survival analyses were then run separately for new and experienced pairs, as some of the factors of interest that may have affected the time to reproductive success were confounded with PairType (i.e., AtLocation, IntSuccess5B, and PairParity). Additionally, the factors that were most important in predicting reproductive success in a new pair may have been very different to those for experienced pairs. For these analyses, a Cox proportional hazards regression analysis was performed (PROC PHREG) to account for the effects of both quantitative and categorical factors on the “time to event” model. Initially, the data were analyzed using a full model (i.e., including all the variables from Table 2 and Table 3). However, in order to increase the statistical power within the survival analyses, additional statistical analyses (described below) were used to provide insight into which factors explained the greatest amount of variability in reproductive success among pairs to justify variable reduction. The final survival analysis models were then reduced to include fewer factors informed by those analyses. When these reduced models were used, the fit of the Cox proportional hazards regression analysis models improved; however, the overall results of the survival analysis did not change. It should be noted that individuals and pairs of otters may appear more than once in the datasets for new and experienced pairs, respectively; therefore, there was some lack of independence in breeding attempts that was not accounted for within the survival analysis (Table 1).
Chi-square tests of association were performed for all categorical variables, specifically to investigate the association between the different levels of each factor and reproductive success. t-tests were used to investigate possible differences in the mean ages of individuals in successful vs. unsuccessful pairs, as well as the mean age difference between males and females in successful vs. unsuccessful pairs. Prior to running the t-tests, the age variables were examined for normality. It should be noted that the distribution of the age difference between the male and female (calculated variable: DiffAge) was positively skewed; therefore, results from a t-test may not be valid. In addition, these tests do not control for any confounding variables, nor do they control for the length of time of the breeding attempt; therefore, these results were primarily used for variable reduction of the survival analysis models.
Conditional random forest (CRF) analyses and least absolute shrinkage and selection operator (LASSO) logistic regression analyses were also used to provide additional insight into which factors explained the greatest amount of variability in reproductive success among pairs (see Bauman et al., 2019 for a detailed description of CRF and LASSO regression techniques for RVA within R). All variables listed in Table 2 and Table 3, excluding AttemptTime, were included in the CRF and LASSO models for new pairs. The models for experienced pairs also excluded AtLocation, IntSuccess5B, and PairParity due to a lack of variability in those factors among this pair type. For both CRF and LASSO, 1000 iterations of the models were run for each pair type. The performance of each iteration was measured using the area under the receiver operating characteristic curve (AUC): AUC values range from 0 to 1, with a value of 0.5 expected as being the same as random chance and values close to 1 representing high accuracy in predicting outcomes [28].
2.2. Contraception Use and Reversals
Of the 195 ASCO breeding pairs in the RVA, there were 69 pairs in which either the female only (N = 49), the male only (N = 4), or both individuals (N = 16) had a prior history of contraceptive treatment for either reproductive suppression or behavioral aggression (Table 1). The contraceptives used in females included melengestrol acetate (MGA) implants, deslorelin (Suprelorin®) implants, medroxyprogesterone acetate (Depo-Provera®) injections, oral megestrol acetate (Ovaban® or Megace®), or a combination of methods. The contraception methods used in males included deslorelin implants only or deslorelin plus reversible vasectomy.
Contraception reversal was defined as producing offspring (live or stillborn) for the first time post-contraceptive treatment. The reversal rates for MGA and deslorelin implants were calculated based on the number of reversals out of the total number of breeding attempts with the potential to end in a reversal (Table 1). Only breeding attempts with females who were treated with MGA implants only (N = 12 pairs, 11 unique females not yet reversed from treatment) and deslorelin only (N = 17 pairs, 15 unique females not yet reversed from treatment) were analyzed, as all other cases involved a combination of contraceptive products (N = 6 pairs, in various combinations). Only breeding attempts with males who were treated with deslorelin only, who had not yet reversed since treatment (N = 13 pairs, 9 unique males) were analyzed; only a single male was treated with both deslorelin and a reversible vasectomy.
Survival analysis (also performed within SAS®) was used to investigate the probability of contraceptive reversal over time. In this case, contraceptive reversal is considered the “event”. For breeding pairs that were not reproductively successful, the data become “censored” at the time the breeding attempt ends, as it is possible the individual may still reverse, given more time. The time to “event” or “censor” was the amount of time between the contraception stop date (as described previously) and “event” or “censor”, regardless of whether or not mate access began immediately post-contraception. The time to reversal was modeled using the Kaplan–Meier method (PROC LIFETEST). For females, a separate survivor function was estimated for each implant type (i.e., MGA and deslorelin) and a chi-square test for the homogeneity of survivor functions was performed. The number of successive treatments and the animal’s age at the start of the breeding attempt were also included as covariates and tested for any association with contraception reversal. Survival analyses were then run separately for MGA and deslorelin implants. Separate survivor functions were estimated for each implant type for cases in which implants were removed vs. cases in which expired implants were left in place. Chi-square tests for the homogeneity of the resulting survivor functions were performed.
2.3. Habitat Complexity and Dimensions Surveys
Two surveys were distributed to ASCO SSP facilities with data included in the RVA (N = 32) in an attempt to understand how habitat complexity (e.g., substrates and features), design, and size (e.g., land and water dimensions) may also influence breeding success. Initial habitat survey questions were generated based on pre-study discussions with S. Duncan (SSP program coordinator). Twenty-four facilities completed the initial habitat survey in 2016 (Supplementary File S1), which asked facilities to indicate all of the substrates their main ASCO habitat had (from a list of 11 substrates: grass, mulch, sand, clay, soil, rocks, boulders, pebbles, leaves, concrete, and gunite), and all of the features present in the habitat (from a list of 16 features: den(s), cave(s), climbing structure(s), bushes, deadfall, hollow log(s), trees, tree and root systems, waterfall(s), floating log pile(s), rafts, island(s), varied levels, sleeping places, live plants, and nest boxes). The follow-up survey in 2019 (Supplementary File S2), completed by 30 facilities, asked the facilities to provide counts and specific dimensions for both the land and water areas of all of the living spaces to which the ASCOs had access. The dimensions provided by the facility (via the survey; see Supplementary File S2) were used to calculate our best estimates of the area (m2) of the largest living space/habitat, land-to-water ratio of the largest living space/habitat, maximum depth of the deepest pool (m), as well as the area (m2) and the volume (liters) of the largest pool. The follow-up survey also asked for the pool entry type (i.e., gradient, step, or drop-off) and the habitat type (i.e., mixed species and/or rotational exhibit). Twenty-three of the thirty-two facilities completed both habitat surveys.
Many of the habitat variables generated from the habitat surveys (e.g., substrates and features) were confounded with one another (e.g., the feature “live plants” was most often also present with the feature “trees”). Therefore, in order to investigate any possible associations between ASCO habitat components and reproductive success, we first used principal component analysis (PCA) to develop a subset of habitat factors that explained the majority of the variation in habitat complexity and size that could then be used as independent factors in other analyses. Habitat substrates or features that were not present and/or absent at five or more facilities (i.e., minimum N = 5) were not included in the PCA (i.e., clay, rocks, climbing structure(s), rafts, island(s), sleeping places, and nest boxes). For the PCA, the presence of a substrate or feature was indicated by a “1” and absence was indicated by a “0”. Additionally, if the total number of substrates in a habitat was >6, then it was scored “1”, else “0”; if the total number of features was >8, it was scored “1”, else “0”; if the facility had >1 pool over 0.61 m deep, it was scored “1”, else “0”; and if the facility had >3 living areas for ASCOs, then it was scored “1”, else “0”. These conversions were performed by grouping based on natural gaps in the data set. The quantitative variables calculated from the habitat dimensions survey were also converted into binary variables prior to PCA due to the lack of normality in the distribution of these variables, which quite often had a bimodal distribution, eliminating data transformation as an option. If the maximum depth of the deepest pool was >1.25 m, then it was scored “1”, else “0”; if the volume of the largest pool was >20,000 L, then it was scored “1”, else “0”; if the area of the largest pool was >20 m2, then it was scored “1”, else “0”; if the largest living area was >55 m2, then it was scored “1”, else “0”; and if the land-to-water ratio of the largest living area was >3 (more than 75% of the habitat is land), then it was scored “1”, else “0”.
PCA was performed 7 times, grouping habitat components by (1) substrates, (2) water features, (3) dens/caves, (4) habitat type, (5) plants, (6) habitat complexity, and (7) size, in order to create independent factors that would have meaningful interpretations, since our goal was to provide recommendations for habitat modifications that might improve reproductive success. The eigenvalue is a measure of how much of the variance of the observed variables a principle component (PC) explains. Any PC with an eigenvalue of ≥1 explains more variance than a single observed variable. Therefore, PCs with eigenvalues of ≥1 were retained as habitat factors and the factor patterns, or loadings, were examined for interpretation. The PC scores for each habitat factor for each facility were then appended to the breeding pair dataset so they could also be included in the RVA. Not all facilities with breeding pairs included in the initial RVA completed both habitat surveys (only 23 of 32 facilities); therefore, not all facilities had PC scores for every habitat factor. Breeding pairs without PC scores for any habitat factors had to be excluded from the modified RVA, thus reducing the size of the dataset for the modified RVA to 157 breeding attempts (from the original 195). Chi-square tests of association were performed for all categorical habitat variables, specifically to investigate the association between the different levels of each factor and reproductive success. Similarly, t-tests were used to compare quantitative habitat variables and PC scores between successful and unsuccessful pairs. The CRF, LASSO regression, and survival analysis (reduced model only) were also repeated as described above, with the addition of the retained habitat factors as explanatory variables, using the modified dataset.
3. Results
Of the 195 breeding attempts made during the ten BTP periods between 2001 and 2019, 110 attempts (56.4%) successfully resulted in offspring (live or stillborn).
3.1. Variable Reduction Analyses
3.1.1. Chi-Square Tests of Association
Among new pairs, there was a significant association between male contraception history (i.e., MContraceptWPriorReversal) and reproductive success (Χ2 = 8.44, p = 0.0037); 59.2% of new pairs in which the male had no prior history of contraceptive use were successful (N = 42/71), whereas only 15.4% of new pairs in which the male was previously contracepted (and had yet to produce offspring post-treatment) were successful (N = 2/13). There were no new pairs in which the male had already reversed from prior contraception use. No other associations between breeding pair characteristics and breeding success were identified by the chi-square tests for new pairs.
Among experienced pairs, we found a significant association between female contraception history and reproductive success (Χ2 = 6.35, p = 0.0417). Experienced pairs in which the female had no prior history of contraceptive use were successful 68.0% of the time (N = 51/75) compared with only 41.9% of experienced pairs in which the female was previously contracepted (N = 13/31). A significant association was also found between individual levels of inbreeding in females (i.e., FHasInbreeding) and success, such that having any level of inbreeding (F > 0) was associated with less reproductive success (Χ2 = 3.84, p = 0.0499).
3.1.2. t-Tests
There were no significant differences observed between successful and unsuccessful new pairs for any of the age-related variables. However, there was a trend for male age, such that males in successful pairs were younger, on average, at the start of the breeding attempt as compared with males in unsuccessful pairs, 6.9 ± 3.7 years old vs. 8.4 ± 4.2 years old, respectively (t = 1.78, p = 0.0782). Among experienced pairs, females in successful pairs were younger, on average, at the start of the breeding attempt compared with females in unsuccessful pairs, 6.7 ± 2.5 years old vs. 7.8 ± 3.2 years old, respectively (t = 2.08, p = 0.0395). There was a similar trend for male age, 7.3 ± 3.7 years old vs. 8.6 ± 4.1 years old, for successful and unsuccessful experienced pairs, respectively (t = 1.72, p = 0.0881). When new and experienced pairs were pooled into a single data set, the trend observed between male age and reproductive success became statistically significant (t = 2.44, p = 0.0154).
3.1.3. Conditional Random Forest (CRF)
On average, the CRF models for both new and experienced pairs were considered “poor”, as the average AUCs were 0.61 and 0.60, respectively [29]. Only variable importance scores for iterations with an AUC > 0.7 (considered “acceptable”) were used to identify which factors may be most important in predicting the success of ASCO breeding pairs. For experienced pairs, the acceptable CRF models (n = 148 iterations) indicated that female contraception history, female age, male age, and a female’s individual level of inbreeding were predictive of reproductive success (Figure 1A). For new pairs (n = 195 iterations), only male age and male contraception history were predictive of reproductive success (Figure 1B).
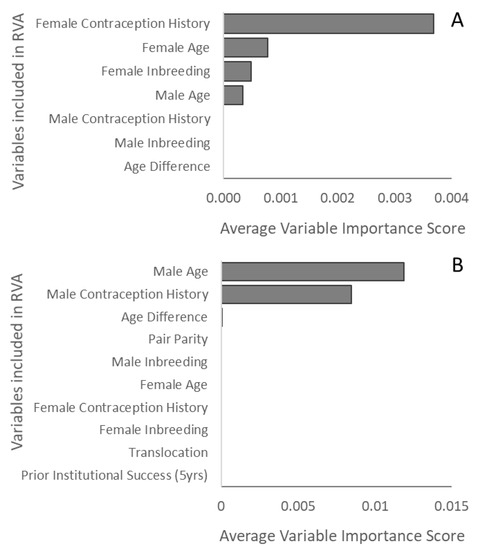
Figure 1.
Average variable importance scores for predicting reproductive success across iterations (ranked “acceptable” or higher) for the conditional random forest models in (A) experienced and (B) new ASCO breeding pairs.
3.1.4. LASSO Regression Analyses
Similar to CRF, the LASSO regression models would be considered “poor”, as the average AUCs were 0.57 and 0.58 for new and experienced pairs, respectively [29]. Therefore, only regression coefficients for iterations with AUC > 0.7 (considered “acceptable”) were evaluated for their relationship with the reproductive success of ASCO breeding pairs. The proportion of acceptable iterations in which each variable included in the RVA had a non-zero regression coefficient are presented in Table 4 and Table 5 for new and experienced pairs, respectively. Variables that were found to be significantly associated with reproductive success in at least 20% of iterations (in any combination) for new pairs included female age, male age, the age difference between the two individuals, and the male’s contraception history (Table 4). Likewise, the variables significantly associated with reproductive success among experienced pairs included female age, male age, the female’s individual level of inbreeding, and the female’s contraception history (Table 5).

Table 4.
Average LASSO regression coefficients resulting from iterations for new pairs with AUC > 0.7 (N = 44). Bold text indicates variables (or variable levels) that were either positively or negatively associated with reproductive success, relative to the reference level (with 95% confidence), and with non-zero coefficients in greater than 20% of iterations.

Table 5.
Average LASSO regression coefficients resulting from iterations for experienced pairs with AUC > 0.7 (N = 72). Bold text indicates variables (or variable levels) that were either positively or negatively associated with reproductive success, relative to the reference level (with 95% confidence), and with non-zero coefficients in greater than 20% of iterations.
For both pair types, increasing male and female age was associated with a decrease in breeding success. A larger age difference between the male and female was also associated with a decrease in breeding success among new pairs. Lastly, new pairs in which the male had never been contracepted had a higher probability of reproductive success than pairs in which the male was previously contracepted, but was yet to produce offspring post-treatment. Among experienced pairs, a decrease in breeding success was associated with any level of inbreeding in the female. Additionally, experienced pairs in which the female had previously been contracepted, but had not yet produced offspring post-treatment, had lower breeding success compared with pairs in which the female had no history of contraception use.
3.2. Reproductive Viability Analysis (RVA)
3.2.1. Survival Analyses—Full Models
The survival functions for new and experienced pairs were significantly different (Χ2 = 10.48, p = 0.0012); thus, the probability of reproductive success after a given amount of time (i.e., breeding attempt time) was different depending on whether the pair was new or experienced (Figure 2). After 6 months together, approximately 23.7% of experienced pairs had repeat success, and 21.5% of new pairs produced offspring together for the first time. After 1 year together, only 45.1% of new pairs were successful, yet 69.1% of experienced pairs produced additional offspring. This disparity became even larger after 2 years of attempted breeding: only 49.8% of new pairs had success vs. 79% of experienced pairs. The average breeding attempt time (±SD) to reproductive success among experienced pairs that produced offspring was 0.62 ± 0.3 years (compared with 0.97 ± 0.8 years for unsuccessful experienced pairs). The average time to reproductive success among new pairs that were successful was 0.81 ± 0.8 years, which was not significantly different from that of experienced pairs, but unsuccessful new pairs were left together for 2.39 ± 1.5 years on average, without ever producing offspring, which was significantly longer than that of the other groups (F6,188 = 19.73, p < 0.0001).
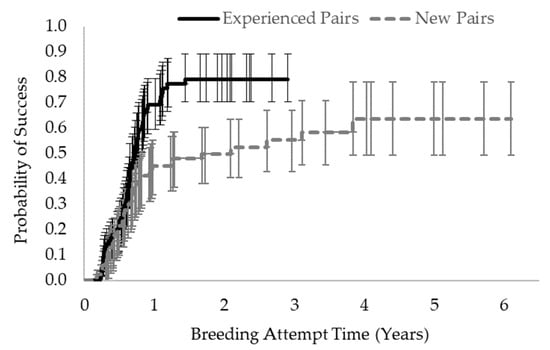
Figure 2.
Probability of reproductive success (i.e., live or stillbirth offspring produced) over time for ASCO breeding pairs by pair type. Error bars indicate the 95% confidence intervals around the probability estimates. Experienced pairs had a significantly higher likelihood of reproductive success than new pairs, particularly after one year of attempted breeding (Χ2 = 10.48, p = 0.0012).
For new pairs, survival analysis indicated that male contraception history was the only variable that significantly affected the probability of breeding success over time (Χ2 = 5.95, p = 0.0147). Pairs in which the male had a prior history of contraceptive use (without prior reversal) had a significantly lower probability of breeding success over time compared with pairs in which the male had never been treated (Figure 3). In contrast, male age was the only variable that significantly affected the probability of breeding success over time among experienced pairs (Χ2 = 4.03, p = 0.0447), such that as the male’s age at the start of the breeding attempt increased, the probability of reproductive success of the pair decreased.
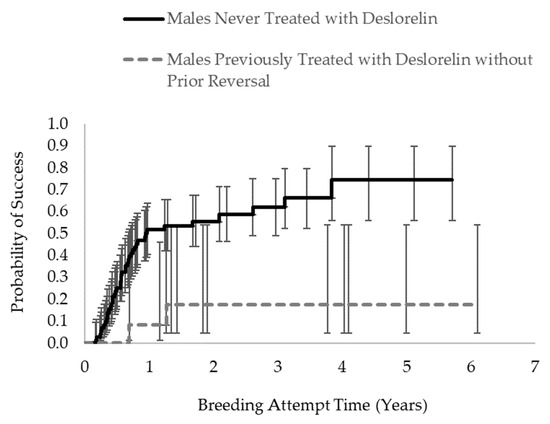
Figure 3.
Probability of reproductive success (i.e., live or stillbirth offspring produced) for new ASCO breeding pairs over time. Error bars indicate the 95% confidence intervals around the probability estimates. Pairs in which the male had never been previously treated with deslorelin had a significantly higher likelihood of reproductive success than pairs in which the male had been previously treated with deslorelin (Χ2 = 5.95, p = 0.0147).
3.2.2. Survival Analyses—Reduced Models
In order to increase our statistical power within the Cox proportional hazards regression analysis, the results from the chi-square tests of association and t-tests, as well as the CRF and LASSO regression analyses (summarized in Table 6), were used to select a subset of variables to include in the final survival function models. The reduced model for new pairs included female age, male age, the age difference between the male and female, and male contraceptive history. The reduced model for experienced pairs included female age, male age, the age difference between the male and female, female contraceptive history, and whether or not the female had a non-zero inbreeding coefficient. Though the overall fit of the survival analysis models improved, the conclusions remained the same as those of the full models: the only significant factors affecting reproductive success over time among new and experienced pairs were male contraception history and male age, respectively (Χ2 = 5.67, p = 0.0173 and Χ2 = 6.15, p = 0.0131, respectively).

Table 6.
Factors significantly associated with breeding success among new and experienced ASCO breeding pairs across various analyses. Statistical significance was declared where p < 0.05.
3.3. Contraception Use and Reversals
When looking at ASCO females who were contracepted with MGA or deslorelin implants only, the contraception reversal rates were 54.5% (N = 6/11) and 53.3% (N = 8/15), respectively (Table 7). Among only the animals that reversed, the median time (±SE) to reversal post-MGA implant treatment (based on the survival function) was 1.95 ± 0.73 years (minimum 0.50 years) and the median time to reversal post-deslorelin treatment was 2.05 ± 0.29 years (minimum 0.82 years). This difference in time to reversal between the two implant types was not statistically significant.

Table 7.
Contraception reversal rates for ASCO treated with MGA implants (females) and deslorelin implants (females and males) only, by number of successive treatments (i.e., implants) and implant removal.
The number of successive deslorelin implant treatments in females was significantly associated with time to reversal, such that more successive treatments prior to attempted breeding were associated with a longer time to reversal (Χ2 = 4.33, p = 0.0375, Figure 4A). Additionally, deslorelin implant removal was also significantly associated with time to reversal: the removal of the implant(s) significantly reduced the time to reversal (Χ2 = 5.32, p = 0.0210, Figure 4B). In contrast, there was no association between the number of successive implants nor implant removal and the time to reversal for females treated with MGA implants. However, the number of animals who received multiple MGA implants and the number of individuals who never had their MGA implants removed were small; therefore, the ability to detect these potential effects was limited (see Table 7). A female’s age at the start of the breeding attempt was not significantly associated with time to reversal for either contraceptive implant type.
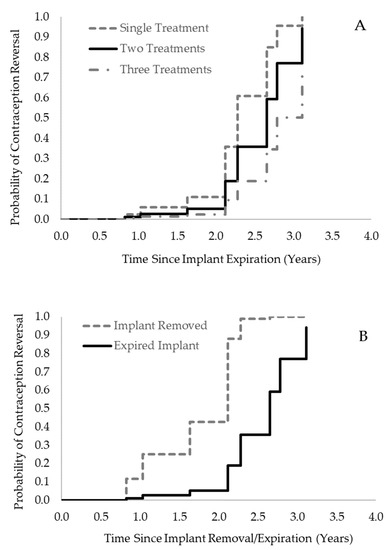
Figure 4.
Probability of contraception reversal (i.e., reproductive success) over time after contraception stop date (implant removal or anticipated expiration date) for ASCO females following deslorelin implant treatment(s). (A) Females treated with successive deslorelin implants prior to attempted breeding had increased time to reversal (Χ2 = 4.33, p = 0.0375). Survival curves presented are for cases in which implants were not removed. (B) Removal of the implant(s) significantly decreased time to reversal (Χ2 = 5.32, p = 0.0210). Survival curves presented are for cases in which females were treated with two deslorelin implants in succession preceding their breeding opportunity.
Among males, the contraception reversal rate post-deslorelin treatment was 33.3% (N = 3/9, Table 7) and the median time (±SE) to reversal was 2.40 ± 0.99 years (based on the survival function, minimum 0.84 years). The male’s age at the start of the breeding attempt, the number of deslorelin implant treatments, and implant removal were not associated with time to reversal.
3.4. Habitat Complexity and Dimensions Surveys
3.4.1. Dimensionality Reduction Using Principal Component Analyses
Table 8 details which habitat components were included in each PCA, the final number of habitat factors generated by each, the names and general interpretation of each new factor, and the percentage of total variation in those habitat components (e.g., substrates, features, etc.) explained by the retained habitat factors.

Table 8.
Habitat components included in each PCA, the final number of habitat factors generated by each (number of PCs with eigenvalues ≥ 1), names and general description for each new factor, and the percentage of total variation in those habitat components explained by the retained habitat factors.
3.4.2. Influence of Habitats on Breeding Success
When investigating the association between reproductive success and each habitat variable separately (without consideration of other factors), the only factor that was found to be predictive of reproductive success among new ASCO pairs was the type of entry into the pool, more specifically pool entries with steps (Table 9). New pairs in habitats with step pool entry types were less likely to be successful than pairs in habitats with gradient and/or drop-off pool entry types (33.3% successful, N = 7/21 vs. 59.6% successful, N = 28/47, respectively, Χ2 = 4.00, p = 0.0455). Among experienced pairs, the presence of sand substrate (vs. absence) was associated with greater reproductive success (76.9% successful, N = 30/39 vs. 52.0% successful, N = 26/50, respectively, Χ2 = 5.83, p = 0.0157), the presence (vs. absence) of floating log piles in the habitat was negatively associated with reproductive success (42.11% successful, N = 8/19 vs. 68.6% successful, N = 48/70, respectively, Χ2 = 4.49, p = 0.0342), and successful pairs had access to more pools over 0.61 m deep, on average compared with unsuccessful pairs (t = −2.01, p = 0.0476). There were no significant differences in the mean PC scores between successful and unsuccessful pairs, new or experienced, for any of the habitat factors identified via PCA. However, among new pairs, there were trends between PC scores for the Water1 and Dens1 habitat factors and pair success. The PC scores for Water1 tended to be lower on average among successful pairs (mean PC score = −0.45) compared with unsuccessful pairs (mean PC score = 0.08, t = 1.63, p = 0.108). Low PC scores for Water1 were associated with a gradient pool entry type, and higher PC scores were associated with a step pool entry type. The PC scores for Dens1 tended to be higher on average among successful pairs (mean PC score = 0.15) compared with unsuccessful pairs (mean PC score = −0.24, t = −1.69, p = 0.0946). Higher PC scores for Dens1 were associated with the presence of dens and/or caves, and lower PC scores were associated with the absence of dens and/or caves.

Table 9.
Factors significantly associated with breeding success among new and experienced ASCO breeding pairs based on different analyses when using the modified RVA dataset (reduced N) and after the incorporation of habitat factors into the analyses (where applicable). Statistical significance was declared where p < 0.05.
When the CRF analysis was repeated to include the habitat factors retained after PCA, the factors identified as most predictive of reproductive success among experienced pairs did not change overall; however, their order of importance changed (Table 9). Similarly, the factors previously identified as important for predicting success among new pairs were still found to be significant, but four additional factors were also identified as important, including the age difference between the male and female, the presence/absence of dens and/or caves (Dens1), gradient vs. step pool entry type (Water1), and whether or not either the male or female (or both individuals) had to be moved between facilities to fulfill the breeding attempt.
When the habitat factors retained after PCA were added to the LASSO regression models, very few models were found to be acceptable or predictive of reproductive success (AUC > 0.7): 34 of 1000 models for new pairs and 14 of 1000 models for experienced pairs; therefore, the results should be interpreted with extreme caution. Based on only the models considered acceptable, using the same criteria as before (i.e., either positively or negatively associated, relative to the reference level, with 95% confidence, and had non-zero coefficients in greater than 20% of iterations), none of the original variables identified as predictive of reproductive success for both new and experienced pairs were found to be significant (Table 9). However, for new pairs, one habitat factor was identified as predictive of reproductive success: Plants1. Higher reproductive success was associated with lower PC scores for Plants1. Low PC scores for Plants1 were associated with a lack of live plants, trees, and tree and root systems in the habitat. No other factors met all criteria to be considered significant predictors of reproductive success.
In addition to the decrease in reproductive success among new pairs associated with contraception use in males (Χ2 = 7.15, p = 0.0075), two of the habitat factors, Water2 and Plants1, were also found to be significant when the length of the breeding attempt was accounted for (survival analysis). Higher PC scores for Water2 were associated with a higher probability of reproductive success, suggesting that a gradient pool entry (vs. a drop-off) and the presence of floating log piles positively influenced breeding success in new pairs (Χ2 = 5.89, p = 0.0152), and, in agreement with the LASSO results, higher reproductive success was associated with lower PC scores for Plants1, suggesting a negative influence of live plants, trees, and tree and root systems in the habitat on success among new pairs (Χ2 = 6.07, p = 0.0138). Among experienced pairs, in contrast with the survival analysis for the original/larger RVA dataset, it was female age, not male age, that was found to be associated with the probability of successful reproduction over time in the reduced dataset, but similar to male age, increasing female age decreased the probability of reproductive success (Χ2 = 5.92, p = 0.0149). Two additional habitat factors were also found to be significantly associated with breeding success: Water1 and HabitatType1, suggesting that a step pool entry type (vs. gradient) decreased breeding success (Χ2 = 4.70, p = 0.0302) and that experienced pairs housed in mixed species or rotational exhibitshave a lower likelihood of success than experienced pairs in a species-dedicated habitat (Χ2 = 4.15, p = 0.0416).
All the factors significantly associated with breeding success among new and experienced ASCO breeding pairs based on the different analyses when using the modified RVA dataset (reduced N) and after the incorporation of habitat factors into the analyses (where applicable) are summarized in Table 9. Male contraception history continued to stand out as a consistent predictor of reproductive success among new pairs, even when considering possible influences of the habitat. It appears that the most important aspects of the habitat to consider for new breeding pairs are the pool entry type, live plant features, and dens/caves, based on these factors appearing multiple times across different analyses. The presence of a step pool entry type, the presence of live plants, trees, and tree and root systems, and the absence of dens and/or caves may hinder reproductive success. The consideration of habitat factors appears to be less important overall for experienced pairs, and rather the male’s and female’s ages, and the female’s contraception history, were likely the most significant predictors of additional litters among established pairs.
4. Discussion
RVA is an exploratory analysis to determine the factors that are most predictive of reproductive success in ex situ animal populations. The results are not only informative for learning more about a species’ biology, but they can inform how zoo-based animal populations are managed, and when habitat features are considered, how habitats should be designed or modified to improve the effectiveness of population and reproductive management. Though multiple statistical approaches can be used to perform RVA, there are some factors that are often identified by multiple methods, which are likely the most important to consider when making future breeding recommendations.
4.1. Contraception
When it comes to establishing new breeding pairs for the ASCO SSP, it seems an important factor to consider is the male’s contraception history, as it was identified as important to, or always negatively associated with, the probability of breeding success by every statistical method used, even after the influence of habitat factors was considered (with the exception of the LASSO regression, which performed very poorly overall). This study is the first to look at the time to reproduction post-deslorelin treatment in ASCO. Our analyses show the time to reversal to be long and highly variable between males (0.84, 2.13, and 4.23 years until successfully siring offspring post-implant expiration, N = 3). In some cases, it appears that deslorelin implants last much longer than predicted, or that treatment may even result in permanent reproductive suppression, as six males failed to produce offspring, despite having breeding opportunities that were >3 years (up to 7.47 years) post-implant expiration, and in some cases, even when provided more than one potential mate (i.e., more than one breeding attempt). These results are similar to the findings of a study on male lion-tailed macaques, which showed potential permanent infertility in some, but not all, males after treatment with deslorelin [30]. Among other otter species, deslorelin has been documented to suppress reproductive hormones for 3–4 years in female sea otters (Enhydra lutris), and more than 2 and 5 years in two male sea otters [31]. Extended reductions in blood testosterone and testis size were also recorded for three additional male sea otters [32]. Therefore, the extended infertility observed in male ASCO post-deslorelin treatment may be present across additional otter species, and the AZA RMC is no longer recommending the use of deslorelin in ASCO males who may receive a future breeding recommendation. Extended infertility in males treated with deslorelin has also been documented in other taxa, including cheetahs [33], black-footed cats [32], and adult tomcats [34].
As the sample sizes were already small for investigating contraception reversal, we did not remove animals whose mates also had a history of contraception use. Therefore, the overall rates of reversal may be higher for individuals who are paired with a mate with no prior contraception use. Three of the six ASCO males who failed to reproduce post-deslorelin treatment were paired with a female (or females) who also had not produced any offspring post-contraceptive treatment, which is a potential confounding factor. However, the RVA did not find any association between female contraception history and reproductive success among new pairs; therefore, the female’s contraception history was unlikely to be the primary reason for the lack of reproductive success from these males. In contrast, the RVA did find the contraception history of the female to be potentially important for predicting breeding success among experienced pairs, though this effect was not statistically significant when the length of the breeding attempt was accounted for, which suggests that, despite deslorelin implants lasting longer than predicted, given enough time, female fertility does eventually return, unless females first reach the age of reproductive senescence, which appears to occur around age 15 [35].
The rate of reversal post-deslorelin treatment observed among female ASCOs in this study (53.3%, N = 8/15) is comparable to that reported for tigers (55.6%, N = 10/18) [36] and across various other taxa (62.5%, N = 10/16) [19]. Traditionally, deslorelin implants are placed between the scapulae. Unfortunately, this makes locating and removing the implants more challenging. However, implant removal can be facilitated using ultrasound [37]. The rate of reversal post-deslorelin treatment was higher (60.0%) among ASCO females from which deslorelin implant removal was confirmed vs. those females whose implants were left in place/expired or implant status was unknown (50.0%). Our analyses also indicate that the time to reversal post-implant treatment was faster among females who had their implants removed. In contrast to this study, there was no association found between implant removal and reversal in tigers [36]. Cowl et al., 2018, found that placing deslorelin in alternative sites, such as the base of the ear or forelimb, had equivalent contraceptive efficacy and a higher rate of reversal compared with the traditional placement site (83.3% reversal rate vs. 50% reversal rate, respectively) [19]. Therefore, one way to improve reproductive success post-deslorelin treatment in ASCO may be to place implants in alternative sites in anticipation of future removal, which may facilitate the ease of removal and decrease the time to reversal.
In addition to implant removal, this study found that the number of successive deslorelin implant treatments in females was significantly associated with time to reversal, such that more successive treatments prior to attempted breeding were associated with a longer time to reversal. To our knowledge, this is the first study to investigate the effect of deslorelin treatment duration (use of subsequent treatments) on reversal.
When considering the results of the RVA together with the information gained on contraception reversibility and time to reversal, it seems apparent that the use of deslorelin in ASCO males and long-term deslorelin use in females (multiple treatments) hinder future reproductive potential. Therefore, the decision to use deslorelin should be carefully considered in genetically valuable individuals and potential breeders, and should perhaps only be used after a pair has produced at least one litter. We also recommend reconsidering the use of deslorelin for aggression management in a single-sex group when individuals may receive a breeding recommendation in the future. In cases where contraception is still needed for females, the use of progestin-based products should be considered, such as MGA implants or oral megestrol acetate (Ovaban®), for occasional or short-term contraception needs within the female’s lifetime to try to minimize the effects of these reversibility-related issues on the reproductive success of breeding pairs. Long-term progestin use in any carnivore species is generally not recommended by the RMC, as it has been associated with endometrial hyperplasia, endometrial mineralization, and hydrometra leading to infertility, even after implant removal in zoo felids [38], an increase in the risk of mammary and endometrial cancers in felids [39,40], as well as endometrial hyperplasia and pyometra in canids [41]. We recommend contacting the AZA RMC for current guidelines prior to contraception use.
4.2. Age
The results of the ASCO RVA indicate that, aside from contraception history, both male and female age are likely the most important factors to consider when developing breeding recommendations for the SSP. For both males and females, reproductive success declined with age. Historically, ASCOs have been as old as 15.7 years (females) and 18.2 years (males) at the start of attempted breeding; however, 11.9 years was the oldest a female has been at the start of a breeding attempt that ended in reproductive success. Therefore, reproductive senescence may occur in many female ASCOs earlier than previously believed. In contrast, male ASCOs as old as 17.6 years old at the start of attempted breeding have been successful, though on average, successful males tend to be much younger. Similar to ASCOs, the RVA for the fennec fox SSP found female age to be one of the factors most significantly associated with reproductive success [20]. Among fennec foxes, the probability of a breeding pair being successful decreases as female age increases, with no offspring produced from females over age 6, even though females have been paired up to 13 years of age [20,23]. The trend also continues with tigers, where breeding success in female tigers declines after age 5 for both experienced and inexperienced breeders [22]. A decline in successful litter production as female age increases has also been documented in Tasmanian devils [21], as well as red wolves, despite being observed in copulatory ties [24,25]. A decline in reproductive success with male age, as observed here in male ASCO, has been similarly observed among fennec fox and Mexican wolf breeding pairs [20,23]; however, this has not been seen among male tigers [22]. Though a decrease in female fertility with age has been documented for many species, often citing issues of declining uterine health associated with age and reproductive management [42,43], additional studies are needed to elucidate the underlying factors related to decreased fertility and/or reproductive success in ASCO males with age.
While the average age difference between the male and female has been larger among successful versus unsuccessful pairs of Tasmanian devils, perhaps due to the importance of male size or experience [21], in ASCOs, the age difference between the male and female appears to be only a minimally important consideration for breeding success. Therefore, as genetically valuable animals (e.g., priority breeders) become older, they should be paired with younger mates, as advancing female and male age decreases the likelihood of success, yet the age difference between the male and female is not generally a concern.
4.3. Inbreeding
The other biological factor that was identified by the RVA as associated with the reproductive success of ASCO breeding pairs was the female’s individual level of inbreeding. In the SSP population, pairs in which the female had any level of inbreeding generally had lower reproductive success. The relationship between inbreeding and reproductive success (i.e., fitness) is not routinely investigated in SSPs, but we can speculate that a non-zero inbreeding coefficient estimated from the pedigree is positively correlated with an increase in homozygosity for some genes that are associated with fitness-related traits, resulting in phenotypes that are detrimental to fertility [44]. These types of effects have been documented among some ex situ populations in relation to males, specifically regarding inbreeding negatively affecting semen parameters, such as motility, sperm morphology, and acrosome integrity [45,46]; however, little is known about potentially similar effects in females (i.e., oocyte parameters).
Unfortunately, the small number of individuals with non-zero inbreeding coefficients in this study made it impossible to investigate how increasing levels of inbreeding, and levels of inbreeding higher than those observed in the population at the time of the study may affect breeding success. Though some inbreeding is often unavoidable in small populations, every effort should be made during the BTP process to minimize the creation of ASCO breeding pairs that would result in offspring with non-zero inbreeding coefficients. Therefore, the SSP may need to consider increasing the target size of the population to minimize the likelihood of (and decrease the rate of) inbreeding accumulation.
4.4. Transfers, Mate Compatibility, and Conspecifics
The RVA did not find any evidence that transferring ASCOs between facilities to form new breeding pairs hindered reproductive success. In fact, within the first 9 months of attempted breeding, new pairs had approximately the same probability of reproductive success as experienced pairs. It was not until about 1 year of attempted breeding that the difference in the probability of reproductive success between new pairs and experienced pairs became evident. This difference in the disparity between pair types over time is additional support that the overall lower reproductive success observed among new pairs is likely not related to possible stress-related issues resulting from relocation, or even prior reproductive experience, but rather may be an issue of mate incompatibility. A lack of an effect of translocation on breeding success has been similarly observed among other pair-bonding, monogamous carnivores, including fennec foxes [20,23], Mexican wolves [20], and red wolves [24]. In contrast, a previous analysis of breeding success among paired tigers (a solitary carnivore) found that, when both individuals were already at the location where the breeding recommendation was to take place, they were more likely to be successful [22].
Currently, breeding and transfer recommendations for ASCO are made with the goals of maintaining demographic stability, as well as preserving genetic diversity [47,48,49]. When population managers meet to develop breeding and transfer plans for a population, they create as many breeding pairs as needed to produce the estimated number of offspring needed to reach or maintain the target population size, and use pedigree analysis to choose which individuals will be paired in order to retain genetic diversity and minimize inbreeding. Unfortunately, this does not account or allow for mate choice, which can lead to some animals being paired together that are simply not compatible, leading to a failure to successfully produce offspring [50]. It is possible the disparity in reproductive success between new pairs and experienced pairs observed over longer breeding attempt times is evidence of this phenomenon among ASCO. Therefore, our management recommendation would be to consider splitting up new ASCO pairs that have not been successful after 1 year of attempted breeding, as the likelihood of success given additional breeding time is minimal and moving animals between facilities does not impact reproductive success. Giving individuals the opportunity to breed with a new mate is likely to be more successful than leaving them paired with an individual they are potentially incompatible with. For facilities that have multiple habitats available for ASCO, housing multiple pairs may provide opportunities for swapping mates, which could potentially allow for some degree of mate choice. However, this is likely dependent on the presence of additional offspring (if one pair has been successful), as the fission and fusion of ASCO groups is incredibly challenging and generally unsuccessful [51], which does impose some challenges and limits on the ability of the SSP to split and repair otters overall.
Regarding experienced pairs, it is generally recommended to house their prior offspring with them, so they can assist in rearing their younger siblings. However, their presence can sometimes interfere with copulation attempts for the sire and dam [15]. Our data sources did not include any information on the cohabitation of breeding pairs with their prior offspring, and this may explain some cases in which experienced pairs failed to produce additional pups, either due to direct interference with copulation or increased stress on the breeding individuals, perhaps as a result of overcrowding.
4.5. Habitat and Other Institutional Factors
Though factors related to animal habitats are not traditionally included in an RVA [20], we hypothesized that environmental factors may influence breeding success in ASCOs, as this has previously been documented among giant river otters [26]. Reproductive success among zoo-managed giant river otters has been linked to habitat size (≥287 m2), the proportion of the habitat that is water (≥47%), having a larger water area (≥50 m2), and vegetation (more variety is better), as well as limited exposure to the public [26]. When the information we gathered on ASCO habitats was incorporated into the RVA, there were no obvious correlations between habitat size and reproductive success for ASCO, as was also found for Tasmanian devils [21]. Instead, pool entry type, live plant features, and the inclusion of dens and/or caves appeared to be the most important aspects of the habitat to consider for future breeding success, particularly for new pairs. A step pool entry type (and absence of gradient pool entry type), the presence of live plants, trees, and tree and root systems, and the absence of dens and/or caves appeared to hinder reproductive success.
The type of entry into the pool in the habitat was the most consistent factor to appear across different analyses. This is not necessarily surprising, as copulation most often occurs in shallow waters in this species [15], which would most often be found at the interface between land and water (i.e., the edge of the pool). It could be inferred that, if there is not a generous amount of shallow water present in the habitat, copulation attempts may be less frequent or incomplete/unsuccessful. Gradient pool entry types with long shorelines would most likely provide the greatest amount of shallow water areas. This study found pairs in habitats with step pool entry types to be only half as successful as pairs in habitats with other pool entry types, and the presence of a gradient pool entry type was positively associated with reproductive success over time, supporting this theory. Therefore, pools in ASCO habitats should be designed with a gradient entry, as opposed to step or drop-off entries, to facilitate successful copulation and increase breeding success.
A variety of live plants are often used in ASCO habitats, as well as log piles, large tree stumps or root systems, hollow logs, etc., as they provide visual complexity and offer foraging, playing, and shelter opportunities for the otters [15]. In situ ASCOs prefer habitats with dense vegetation, as it provides cover and protection from predators, as well as concealed sites in which they can build their nests [11]. It is not clear why the presence of live plants, trees, and tree and root systems would be associated with a decrease in breeding success in zoos. It is possible that the presence of live plants is actually correlated with another factor that is the actual driver of lower reproductive success, but that we did not measure or account for in the surveys. It is also possible that this could be a spurious artifact of the low survey response rate (complete data from only 23 institutions). Regardless, this effect of plantings does not appear to be very strong based on our analyses; however, it is certainly another potential factor to consider if an institution continues to struggle with breeding success in this species, despite attempts with multiple pairs of animals.
General husbandry guidelines for otters indicate that all animals should be provided with dens and/or caves, which provide a choice and/or opportunity for the animals to sleep together or separately [15]. In situ, ASCOs often build their natal nests inside dens and/or caves [8]. Based on our habitat survey, only 30% of ASCO habitats contained a den, and only 25% contained a cave, and these were often not mutually exclusive. Our analyses found that the presence of a den and/or cave was positively associated with reproductive success. Therefore, another strategy for increasing overall breeding success for ASCOs at the population level would be to add dens/caves and potential nesting materials into existing ASCO habitats.
5. Conclusions
The RVA for the ASCO SSP described herein revealed:
- Use of deslorelin in males and long-term deslorelin use in females (multiple treatments) hinders future reproductive potential, and its use should therefore be considered carefully in genetically valuable individuals and potential breeders;
- Population managers should consider re-pairing individuals in pairs that have not been successful after 1 year of attempted breeding, after which the likelihood of success is low;
- Genetically valuable animals should be paired with younger mates, as advancing male and female age decreases the likelihood of success, yet the age difference between the male and female is not generally a concern;
- Pools in ASCO habitats should be designed with a gradient entry, as opposed to step or drop-off entries, to facilitate successful copulation and increase breeding success;
- Including dens and/or caves in ASCO living spaces may improve breeding success.
With the ability of the SSP to put several of these recommendations into practice relatively quickly, a higher (and more consistent) rate of reproductive success can likely be achieved, improving the long-term sustainability of the North American ex situ Asian small-clawed otter population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jzbg4030042/s1, File S1: ASCO Initial Habitat Survey; File S2: ASCO Follow-Up Habitat Survey.
Author Contributions
Conceptualization, A.D.F., M.M.M., M.A., S.D. and D.M.P.; methodology, A.D.F., M.M.M., M.A. and D.M.P.; software, A.D.F.; validation, A.D.F.; formal analysis, A.D.F. and M.M.M.; investigation, A.D.F., M.M.M., M.A. and S.D.; resources, A.D.F. and S.D.; data curation, A.D.F., M.M.M. and M.A.; writing—original draft preparation, A.D.F.; writing—review and editing, A.D.F., M.M.M., M.A., S.D. and D.M.P.; visualization, A.D.F.; supervision, D.M.P.; project administration, A.D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
An anonymized version of the data presented in this study may be available from the corresponding author upon reasonable request and valid justification. The data are not publicly available due to the inclusion of confidential animal and institutional information.
Acknowledgments
We would like to acknowledge all the individuals and facilities who have been involved in the breeding and management of the AZA ex situ ASCO population. Special thanks to Sara Sullivan, Paul Senner, and Tallie Wiles for their assistance in data collection, Jane Habbegger for data entry, and Seeyoon Lee for reviewing literature.
Conflicts of Interest
One author, Sarah Duncan, is the program coordinator and studbook keeper for the ASCO SSP and was the primary contact with AZA facilities to make interim breeding recommendations and collect information on which the breeding recommendations outlined in the BTPs were actually attempted. Regarding the habitat survey, some questions were generated based on pre-study discussions with S. Duncan, which focused on which substrates and features several facilities may use. We do not believe that the analyses or results were biased in any way based on S. Duncan’s involvement in the initial habitat survey design and data collection.
References
- Powell, D.M.; Dorsey, C.L.; Faust, L.J. Advancing the science behind animal program sustainability: An overview of the special issue. Zoo Biol. 2019, 38, 5–11. [Google Scholar] [CrossRef]
- Traylor-Holzer, K.; Leus, K.; Byers, O. Integrating ex situ management options as part of a One Plan Approach to species conservation. In The Ark and Beyond: The Evolution of Zoo and Aquarium Conservation; Minteer, B.A., Maienschein, J., Collins, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2018; pp. 129–141. [Google Scholar]
- Lees, C.M.; Wilcken, J. Sustaining the ark: The challenges faced by zoos in maintaining viable populations. Int. Zoo Yearb. 2009, 43, 6–18. [Google Scholar] [CrossRef]
- Faust, L.J.; Long, S.T.; Perišin, K.; Simonis, J.L. Uncovering challenges to sustainability of AZA Animal Programs by evaluating the outcomes of breeding and transfer recommendations with PMCTrack. Zoo Biol. 2019, 38, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Dorsey, C.; Boyle, P. Status of Association of Zoos and Aquariums’ Cooperatively Managed Populations. World Assoc. Zoos Aquar. Mag. 2011, 12, 15–18. [Google Scholar]
- Basnet, A.; Ghimire, P.; Timilsina, Y.P.; Bist, B.S. Otter research in Asia: Trends, biases and future directions. Glob. Ecol. Conserv. 2020, 24, e01391. [Google Scholar] [CrossRef]
- Dirgantara, A.P.; Megantara, E.N.; Husodo, T.; Febrianto, P.; Wulandari, I.; Shanida, S.S. The existence of Asian small-clawed otter (Aonyx cinereus Illiger, 1815) in the UCPS Hydropower, Cianjur, West Java, Indonesia. Biodiversitas 2021, 22, 4391–4401. [Google Scholar] [CrossRef]
- Hussain, S.A.; Gupta, S.K.; de Silva, P.K. Biology and ecology of Asian small-clawed otter Aonyx cinereus (Illiger, 1815): A review. IUCN Otter Spec. Group Bull. 2011, 28, 63–75. [Google Scholar]
- Mohapatra, P.P.; Palei, H.S.; Hussain, S.A. Occurrence of Asian small-clawed otter Aonyx cinereus (Illiger, 1815) in Eastern India. Curr. Sci. India 2014, 3, 367–370. [Google Scholar]
- Perinchery, A.; Jathanna, D.; Kumar, A. Factors determining occupany and habitat use by Asian small-clawed otters in the Western Ghats, India. J. Mammal. 2011, 92, 796–802. [Google Scholar] [CrossRef]
- Prakash, N.; Mudappa, D.; Shankar Raman, T.R.; Kumar, A. Conservation of the Asian small-clawed otter (Aonyx cinereus) in human-modified landscapes, Western Ghats, India. Trop. Conserv. Sci. 2012, 5, 67–78. [Google Scholar] [CrossRef]
- Wright, L.; de Silva, P.K.; Chan, B.; Reza Lubis, I.; Basak, S. Aonyx cinereus. The IUCN Red List of Threatened Species 2021: e.T44166A164580923. Available online: https://www.iucnredlist.org/species/44166/164580923 (accessed on 8 February 2023).
- Bateman, H.L.; Bond, J.B.; Campbell, M.; Barrie, M.; Riggs, G.; Snyder, B.; Swanson, W.F. Characterization of basal seminal traits and reproductive endocrine profiles in North American river otters and Asian small-clawed otters. Zoo Biol. 2009, 28, 107–126. [Google Scholar] [CrossRef]
- Reed-Smith, J. North American (Nearctic) River Otter (Lontra canadensis) Husbandry Notebook, 4th ed.; John Ball Zoo: Grand Rapids, MI, USA, 2012; pp. 69–99. [Google Scholar]
- Reed-Smith, J.; Lombardi, C.; Henry, B.; Myers, G.; Foti, J.; Sabalones, J. Caring for Asian Small-Clawed, Cape Clawless, Nearctic, and Spotted-Necked Otters, 3rd ed.; 2009; pp. 6–78. Available online: http://www.otterspecialistgroup.org/osg-newsite/wp-content/uploads/2018/05/Caring-for-Selected-Otter-Species.pdf (accessed on 1 February 2023).
- Duncan, S.; Sullivan, S. Population Analysis & Breeding and Transfer Plan: Asian Small-Clawed Otter (Aonyx cinereus) AZA Species Survival Plan® Yellow Program; Population Management Center, Lincoln Park Zoo: Chicago, IL, USA, 2019; pp. 5–10. [Google Scholar]
- Agnew, M.; Asa, C.S.; Franklin, A.D.; McDonald, M.M.; Cowl, V.B. Deslorelin (Suprelorin®) use in North American and European zoos and aquariums: Taxonomic scope, dosing, and efficacy. J. Zoo Wildl. Med. 2021, 52, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Asa, C.; Moresco, A. Fertility control in wildlife: Review of current status, including novel and future technologies. In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology; Comizzoli, P., Brown, J., Holt, W., Eds.; Springer: Cham, Switzerland; New York, NY, USA, 2019; Volume 1200, pp. 507–543. [Google Scholar]
- Cowl, V.B.; Walker, S.L.; Feltrer Rambaud, Y. Assessing the efficacy of deslorelin acetate implants (Suprelorin) in alternative placement sites. J. Zoo Wildl. Med. 2018, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bauman, K.; Sahrmann, J.; Franklin, A.; Asa, C.; Agnew, M.; Traylor-Holzer, K.; Powell, D. Reproductive viability analysis (RVA) as a new tool for ex situ population management. Zoo Biol. 2019, 38, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Keeley, T.; Ritky, K.; Russell, T. A retrospective examination of factors associated with breeding success of Tasmanian devils in captivity (2006 to 2012). J. Zoo Aquar. Res. 2023, 11, 211–219. [Google Scholar] [CrossRef]
- Saunders, S.P.; Harris, T.; Traylor-Holzer, K.; Beck, K.G. Factors influencing breeding success, ovarian cyclicity, and cub survival in zoo-managed tigers (Panthera tigris). Anim. Reprod. Sci. 2014, 144, 38–47. [Google Scholar] [CrossRef]
- Franklin, A.D.; Lacy, R.C.; Bauman, K.L.; Traylor-Holzer, K.; Powell, D.M. Incorporating drivers of reproductive success improves population viability analysis. Anim. Conserv. 2021, 24, 386–400. [Google Scholar] [CrossRef]
- Franklin, A.D.; Waddell, W.T.; Behrns, S.; Goodrowe, K.L. Estrous cyclicity and reproductive success are unaffected by translocation for the formation of new reproductive pairs in captive red wolves (Canis rufus). Zoo Biol. 2020, 39, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lockyear, K.M.; Waddell, W.T.; Goodrowe, K.L.; MacDonald, S.E. Retrospective investigation of captive red wolf reproductive success in relation to age and inbreeding. Zoo Biol. 2009, 28, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Metrione, L.C.; Bateman, H.L.; Swanson, W.F.; Penfold, L.M. Characterization of the behavior and reproductive endocrinology of giant river otters (Pteronura brasiliensis) in managed care. Zoo Biol. 2018, 37, 300–309. [Google Scholar] [CrossRef]
- Ballou, J.D.; Lacy, R.C.; Pollak, J.P. PMx: Software for Demographic and Genetic Analysis and Management of Pedigreed Populations (Version 1.6.5.20220325); Chicago Zoological Society: Brookfield, IL, USA, 2022; Available online: http://www.scti.tools/PMx (accessed on 16 March 2023).
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 173–181. [Google Scholar]
- Penfold, L.M.; Norton, T.; Asa, C.S. Effects of GnRH agonists on testosterone and testosterone-stimulated parameters for contraception and aggression reduction in male lion-tailed Macaques (Macaca silenus). Zoo Biol. 2021, 40, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.; Belting, T.; Rifenbury, K.; Fisher, G.; Boutelle, S.M. Preliminary findings of fecal gonadal hormone concentrations in six captive sea otters (Enhydra lutris) after deslorelin implantation. Zoo Biol. 2013, 32, 307–315. [Google Scholar] [CrossRef]
- Bertschinger, H.J.; Asa, C.S.; Calle, P.P.; Long, J.A.; Bauman, K.; DeMatteo, K.; Jöchle, W.; Trigg, T.E.; Human, A. Control of reproduction and sex related behaviour in exotic wild carnivores with the GnRH analogue deslorelin. J. Reprod. Fertil. Suppl. 2001, 57, 275–283. [Google Scholar]
- Bertschinger, H.J.; Trigg, T.E.; Jöchle, W.; Human, A. Induction of contraception in some African wild carnivores by downregulation of LH and FSH secretion using the GnRH analogue deslorelin. Reprod. Suppl. 2002, 60, 41–52. [Google Scholar] [PubMed]
- Romagnoli, S.; Baldan, A.; Ferro, S.; Righetti, C.; Scenna, L.; Gabai, G.; Badon, T.; Fontaine, C.; Mollo, A.; Stelletta, C.; et al. Length of efficacy and effect of implant location in adult tom cats treated with a 9.4 mg deslorelin subcutaneous implant. J. Feline Med. Surg. 2019, 21, 507–519. [Google Scholar] [CrossRef]
- ZIMS Survival, Growth, & Reproduction Report (Aonyx cinereus), Data Extraction Date: 17 February 2022. Species360 Zoological Information Management System. Available online: http://zims.Species360.org (accessed on 8 March 2023).
- Guthrie, A.; Strike, T.; Patterson, S.; Walker, C.; Cowl, V.; Franklin, A.D.; Powell, D.M. The past, present and future of hormonal contraceptive use in managed captive female tiger populations with a focus on the current use of deslorelin acetate. Zoo Biol. 2021, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Moresco, A.; Dadone, L.; Arble, J.; Klaphake, E.; Agnew, D.W. Location and removal of deslorelin acetate implants in female African lions (Panthera leo). J. Zoo Wildl. Med. 2014, 45, 397–401. [Google Scholar] [CrossRef]
- Munson, L.; Gardner, A.; Mason, R.J.; Chassy, L.M.; Seal, U.S. Endometrial hyperplasia and mineralization in zoo felids treated with melengestrol acetate contraceptives. Vet. Pathol. 2002, 39, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Harrenstien, L.A.; Munson, L.; Seal, U.S.; The American Zoo and Aquarium Association Mammary Cancer Study Group. Mammary cancer in captive wild felids and risk factors for its development: A retrospective study of the clinical behavior of 31 cases. J. Zoo Wildl. Med. 1996, 27, 468–476. [Google Scholar]
- Munson, L.; Moresco, A.; Calle, P.P. Adverse effects of contraceptives. In Wildlife Contraception: Issues, Methods and Application; Asa, C.S., Porton, I.J., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 66–82. [Google Scholar]
- Asa, C.S.; Bauman, K.L.; Devery, S.; Zordan, M.; Camilo, G.R.; Boutelle, S.; Moresco, A. Factors associated with uterine endometrial hyperplasia and pyometra in wild canids: Implications for Fertility. Zoo Biol. 2014, 33, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Crosier, A.E.; Comizzoli, P.; Baker, T.; Davidson, A.; Munson, L.; Howard, J.; Marker, L.L.; Wildt, D.E. Increasing age influences uterine integrity, but not ovarian function or oocyte quality, in the cheetah (Acinonyx jubatus). Biol. Reprod. 2011, 85, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Penfold, L.M.; Powell, D.; Traylor-Holzer, K.; Asa, C.S. “Use it or lose it”: Characterization, implications, and mitigation of female infertility in captive wildlife. Zoo Biol. 2014, 33, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D.; Willis, J.H. The genetics of inbreeding depression. Nat. Rev. Genet. 2009, 10, 783–796. [Google Scholar] [CrossRef]
- Lockyear, K.M.; MacDonald, S.E.; Waddell, W.T.; Goodrowe, K.L. Investigation of captive red wolf ejaculate characteristics in relation to age and inbreeding. Theriogenology 2016, 86, 1369–1375. [Google Scholar] [CrossRef]
- Gomendio, M.; Cassinello, J.; Roldan, E.R.S. A comparative study of ejaculate traits in three endangered ungulates with different levels of inbreeding: Fluctuating asymmetry as an indicator of reproductive and genetic stress. Proc. R. Soc. Lond. B. 2000, 267, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Ballou, J.D.; Lacy, R.C. Identifying genetically important individuals for management of genetic variation in pedigreed populations. In Population Management for Survival and Recovery: Analytical Methods and Strategies in Small Population Conservation; Ballou, J.D., Gilpin, M., Foose, T.J., Eds.; Columbia University Press: New York, NY, USA, 1995; pp. 76–111. [Google Scholar]
- Montgomery, M.E.; Ballou, J.D.; Nurthen, R.K.; England, P.R.; Briscoe, D.A.; Frankham, R. Minimizing kinship in captive breeding programs. Zoo Biol. 1997, 16, 377–389. [Google Scholar] [CrossRef]
- Rodriguez-Clark, K.M. Genetic theory and evidence supporting current practices in captive breeding for conservation. In Genetics and the Extinction of Species; Landweber, L.F., Dobson, A.P., Eds.; Princeton University Press: Princeton, NJ, USA, 1999; pp. 47–65. [Google Scholar]
- Martin, M.S.; Shepherdson, D.J. Role of familiarity and preference in reproductive success in ex situ breeding programs. Conserv. Biol. 2012, 26, 649–656. [Google Scholar] [CrossRef]
- Wiles, T.; (Smithsonian’s National Zoo and Conservation Biology Institute, Washington, DC, USA). Personal communication, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).