Abstract
Photobiomodulation therapy (cold laser or low-level laser therapy) has been evaluated in human and small animal medicine; however, there is a lack of knowledge about the role photobiomodulation therapy could play in reptile rehabilitation and release. This study used a quantifiable unit, Hounsfield units (bone density measurement), in computed tomography (CT) to evaluate if photobiomodulation therapy showed a significant healing difference between groups treated with photobiomodulation and those that were not. This study included 20 eastern box turtles (Terrapene carolina) presented to a rehabilitation center that sustained shell fractures without penetrating the coelom. They all received similar medical treatments, except that the photobiomodulation group received 250 Hz of red light laser for three minutes three times a week for eight weeks. The turtles were evaluated over the course of two months of therapy. Computed tomography scans were performed prior to therapy, at the midpoint of treatment (one month postinjury), and at the end of the study (two months postinjury). The average Hounsfield units of the fractures were evaluated using nonparametric means, the Wilcoxon/Kruskal–Wallis tests (ranked sums), and found that there were no significant differences in shell density between the photobiomodulation and control groups amongst the scans. This study did find that there was a significant difference (p = 0.0455) between the two groups in regard to the width of the fracture between pre- and post-treatment scans. This study found that the photobiomodulation group had a significantly decreased width of the fracture site between pre-treatment and post-treatment measurements, showing that photobiomodulation could be a relatively easy and effective treatment to promote healing of fractured turtle shells.
1. Introduction
Fast wound healing is essential in veterinary medicine, particularly in wildlife rehabilitation settings where rehabilitators are hoping to release patients back to their habitat as quickly as possible. Photobiomodulation therapy (cold laser or low-level laser therapy) has been studied in healing and physical therapy in humans as well as small animals and horses [1]. Photobiomodulation treatment has also been studied in small animals for wound healing and has been shown to increase neovascularization of tissues [2], growth factors [3,4], and wound tensile strength [5].
Photobiomodulation therapy, unfortunately, has not been extensively studied among reptiles. Due to little being known about photobiomodulation therapy and reptile healing, there is an increasing interest in how photobiomodulation therapy can be used to promote healing in our reptiles and amphibians, especially in a wildlife rehabilitation or zoological medicine setting. One amphibian study, evaluating photobiomodulation in combination with silver sulfadiazine 1% cream, was used on uncomplicated full thickness dermal lesions in frogs [6]. This study showed no significant difference between control and treatment groups; however, despite no significant differences being found, Archibald et al. found that visually the wounds appeared to epithelialize faster in the photobiomodulation therapy group compared to the control group [6]. This study suggests that photobiomodulation therapy may be beneficial in their healing despite there being no significant difference between treatment and control groups for small uncomplicated wounds [6].
Photobiomodulation therapy is a light therapy that triggers biochemical changes that include absorption of photons by cellular photoreceptors; photobiomodulation laser therapy acts specifically on the mitochondrial photoreceptors, stimulating cell proliferation and fibroblastic regeneration [7]. During photobiomodulation therapy, there is excitation of cellular signaling within the mitochondria, including the pathways that produce adenosine triphosphate (ATP), reactive oxygen species (ROS), and nitric oxide (NO), all of which are beneficial to cell healing [8]. Photobiomodulation therapy works at not only the cellular level but also at the systemic level, providing system upregulation and full system healing. At the cellular level, photobiomodulation therapy produces increases in metabolism, proliferation, migration, synthesis, and secretion of proteins as well as upregulating different types of cells including fibroblasts, keratinocytes, endothelial cells, lymphocytes, muscle cells, and stem cells [8]. In addition, at the systemic level, photobiomodulation laser therapy influences the inflammatory pathway, including the cytokines and chemokines causing decreased inflammation [8]. Working at the cellular and systemic level allows photobiomodulation therapy to promote faster healing and help reduce pain [8].
In human medicine, photobiomodulation therapy has been studied extensively, and the research has shown that a combination of medications with photobiomodulation therapy can work to decrease chronic pain associated with illnesses such as osteoarthritis [7]. In addition, photobiomodulation therapy has been studied for pain and edema reduction in women with breast cancer lymphedema; that study found that this therapy provided greater reduction in arm edema and pain in women who received the laser therapy as compared to those who did not receive the treatment [9]. There are also studies evaluating treating human wounds with photobiomodulation therapy that show that the therapy promotes early epithelialization in human wound healing [8].
As in humans, photobiomodulation therapy has been studied in small and large animals; however, there have been conflicting results throughout the different studies. A study evaluating photobiomodulation therapy on acute, full-thickness wounds of dogs showed that there was no significant difference between the photobiomodulation treatment group and the control group in the healing of the wounds. Additionally, in another study, there was also no significant difference between the photobiomodulation therapy and control groups, but the photobiomodulation therapy group had visual clinical improvement [10,11]. In contrast, in equine medicine, horses with metacarpal wounds healed significantly faster when treated with photobiomodulation therapy as opposed to the control groups [12]. In addition, photobiomodulation therapy evaluated on full-thickness teat wounds in dairy cattle that were surgically repaired showed that those treated with photobiomodulation therapy had enhancement of the healing process compared to those that were not treated, including significantly greater tensile strength of the suture used, minimized inflammation, reduced formation of edema, and improvement of skin regeneration and collagen synthesis [13]. In another canine study, researchers evaluated scar healing on dogs undergoing thoracolumbar hemilaminectomies [14]. They found that those in the photobiomodulation treatment group showed improved healing within a week and continued to have healing significantly increased at 20 days postoperation compared to the control groups [14]. In one of the newer research articles evaluating photobiomodulation therapy on canine healing, Hoisang et al. found that 830 nm photobiomodulation therapy or simultaneous superpulsed and multiple wavelengths showed a significant difference in swifter healing time compared to the non-photobiomodulation therapy group [15].
Deciding on specific doses of photobiomodulation therapy is a common issue that most have when using photobiomodulation therapy for the first time. Doses have been shown to depend on tissue type, anatomic site, tissue depth, species, coat color and length, skin color, and even body condition score [16]. However, newer data have shown that low-laser therapy specifically ranging from 3 to 6 J/cm2 was more effective than the previous doses of 10 or above J/cm2 on mammals regardless of species [17]. However, many researchers and clinicians rely upon previous research as well as the Multi Radiance Medical ActiVet Pro Treatment Protocol Manual as the basis of their photobiomodulation therapy. There are published doses for certain conditions, including superficial wounds, deep wounds, and chronic diseases in companion animals, equine animals, farm animals, and large zoo species, as well as doses published for exotic species, including reptiles, small mammals, and avian species [8].
Turtle shells, in contrast to our mammals, amphibians, and avian species, are made of bone and keratin as opposed to epithelial tissue [18]. There are a few studies looking into photobiomodulation therapy and bone healing. In one case report, a 10-week-old male Xoloitzcuintle (Mexican hairless dog) that broke its tibia and was treated with surgical fixation as well as photobiomodulation therapy with a static magnetic field showed accelerated and improved bone healing following the surgical procedure [19]. Another study of rabbits evaluated photobiomodulation therapy as a treatment for osteochondral lesions of the knee, and after 24 weeks of photobiomodulation therapy, the treated knees had better cell morphology and repaired osteocartilaginous tissue compared to the non- treated knees [20]. In addition, another study evaluating advances in photobiomodulation therapy in bone healing concluded that laser therapy photoactivated osteoblastic cells, could accelerate proliferation of osteoprogenitor cells and enhance osteoblast calcification, and may also promote bone regeneration and healing [21]. Further, a newer study in canids, which was a randomized double-blinded control trial evaluating photobiomodulation therapy on osteoarthritic dogs, found that dogs that received photobiomodulation therapy had reduced pain and clinical signs as compared to those that were not treated [22].
In addition, there is a single reptile case report that used photobiomodulation therapy in an adult, wild-caught, intact female Aubrey’s flapshell turtle (Cycloderma aubryi) with osteolytic lesions due to a systemic bacterial infection [23]. This study found that the osteomyelitic lesions treated with photobiomodulation therapy showed grossly visible improvement compared to the lesions that were untreated; the researchers then treated all lesions with photobiomodulation therapy, and the turtle made a full recovery [23]. These studies support that photobiomodulation therapy could be beneficial in the healing of injured turtle shells despite their composition of keratin and bone, as therapy with photobiomodulation has been shown to promote these cells as well as epithelialization.
This study aimed to investigate the role of photobiomodulation therapy in the healing of uncomplicated shell fractures in chelonians. Additionally, this study further aimed to quantify fracture healing by the use of computed tomography (CT) and Hounsfield units, a quantitative measurement of radio density, to better understand the healing of the shell throughout the CT scans [24]. The authors hypothesized that photobiomodulation therapy would lead to increased healing rates of shell fractures in the photobiomodulation treated group compared to the control group.
2. Materials and Methods
2.1. Animals/Inclusion Criteria
This study was performed between the summer and fall months of 2019. Unknown age and sex wild-caught injured box turtles (Terrapene carolina) were brought into the North Carolina State College of Veterinary Medicine Turtle Rescue Team (Raleigh, NC, USA) for medical treatment. The North Carolina State Turtle Rescue Team provides veterinary care and rehabilitation to injured wild chelonians in North Carolina and releases them back to their natural habitat once they are deemed healthy. Box turtles presented with a wide variety of presenting causes for their shell fracture, including being hit by a motor vehicle, farm equipment/lawn mower altercations, and attack by domestic animals, in addition to other potential causes of injury. Box turtles were enrolled on the basis of having a carapace fracture that was determined to not breach the coelom and it appearing likely that the turtle would have a releasable outcome. Box turtles were enrolled during the months of June 2019 through September 2019. A total of 20 eastern box turtles were enrolled.
All animals, sampling, and procedures were covered under the North Carolina State University IACUC #19-036-O. All turtles, once the study was completed, were released back to the area in which they were originally found, away from roads or highways, once they were diagnosed as being healed appropriately for release.
2.2. Procedure
All fractures were evaluated upon intake; initial cleaning and pain medications were provided; and then animals were placed in one group (control) or the treatment group (photobiomodulation therapy) on an alternating basis. Fractures were generally 40–60 mm in length across both groups. All turtles received a computed tomography (CT) scan (see Figure 1) prior to starting long-term treatments, and this initial CT scan occurred within 12 h of intake. Another CT scan was completed again one month later (halfway through treatment) and then at the end of the treatment period (two months after the initial CT scan). All turtles received pain medications upon arrival, Ketorolac 0.25 mg/kg (Fresenius Kabi, LLC NovaPlus, 30 mg/mL, Wilson, NC, USA,) intramuscularly every 24 h for three days, and then were switched to Ketoprofen 2 mg/kg (Zoetis, 100 mg/mL, Parsippany-Troy Hills, NJ, USA) intramuscularly every 48 h for eight doses. All animals were started on an antibiotic, Ceftazidime 20 mg/kg (WG Critical Care LLC, 100 mg/mL, Paramus, NJ, USA) intramuscularly every 72 h for six doses. Turtles were closely monitored every day for four weeks by veterinarians and volunteers. The turtles were evaluated for mentation, eating, and urination/defecation daily as well as given a physical examination once daily. Additionally, the fracture site was flushed with saline, and silver sulfadiazine (Ascend Laboratories, Parsippany NJ, USA) was applied once a day for three weeks. Any turtles that had a fracture that could be stabilized underwent a stabilization surgery, which might include hardware applied to the shell the following day after intake; this procedure was performed on 2–3 turtles per group. The turtles that were placed in the photobiomodulation therapy group were treated with 250 Hz red light (690 nm wavelength red LEDs) for three minutes along the fracture site every Monday, Wednesday, and Friday throughout their stay for a total of two months (based on dosing information from Multi Radiance Medical ActiVet Pro Treatment Protocol Manual). The laser (class 3B solid-state superpulsed laser- MR4 ACTIVet veterinary laser, Multi Radiance Medical, Solon, OH, USA) was held approximately 5–8 mm above the fracture site and moved back and forth, slowly, over the fracture for three minutes. Protective eyewear was worn by personnel administering the therapy, and all turtle eyes were shielded with black fabric during treatment. All turtles were placed in a low soak or full soak each day, depending on where the fracture site was located along the shell and how it was healing.

Figure 1.
Turtle under CT scan with phantom bone.
All turtles were kept in the Turtle Rescue Team lab, which has an ambient temperature of 24–30 degrees Celsius (75–85 degrees Fahrenheit), and a UVB light, ZooMed Reptisun 10.0 (Zoo Med Laboratories, San Luis Obispo, CA, USA), was provided. UVB and light were on a 12 h cycle. Each turtle was kept in a 25 × 43 cm black plastic bin (Cambro, Mebane, NC, USA) that housed an animal hide container and a water dish. The substrate consisted of newspapers that were changed daily to better evaluate urination/defecation. Each turtle was also given “outside” time, when the weather permitted, in which turtles were taken outside and allowed to bask in the sun for a few hours. All turtles were given a combination of green leaf lettuce/red leaf lettuce/romaine, fruits (strawberries, blueberries, grapes), and occasionally mealworms.
2.3. Analysis
An individual (AS) blinded to the groups in which each turtle was placed evaluated the CT scans that were performed at each timepoint. Using Hounsfield units, a quantitative measurement for radiodensity, the blinded individual took measurements within the fracture site at the proximal, middle, and distal aspects for a single CT slice; for an example, see Figure 2. In addition, measurements of normal shell were also taken to the left and right of the fracture site. The measurements for normal shell were then averaged at each site, creating two sets of data for each fracture site: abnormal (fracture) and normal (shell). Each turtle, once the measurements were completed, had six sets of data for each CT scan.
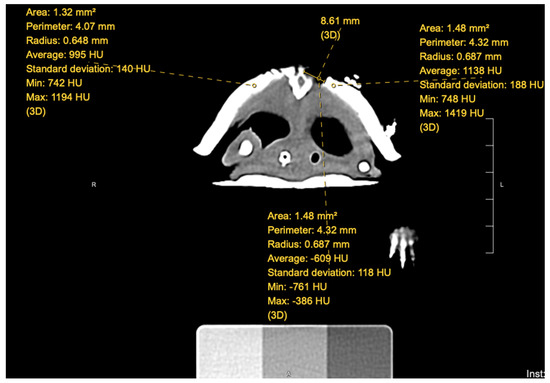
Figure 2.
Measurements of the turtle’s fracture site on a single CT slice. Hounsfield units of both fracture and normal shell along with the measurement of the width of the fracture.
In addition, the fracture width was measured in millimeters by pre- and post-treatment CT scans by the same blinded individual (AS). Prior to statistical analysis, and unblinding the measurement taker (AS), the individual was asked to place each turtle in a category of control group or treatment group based solely on the information gathered from the CT scans. Only after the information was gathered was the blinded individual made aware of what turtles were within the photobiomodulation therapy and control groups.
Once the information was gathered, it was analyzed using JMP software (SAS institute, Cary, NC, USA, version 15). Each set of data was analyzed (proximal, middle, and distal) between control groups and treated photobiomodulation therapy groups. A one- way ANOVA was completed along with a nonparametric means test: the Wilcoxon/Kruskal–Wallis tests (ranked sums). An additional test, the median test, was run, evaluating the median width of fracture healing between the control and the photobiomodulation therapy groups.
3. Results
The only information the blinded individual (AS) had to reference for the placement of turtles into either control group or photobiomodulation therapy group was the measurements obtained from each of the CT scans. The individual had a 60% accuracy rate at determining which turtle was in which group (control vs. photobiomodulation therapy), based on the information gathered from the CT scans alone.
There was no significant difference (p = 0.203, S = 101, Z = 1.27) found between fracture healing, in terms of Hounsfield units, between the two groups on any of the CT scans (pre-, mid-, or post-treatment). There was, however, a significant difference in the width of the fracture between the two groups (p = 0.045, S = 2, Z = 2), with the photobiomodulation therapy group showing a greater reduction in the width of the fracture between pre- and post-treatment CT scans compared to the control group.
4. Discussion
The findings of this study showed no significant difference in fracture healing in terms of Hounsfield units, but there was significant difference between the width of the fracture between the photobiomodulation therapy and control groups. In evaluating the findings and comparing them to references (specifically 10–14), we note these are similar findings. In comparing the current study to the amphibian model, similar evidence is found; however, there was significant difference in the width of the fracture site demonstrated in the current study [6]. However, both the amphibian study and the current study found that visually the wounds appeared to show better healing in the photobiomodulation therapy group compared to the control group.
The authors note that the canine studies that did not find significant differences between the photobiomodulation and non-photobiomodulation therapy groups had the same animal as its own control [10,11]; the dog in the study had either a separate wound on its body or half of a wound that was treated with cold laser therapy and the other wound or half of a wound treated as the control simultaneously [10,11]. The authors hypothesize that the reason there was no significant difference in healing is because photobiomodulation laser therapy upregulates the entire system, not just a specific area due to cellular signaling [25].
This current study is one of the first to the authors’ knowledge evaluating the effect of photobiomodulation therapy on the fracture healing of chelonian shells. This study aimed to replicate the use of photobiomodulation and its potential for consideration by wildlife rehabilitators, specifically if they may consider purchasing their own photobiomodulation unit for wound healing; however, the question arises: is it worth the rehabilitator’s resources (time and money) to pursue photobiomodulation therapy on injured patients if there is no significant difference between photobiomodulation therapy and control groups?
This study provided a concept of how photobiomodulation therapy can be used for chelonian shell healing in a wildlife rehabilitation setting; however, there were some drawbacks to this study. The first drawback was the small sample size. This study could only evaluate whatever animals were brought into the Turtle Rescue Team clinic during the summer months of 2019. Therefore, this study was only able to enroll 20 eastern box turtles (Terrapene carolina) in total, which may have been why no significant difference was found between fracture healing along all sites (proximal, middle, distal) between the photobiomodulation therapy group and the control group at the three different timepoints (pre-, mid-, and post-treatment scans). Ideally, if this study were repeated, a greater sample size of 25–30 turtles per treatment group would help improve statistical analysis.
Another limitation of this study was that the fracture site evaluated by the blinded individual was 1) not always the same diameter for the Hounsfield unit measurement and 2) not necessarily in the exact same spot on every scan (pre-, mid-, and post-treatment CT scans) between individual scans. Although the diameter measured for Hounsfield units was measured as relatively the same across the individual scans, between individuals, this diameter could be anywhere between 1.17 mm2 to 2.16 mm2, about a 1 mm2 difference. In addition, exact placement of measurements was determined by acknowledging the anatomy of the previously measured CT slice and finding the relatively same area within the next scan. Even though the measurements were as close as possible to the exact same site based on anatomy, this study cannot guarantee that each fracture was measured at the exact same location for every scan. Future studies should consider markers along the shell at the areas of measurement, as oftentimes, the hardware used to repair the turtle was used to help the blinded individual evaluate similar CT slices across scans.
This study did demonstrate that the relative width of the fracture site between pre- and post-treatment scans was significantly different, since the photobiomodulation therapy group had a greater reduction of the fracture width over the two months of treatment compared to the control group. The authors hypothesize that this is most likely due to the photobiomodulation therapy stimulating new keratin cells and osteoprogenitor cells to fill in the fracture more quickly compared to the control group [21]. This has been proven by multiple studies evaluating photobiomodulation in small and large animals as well as exotic medicine [1,2,3,4,5]. In addition, photobiomodulation therapy does promote and upregulate keratinocytes, which is a predominant cell of regrowing/healing turtle shells [8]. It is hypothesized that there was no significant difference seen with the Hounsfield units because it was measured in the center of the fracture for each scan, whereas healing was suspected to be coming in from the outer edges of the fracture.
Additional research is needed to evaluate photobiomodulation therapy in terms of if these animals are able to be released earlier than controls. Records were unfortunately moved to a digitized system starting the year of 2019/2020, at which time records were being scanned into the system of RaptorMed (Huntersville, NC, USA, 2010). Previously, the records had been written on paper. Unfortunately, the dates of release were lost for most of the turtles in this study during the translation of the paper records into the new system; therefore, no statistical analysis was able to be evaluated regarding the release date information. The one turtle that appeared to be released the soonest of the turtles that had release dates stayed with Turtle Rescue Team for four days after the final CT scan prior to release. This turtle was part of the photobiomodulation therapy group. Further research should evaluate this aspect, as release of rehabilitated chelonians is important in terms of success for wildlife rehabilitation.
In addition to the release dates being lost, most of the identifying information on the turtle’s approximate age (juvenile or adult) as well as sex (male or female) was also lost in the transition from paper records to the digital system. Most of the turtles were marked as having an unknown age and sex within the digital recordkeeping system. The authors believe it would also be interesting to evaluate photobiomodulation therapy and the healing of fractures between age groups (juvenile and adult) as well as between sex (male and female). Additional research could also evaluate photobiomodulation therapy and fracture healing between different species of chelonians, including between terrestrial species and aquatic species.
Additional research in this area would include a larger population size for further evaluation. This study was limited by the number of injured turtles brought into the Turtle Rescue Team lab for that one-year study period. A study period lasting throughout additional years with photobiomodulation therapy compared to controls would be ideal. Additionally, placing markers along the CT scan (either on the shell or alongside the turtle) would be helpful in ensuring that fractures are evaluated at the exact same location across individual CT scans. Alternatively, this design, although recommended in the Multi Radiance Medical ActiVet Pro Treatment Protocol Manual, may involve inappropriate dosage/frequency of treatment and should also be investigated further in future studies [26]. Future studies are encouraged to have separate treatment groups with possible different dosages/frequencies to compare healing along with the control group, since little is known about the appropriate wavelength and frequency of photobiomodulation for the reptile species.
5. Conclusions
This study concludes that there is no significant difference between the two groups (photobiomodulation therapy and control groups) in terms of Hounsfield units, a quantitative measure of radiodensity on CT scans. This study did show a significant difference in terms of fracture width between the photobiomodulation therapy and control groups, meaning that the photobiomodulation therapy (low-level laser or cold laser therapy) group had a smaller fracture width at the end of the study compared to the control group. This finding suggests that photobiomodulation therapy might be a good adjunct to conventional rehabilitation therapy for chelonians with shell fractures that are not classified as coelomic breach.
Author Contributions
Conceptualization C.M. and T.M.H.; gathering data A.R.S.; running statistics A.R.S. and T.M.H.; writing A.R.S.; review and editing C.M. and T.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Computed tomography scans were paid for by Multi Radiance Medical (Solon, OH, USA), funding account 110668, a laser therapy device manufacturer. Multi Radiance Medical had no role in the study design, data collection, data analysis, or writing of this manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to this study being incorporated under the North Carolina State IACUC #19-036-O.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to university limitations.
Acknowledgments
The authors would like to thank the North Carolina State University College of Veterinary Medicine Radiology Service, specifically Spencer Fennik and Nathan Nelson, for helping with the reading of the CT scans and helping with measurements; Jim Robey, the CT scan operator who helped position and scan all the turtles; the North Carolina State University Turtle Rescue Team students and volunteers; and Kent Passingham and Gregory Lewbart for allowing this study to take place in their laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pryor, B.; Millis, D.L. Therapeutic Laser in Veterinary Medicine. Veter. Clin. N. Am. Small Anim. Pr. 2015, 45, 45–56. [Google Scholar] [CrossRef]
- Maeda, T. Histological, thermographic and thermometric study in vivo and excised 830 nm diode laser irradiated rat skin. Laser Ther. 1990, 2, 32. [Google Scholar]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed. Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef]
- Mvula, B.; Mathope, T.; Moore, T.; Abrahamse, H. The effect of low level laser therapy on adult human adipose derived stem cells. Lasers Med. Sci. 2008, 23, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Parizotto, N.A.; Baranauskas, V. Structural analysis of collagen fibrils after HeNe laser photostimulated regenerating rat tendon. In Proceedings of the 2nd Congress World Association for Laser Therapy, Kansas City, KS, USA, 2–5 September 1998. [Google Scholar]
- Archibald, K.E.; Harrison, T.; Troan, B.; Smith, D.; Minter, L. Effect of multiradiance low-level laser therapy and topical silver sulfadiazine on healing characteristics of dermal wounds in marine toads (Rhinella marina). Vet. Med. Int. 2020, 2020, 8888328. [Google Scholar] [CrossRef]
- Dima, R.; Francio, V.T.; Towery, C.; Davani, S. Review of Literature on Low-level Laser Therapy Benefits for Nonpharmacological Pain Control in Chronic Pain and Osteoarthritis. Trials 2017, 5, 6. [Google Scholar]
- Riegel, R.J.; Godbold, J.C., Jr. (Eds.) Laser Therapy in Veterinary Medicine: Photobiomodulation; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Smoot, B.; Chiavola-Larson, L.; Lee, J.; Manibusan, H.; Allen, D.D. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: A systematic review and meta-analysis. J. Cancer Surviv. 2015, 9, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Kurach, L.M.; Stanley, B.J.; Gazzola, K.M.; Fritz, M.C.; Steficek, B.A.; Hauptman, J.G.; Seymour, K.J. The effect of low-level laser therapy on the healing of open wounds in dogs. Vet. Surg. 2015, 44, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Perego, R.; Proverbio, D.; Spada, E.; Zuccaro, A. First experience with photobiomodulation (PBM) in post-surgical wound healing in dogs. J. Vet. Clin. Pract. Pet Care 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Jann, H.W.; Bartels, K.; Ritchey, J.W.; Payton, M.; Bennett, J.M. Equine wound healing: Influence of low level laser therapy on an equine metacarpal wound healing model. Photonics Lasers Med. 2012, 1, 117–122. [Google Scholar] [CrossRef]
- Ghamsari, S.M.; Taguchi, K.; Abe, N.; Acorda, J.A.; Sato, M.; Yamada, H. Evaluation of low level laser therapy on primary healing of experimentally induced full thickness teat wounds in dairy cattle. Vet. Surg. 1997, 26, 114–120. [Google Scholar] [CrossRef]
- Wardlaw, J.L.; Gazzola, K.M.; Wagoner, A.; Brinkman, E.; Burt, J.; Butler, R.; Gunter, J.M.; Senter, L.H. Laser therapy for incision healing in 9 dogs. Front. Vet. Sci. 2019, 5, 349. [Google Scholar] [CrossRef] [PubMed]
- Hoisang, S.; Kampa, N.; Seesupa, S.; Jitpean, S. Assessment of wound area reduction on chronic wounds in dogs with photobiomodulation therapy: A randomized controlled clinical trial. Vet. World 2021, 14, 2251. [Google Scholar] [CrossRef]
- Fesseha, H. Laser Therapy and its Potential Application in Veterinary Medicine—A Review. J. Light Laser Curr. Trends 2020, 3, 007. [Google Scholar]
- Andrade, F.D.S.D.S.D.; Clark, R.M.D.O.; Ferreira, M.L. Effects of low-level laser therapy on wound healing. Rev. Col. Bras. Cir. 2014, 41, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T.H.; Innis, J.N. Chelonin Taxonomy, Anatomy, and Physiology. In Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed.; Divers, S.J., Stahl, S.J., Mader, D.R., Eds.; Elsevier: St. Louis, MO, USA, 2019; pp. 31–49. [Google Scholar]
- Asteinza Castro, I.M.; Morga, A.A.; Johnson, D.S. Photobiomodulation therapy combined with static magnetic field in tibial fracture healing of a dog: A case report. Vet. Med. Sci. 2023, 9, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Guzzardella, G.A.; Tigani, D.; Torricelli, P.; Fini, M.; Martini, L.; Morrone, G.; Giardino, R. Low-power diode laser stimulation of surgical osteochondral defects: Results after 24 weeks. Artif. Cells Blood Substit. Biotechnol. 2001, 29, 235–244. [Google Scholar] [CrossRef]
- Barber, A.; Luger, J.; Karpf, A.; Salame, K.; Shlomi, B.; Kogan, G.; Nissan, M.; Alon, M.; Rochkind, S. Advances in laser therapy for bone repair. Laser Ther. 2000, 13, 80–85. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Carreira, L.M. A randomized double-blinded controlled trial on the effects of photobiomodulation therapy in dogs with osteoarthritis. Am. J. Vet. Res. 2022, 83, ajvr.22.03.0036. [Google Scholar] [CrossRef]
- DiRuzzo, S.; Praschag, P.; Miller, L.; Brodsky, M. Successful Treatment of Severe Ulcerative Dermatitis in an Aubry’s Flapshell Turtle (Cycloderma aubryi). J. Herp. Med. Surg. 2022, 32, 262–270. [Google Scholar] [CrossRef]
- DenOtter, T.D.; Schubert, J. Hounsfield Unit. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bayat, M.; Vasheghani, M.; Razavi, N.; Taheri, S.; Rakhshan, M. Effect of low-level laser therapy on the healing of second-degree burns in rats: A histological and microbiological study. J. Photochem. Photobiol. B Biol. 2005, 78, 171–177. [Google Scholar] [CrossRef]
- Multi Radiance Medical. ActiVet Pro Treatment Protocol Manual; Multi Radiance Medical: Solon, OH, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).