Centers of Endemism and The Potential of Zoos and Botanical Gardens in Conservation of Endemics
Abstract
1. Introduction
2. What Are Centers of Endemism?
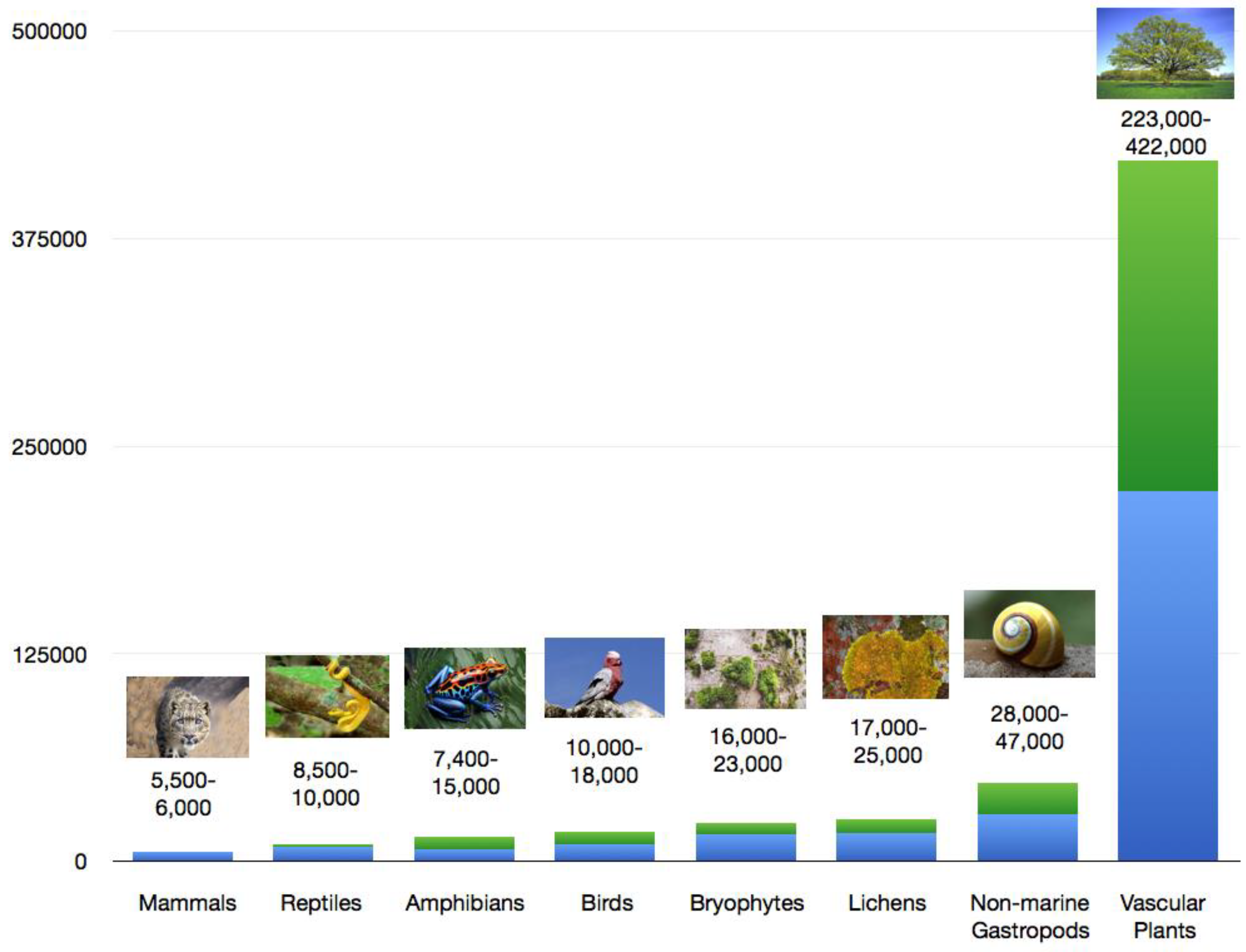
3. Endemism in Different Groups of Organisms
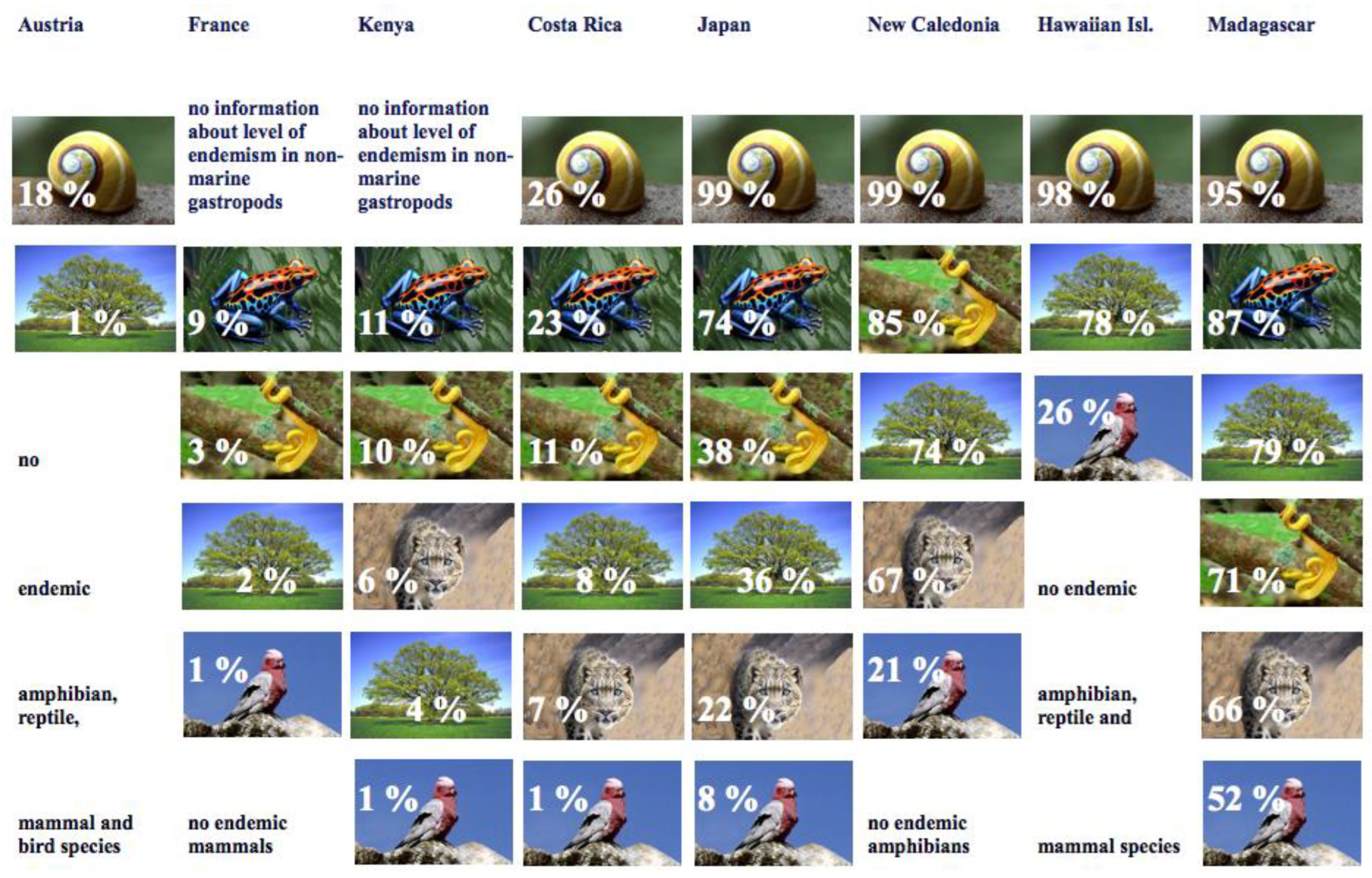
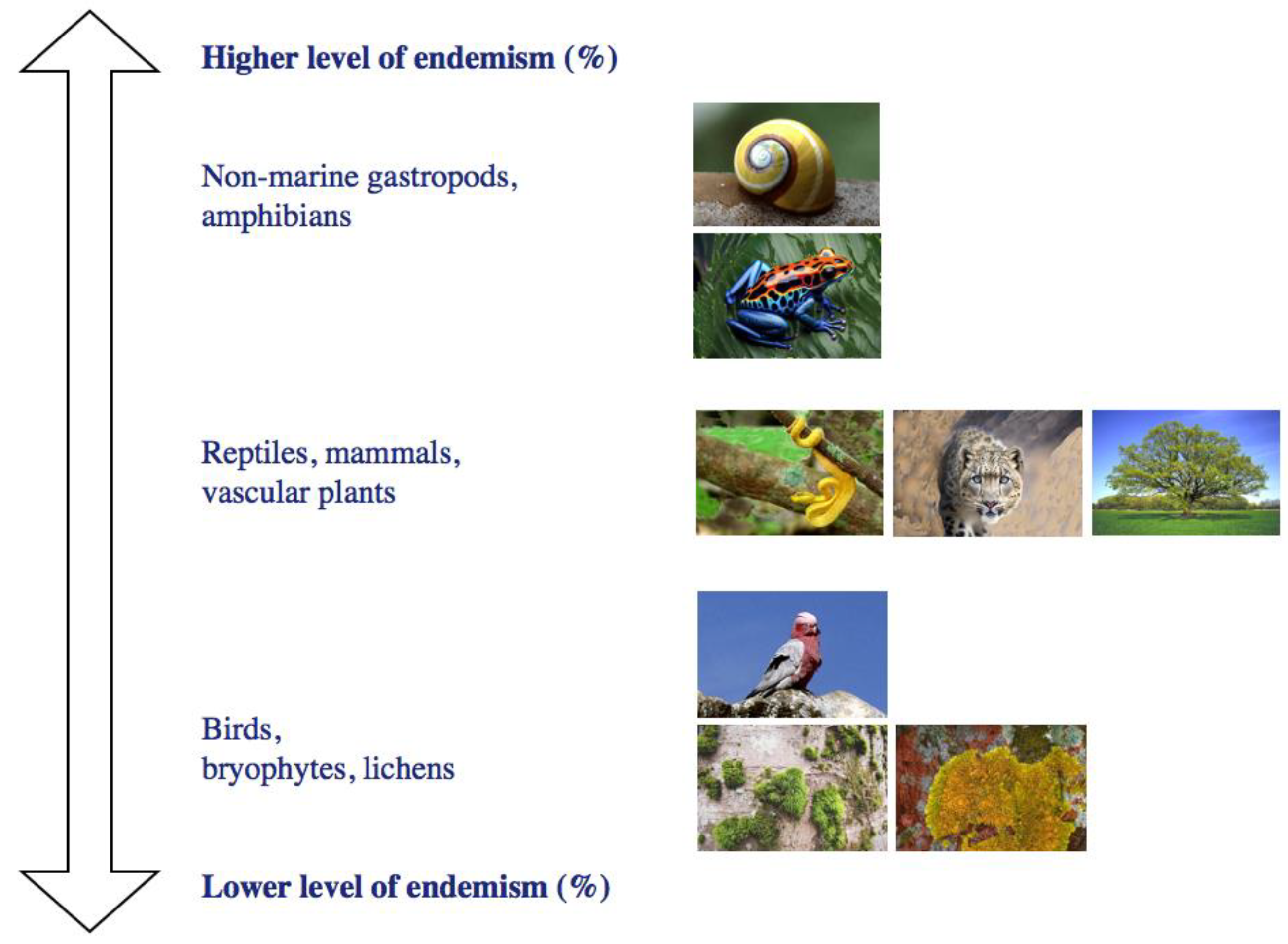
4. How Are Endemics Distributed and Related to Environmental Heterogeneity in Space?
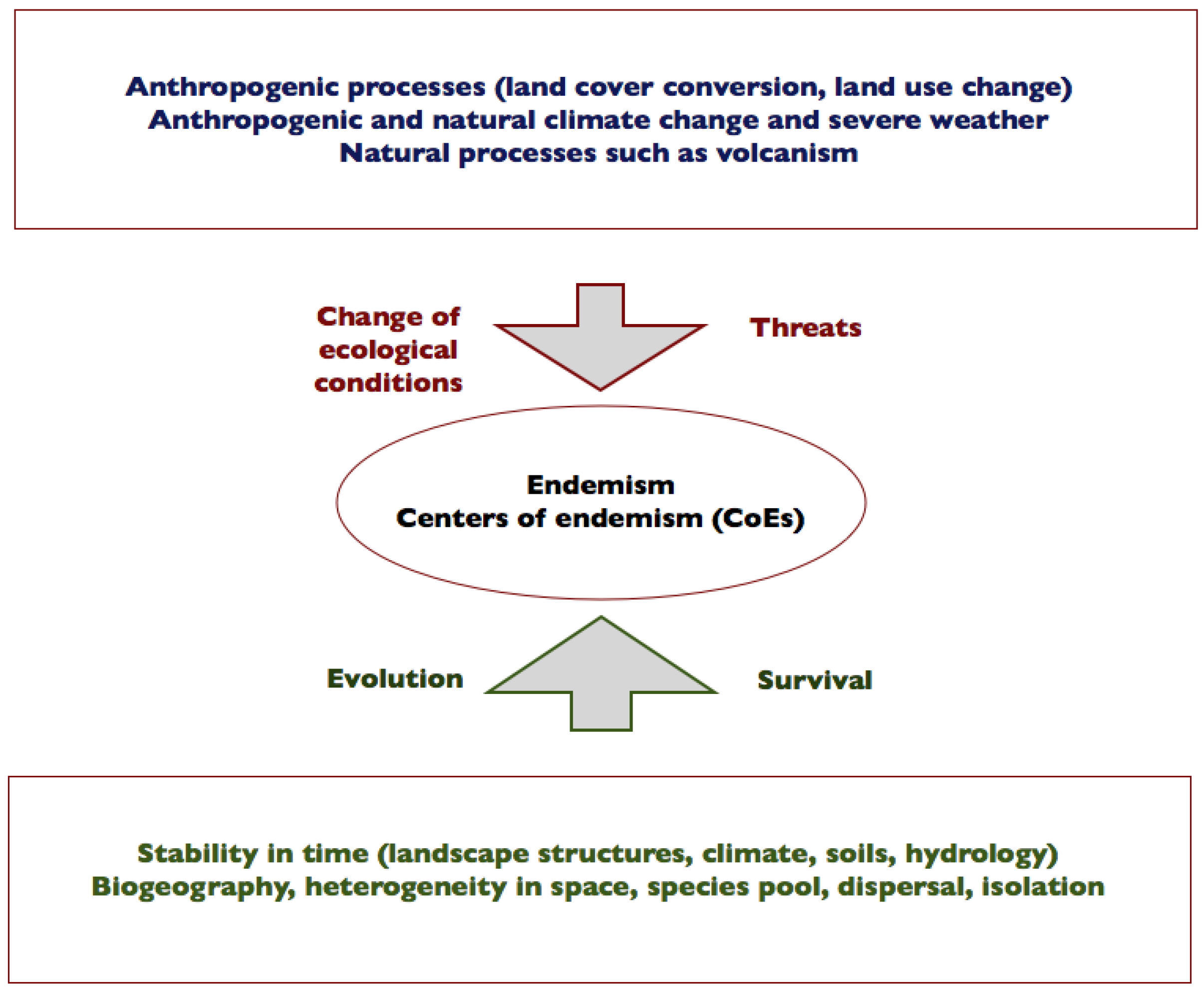
5. How Is Endemism Related to Continuity, Change in Time and Isolation?
6. How Important Are Zoos and Botanical Gardens for Endemics and Vice Versa?
7. Conclusions and Outlook: What Are The Perspectives for Endemics and Centers of Endemism?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Candolle, A.B. Essai elementaire de geographie botanique. Dict. Des. Sci. Nat. 1820, 18, 1–64. [Google Scholar]
- Bramwell, D. (Ed.) Plants and Islands; Academic Press: London, UK, 1979; ISBN 0-12-125460-7. [Google Scholar]
- Hendrych, R. Material and notes about the geography of the highly stenochoric to monotopic endemic species of the European flora. Acta Univ. Carol.-Biol. 1982, 3, 335–372. [Google Scholar]
- Cardona, M.A.; Contandriopoulos, J. Endemism and evolution in the islands of the Western Mediterranean. In Plants and Islands; Bramwell, D., Ed.; Academic Press: London, UK; New York, NY, USA; Toronto, ON, Canada, 2007; pp. 133–170. [Google Scholar]
- Clark, V.R.; Barker, N.P.; Mucina, L. The Sneeuberg: A new centre of floristic endemism on the Great Escarpment. S. Afr. J. Bot. 2008, 75, 196–238. [Google Scholar] [CrossRef]
- Bruchmann, I. Plant Endemism in Europe: Spatial Distribution and Habitat Affinities of Endemic Vascular Plants. Ph.D. Thesis, University of Flensburg, Flensburg, Germany, 2011. [Google Scholar]
- Anádon-Irizarry, V.; Wege, D.C.; Upgren, A.; Young, R.; Boom, B.; Le’on, Y.M.; Arias, Y.; Koenig, K.; Morales, A.L.; Burke, W.; et al. Sites for priority biodiversity conservation in the Caribbean Islands Biodiversity Hotspot. J. Threat. Taxa 2012, 4, 2806–2844. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Harter, D.E.V.; Steinbauer, M.J.; Puyol, D.G.; Fernandez-Palacios, J.M.; Jentsch, A.; Beierkuhlein, C. Climate vs. topography—Spatial patterns of plant species diversity and endemism on a high-elevation island. J. Ecol. 2015, 103, 1621–1633. [Google Scholar] [CrossRef]
- Myers, N. The biodiversity challenge: Expended hotspots analysis. Environmentalist 1990, 10, 243–256. [Google Scholar] [CrossRef]
- van Wyk, A.E.; Smith, G.F. Regions of Floristic Endemism in Southern Africa: A Review with Emphasis on Succulents; Umdaus Press: Pretoria, South Africa, 2001; ISBN 1919766197. [Google Scholar]
- Melendo, M.; Gimenez, E.; Cano, E.; Gomez-Mercado, F.; Valle, F. The endemic flora in the south of the Iberian Peninsula: Taxonomic composition, biological spectrum, pollination, reproductive mode and dispersal. Flora 2003, 198, 260–276. [Google Scholar] [CrossRef]
- Barthlott, W.; Mutke, J.; Rafiqpoor, D.; Kier, G.; Kreft, H. Global centers of vascular plant diversity. Nova Acta Leopold. 2005, 92, 61–83. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2, 2022. Available online: https://www.iucnredlist.org (accessed on 26 January 2023).
- Hobohm, C.; Tucker, C. The increasing importance of endemism, responsibility, the media and education. In Endemism in Vascular Plants; Hobohm, C., Ed.; Plant and Vegetation Series; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9, pp. 3–9. [Google Scholar]
- Burgess, N.D.; Clarke, G.P.; Rodgers, W.A. Coastal forests of eastern Africa: Status, endemism patterns and their potential causes. Biol. J. Linn. Soc. 1998, 64, 337–367. [Google Scholar] [CrossRef]
- Chambers, T.C.; Drinnan, A.N.; McLoughlin, S. Some morphological features of Wollemi Pine (Wollemia nobilis: Araucariaceae) and their comparison to cretaceous plant fossils. Int. J. Plant. Sci. 1998, 159, 160–171. [Google Scholar] [CrossRef]
- Ackerman, J.D.; Trejo-Torres, J.C.; Crespo-Chuy, Y. Orchids of the West-Indies: Predictability of diversity and endemism. J. Biogeogr. 2007, 34, 779–786. [Google Scholar] [CrossRef]
- Clark, V.R.; Bentley, J.; Dold, A.P.; Zikishe, V.; Barker, N.P. The rediscovery of the Great Winterberg endemic Lotononis harveyi B.–E.van Wyk after 147 years, and notes on the poorly known Amathole endemic Macowania revoluta Oliv. (Southern Great Escarpment, South Africa). PhytoKeys 2016, 62, 113–124. [Google Scholar] [CrossRef]
- Lesica, P.; Yurkewycz, R.; Crone, E.E. Rare plants are common where you find them. Am. J. Bot. 2006, 93, 454–459. [Google Scholar] [CrossRef]
- Bevill, R.L.; Louda, S.M. Comparisons of related rare and common species in the study of plant rarity. Conserv. Biol. 1999, 13, 493–498. [Google Scholar] [CrossRef]
- Lavergne, S.; Thompson, J.D.; Garnier, E.; Debussche, M. The biology and ecology of narrow endemic and widespread plants: A comparative study of trait variation in 20 congeneric pairs. Oikos 2004, 107, 505–518. [Google Scholar] [CrossRef]
- Casazza, C.; Barberis, G.; Minuto, L. Ecological characteristics and rarity of endemic plants of the Italian Maritime Alps. Biol. Conserv. 2005, 123, 361–371. [Google Scholar] [CrossRef]
- Hartley, S.; Kunin, W. Scale dependency of rarity, extinction risk, and conservation priority. Conserv. Biol. 2003, 17, 1559–1570. [Google Scholar] [CrossRef]
- Rabinowitz, D. Seven forms of rarity. In The Biological Aspects of Rare Plant Conservation; Synge, H., Ed.; John Wiley & Sons: Chichester, UK; New York, NY, USA; Brisbane, Australia; Toronto, ON, Canada, 1981; pp. 205–217. [Google Scholar]
- Rabinowitz, D.; Cairns, S.; Dillon, T. Seven forms of rarity and their frequency in the flora of British Isles. In Conservation Biology: Science of Scarcity and Diversity; Soulé, M.E., Ed.; Sinauer Associates: Sunderland, MA, USA, 1986; pp. 182–204. [Google Scholar]
- Barker, N.P.; Fish, L. Rare and infrequent southern African grasses: Assessing their conservation status and understanding their biology. Biodivers. Conserv. 2007, 16, 4051–4079. [Google Scholar] [CrossRef]
- Caiafa, A.N.; Martins, F.R. Forms of rarity of tree species in the southern Brazilian Atlantic rainforest. Biodivers. Conserv. 2010, 19, 2597–2618. [Google Scholar] [CrossRef]
- Choe, H.; Thorne, J.H.; Hijmans, R.; Seo, C. Integrating the Rabinowitz rarity framework with a national plant inventory in South Korea. Ecol. Evol. 2019, 9, 1353–1363. [Google Scholar] [CrossRef]
- Toledo, L.F.; Becker, C.G.; Haddad, C.F.; Zamudio, K.R. Rarity as an indicator of endangerment in neotropical frogs. Biol. Conserv. 2014, 179, 54–62. [Google Scholar] [CrossRef]
- McClain, C.R. The commonness of rarity in a deep-sea taxon. Oikos 2021, 130, 863–878. [Google Scholar] [CrossRef]
- Jetz, W.; Rahbek, C.; Colwell, R.K. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol. Lett. 2004, 7, 1180–1191. [Google Scholar] [CrossRef]
- Kruckeberg, A.R.; Rabinowitz, D. Biological aspects of endemism in higher plants. Annu. Rev. Ecol. Syst. 1985, 16, 447–479. [Google Scholar] [CrossRef]
- Riemann, H.; Ezcurra, E. Endemic regions of the vascular flora of the peninsula of Baja California, Mexico. J. Veg. Sci. 2007, 18, 327–336. [Google Scholar] [CrossRef]
- Nowak, A.; Nobis, M. Tentative list of endemic vascular plants of the Zeravshan Mts in Tajikistan: Distribution, habitat preferences and conservation status of species. Biodivers. Res. Conserv. 2010, 19, 65–80. [Google Scholar] [CrossRef]
- Brochmann, C.; Rustan, O.H.; Lobin, W.; Kilian, N. The endemic vascular plants of the Cape Verde Islands, W. Africa. Sommerfeltia 1997, 24, 1–363. [Google Scholar] [CrossRef]
- Izquierdo, I.; Martín, J.L.; Zurita, N.; Arechavaleta, M. (Eds.) Lista de Especies Silvestres de Canarias (Hongos, Plantas, y Animales Terrestres); Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias: La Laguna, Spain, 2004; ISBN 84-89729-23-9. [Google Scholar]
- Latheef, S.A.; Prasad, B.; Bavaji, M.; Subramanyam, G. A database on endemic plants at Tirumala hills in India. Bioinformation 2008, 2, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.R.; Timberlake, J.R.; Hyde, M.A.; Mapaura, A.; Palgrave, M.C.; Wursten, B.T.; Ballings, P.; Burrows, J.E.; Linder, H.P.; McGregor, G.K.; et al. A first comprehensive account of floristic diversity and endemism on the Nyanga Massif, Manica Highlands (zimbabwe-Mozambique). Kirkia 2017, 19, 1–53. [Google Scholar]
- Lowry, P.P., II. Diversity, endemism, and extinction in the flora of New Caledonia: A review. In Rare, Threatened, and Endangered Floras of Asia and the Pacific Rim; Peng, C.I., Lowry, P.P., II, Eds.; Institute of Botany, Academica Sinica: Taipei, China, 1998; Volume 16, pp. 181–206. [Google Scholar]
- Cowling, R.M.; Lombard, A.T. Heterogeneity, speciation/extinction history and climate: Explaining regional plant diversity patterns in the Cape Floristic Region. Divers. Distrib. 2002, 8, 163–179. [Google Scholar] [CrossRef]
- Giménez, E.; Melendo, M.; Valle, F.; Gomez-Mercado, F.; Cano, E. Endemic flora biodiversity in the south of the Iberian Peninsula: Altitudinal distribution, life forms and dispersal modes. Biodivers. Conserv. 2004, 13, 2641–2660. [Google Scholar] [CrossRef]
- Helme, N.A.; Trinder-Smith, T.H. The endemic flora of the Cape Peninsula, South Africa. S. Afr. J. Bot. 2006, 72, 205–210. [Google Scholar] [CrossRef]
- Kier, G.; Kreft, H.; Lee, T.M.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322–9327. [Google Scholar] [CrossRef] [PubMed]
- Fenu, G.; Mattana, E.; Congiu, A.; Bacchetta, G. The endemic vascular flora of Supramontes (Sardinia), a high priority plant conservation area. Candollea 2010, 65, 347–358. [Google Scholar] [CrossRef]
- Huang, J.-H.; Chen, B.; Liu, C.; Lai, J.; Zhang, J.; Ma, K. Identifying hotspots of endemic woody seed plant diversity in China. Divers. Distrib. 2011, 18, 673–688. [Google Scholar] [CrossRef]
- Anguinano, M.; Dean, E.; Starbuck, T.; Rodriguez, A.; Munguía-Lino, G. Diversity, species richness distribution and centers of endemism of Lycianthes (Capsiceae, Solanaceae) in Mexicao. Phytotaxa 2021, 514, 39–60. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Normand, S.; Skov, F. Plio-Pleistocene climate change and geographic heterogeneity in plant-diversity relationships. Ecography 2009, 32, 13–21. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T. Environmental and historical effects on richness and endemism patterns of carabid beetles in the western Palearctic. Ecography 2009, 32, 705–714. [Google Scholar] [CrossRef]
- Fritz, S.A.; Rahbek, C. Global patterns of amphibian phylogenetic diversity. J. Biogeogr. 2012, 39, 1373–1382. [Google Scholar] [CrossRef]
- Voskamp, A.; Baker, D.J.; Stephens, P.A.; Valdes, P.J.; Willis, S.G. Global patterns in the divergence between phylogenetic diversity and species richness in terrestrial birds. J. Biogeogr. 2017, 44, 709–721. [Google Scholar] [CrossRef]
- Taylor, P.J.; Kearney, T.; Dalton, D.L.; Chakona, C.; Kelly, C.M.R.; Barker, N.P. Biomes, geology and past climate drive speciation of laminate-toothed rats on South African mountains (Murinae: Otomys). Zool. J. Linn. Soc. 2019, 20, 1–21. [Google Scholar] [CrossRef]
- Hobohm, C.; Beierkuhnlein, C.; Börtitz, C.; Clark, V.R.; El Balti, N.; Fichtner, A.; Franklin, S.; Gaens, T.; Härdtle, W.; Hansen, A.S.; et al. Land Use Change and the Future of Biodiversity. Environ. Chall. Solut. 2021, 19, 451–483. [Google Scholar] [CrossRef]
- Fjeldsa, J.; Lovett, J.C. Geographical patterns of old and young species in African forest biota: The significance of specific montane areas as evolutionary centres. Biodiv. Cons. 1997, 6, 325–346. [Google Scholar] [CrossRef]
- Goldie, X.; Gillman, L.; Crisp, M.; Wright, S. Evolutionary speed limited by water in arid Australia. Proc. Biol. Sci. 2010, 277, 2645–2653. [Google Scholar] [CrossRef]
- Bruchmann, I.; Hobohm, C. Factors that create and increase endemism. In Endemism in Vascular Plants; Hobohm, C., Ed.; Plant and Vegetation Series; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9, pp. 51–68. [Google Scholar]
- Alagador, D.; Cerdeira, J.O.; Araujo, M.B. Climate change, species range shifts and dispersal corridors: An evaluation of spatial conservation models. Methods Ecol. Evol. 2015, 7, 853–866. [Google Scholar] [CrossRef]
- Zobel, M. The species pool concept as a framework for studying patterns of plant diversity. J. Veg. Sci. 2016, 27, 8–18. [Google Scholar] [CrossRef]
- Zizka, G.; Klemmer, K. Pflanzen- und Tierwelt der Galapagos-Inseln—Entstehung, Erforschung, Gefährdung und Schutz. Kleine Senckenberg. 1994, 20, 1–151. [Google Scholar]
- Wilmé, L.; Ravokatra, M.; Dolch, R.; Schuurman, D.; Mathieu, E.; Schuetz, H.; Waeber, P.O. Toponyms for centers of endemism in Madagascar. Madag. Conserv. Dev. 2012, 7, 30–40. [Google Scholar] [CrossRef]
- Cordier, J.M.; Lescano, J.N.; Ríos, N.E.; Leynaud, G.C.; Nori, J. Climate change threatens micro-endemic amphibians of an important South American high-altitude center of endemism. Amphib.-Reptil. 2019, 41, 233–243. [Google Scholar] [CrossRef]
- Olefeld, J.L.; Bock, C.; Jensen, M.; Vogt, J.C.; Sieber, G.; Albach, D.; Boenigk, J. Centers of endemism of freshwater protists deviate from pattern of taxon richness on a continental scale. Sci. Rep. 2020, 10, 14431. [Google Scholar] [CrossRef] [PubMed]
- Suissa, J.S.; Sundue, M.A. Diversity Patterns of Neotropical Ferns: Revisiting Tryon’s Centers of Richness and Endemism. Am. Fern J. 2020, 110, 211–232. [Google Scholar] [CrossRef]
- Robuchon, M.; Pavoine, S.; Véron, S.; Delli, G.; Faith, D.P.; Mandrici, A.; Pellens, R.; Dubois, G.; Leroy, B. Revisiting species and areas of interest for conserving global mammalian phylogenetic diversity. Nat. Comm. 2021, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.D.; Heywood, V.H.; Hamilton, A.C. (Eds.) Centres of Plant Diversity: Vol 1, Europe, Africa, South West Asia and the Middle East; IUCN Publications: Cambridge, UK, 1994; ISBN 2-8317-0197-X. [Google Scholar]
- Davis, S.D.; Heywood, V.H.; Hamilton, A.C. (Eds.) Centres of Plant Diversity: Vol 2, Asia, Australasia and the Pacific; IUCN Publications: Cambridge, UK, 1995; ISBN 2-8317-0198-8. [Google Scholar]
- Davis, S.D.; Heywood, V.H.; Herrera-MacBryde, O.; Villa-Lobos, J.; Hamilton, A.C. (Eds.) Centres of Plant Diversity: Vol 3, the Americas; IUCN Publications: Cambridge, UK, 1997; ISBN 2-8317-0199-6. [Google Scholar]
- Waeber, P.; Wilmé, L.; Mercier, J.L.; Rakotozafy, L.M.A.; Garcia, C.; Sorg, J.P. The role of lakes in the context of the centers of endemism. Akon’ny Ala 2015, 32, 34–47. [Google Scholar]
- Clark, V.R.; Dold, A.P.; McMaster, C.; McGregor, G.; Bredenkamp, C.; Barker, N. Rich sister, poor cousin: Plant diversity and endemism in the Great Winterberg-Amatholes (Great Escarpment, Eastern Cape, South Africa). S. Afr. J. Bot. 2014, 92, 159–174. [Google Scholar] [CrossRef]
- Clayton, W.D. Chorology of the genera of Gramineae. Kew Bull. 1975, 30, 111–132. [Google Scholar] [CrossRef]
- Fattorini, S. A history of chorological categories. Hist. Philos. Life Sci. 2016, 38, 12. [Google Scholar] [CrossRef]
- Linder, H.P.; Lovett, J.; Mutke, J.M.; Barthlott, W.; Jürgens, N.; Rebelo, T.; Küper, W. A numerical re-evaluation of the sub-Saharan phytochoria of mainland Africa. In Plant Diversity and Complexity Patterns: Local, Regional and Global Dimensions, Proceedings of the International Symposium, Royal Danish Academy of Sciences and Letters, Copenhagen, Denmark, 25–28 May 2003; Det Kongelige Danske Videnskabernes Selskab: Copenhagen, Denmark, 2005; pp. 229–252. [Google Scholar]
- Bradshaw, P.L.; Colville, J.F.; Linder, H.P. Optimising regionalisation techniques: Identifying centres of endemism in the extraordinarily endemic-rich Cape Floristic Region. PLoS ONE 2015, 10, e0132538. [Google Scholar] [CrossRef]
- Hobohm, C.; Tucker, C. How to quantify endemism. Plant Veg. 2014, 9, 11–48. [Google Scholar]
- Rosauer, D.; Laffan, S.W.; Crisp, M.D.; Donnellan, S.C.; Cool, L.G. Phylogenetic endemism: A new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol. 2009, 18, 4061–4072. [Google Scholar] [CrossRef] [PubMed]
- Chiarucci, A.; Beierkuhnlein, C.; Essl, F.; Fernández-Palacios, J.M.; Jentsch, A.; Hobohm, C.; Kreft, H.; Krestov, P.V.; Löbel, S.; Steinbauer, M.J.; et al. Global patterns of vascular plant species richness and endemicity, a new approach to identify hotspots and cold spots. In Biodiversity & Vegetation: Patterns, Processes, Conservation; Mucina, L., Price, J.N., Kalwij, J.M., Eds.; Kwongan Foundation: Perth, Australia, 2014; p. 78. [Google Scholar]
- Herrera, J.P. Prioritizing protected areas in Madagascar for lemur diversity using a multidimensional perspective. Biol. Cons. 2017, 207, 1–8. [Google Scholar] [CrossRef]
- Cowling, R.M.; Samways, M.J. Predicting global patterns of endemic plant species richness. Biodivers. Lett. 1995, 2, 127–131. [Google Scholar] [CrossRef]
- Hobohm, C.; Janišová, M.; Steinbauer, M.; Landi, S.; Field, R.; Vanderplank, S.; Beierkuhnlein, C.; Grytnes, J.-A.; Vetaas, O.R.; Fildelis, A.; et al. Global endemics-area relationships of vascular plants. Perspect. Ecol. Cons. 2019, 17, 41–49. [Google Scholar] [CrossRef]
- Daru, B.H.; Farooq, H.; Antonelli, A.; Faurby, S. Endemism patterns are scale dependent. Nat. Commun. 2020, 11, 2115. [Google Scholar] [CrossRef]
- Shipley, B.; McGuire, J.L. Interpreting and integrating multiple endemism metrics to identify hotspots for conservation priorities. Biol. Cons. 2021, 265, 109403. [Google Scholar] [CrossRef]
- Jacobs, J.G.; Lashley, M.A.; Cove, M.V. Fawn counts and adult female site use are mismatched indicators of habitat quality in an endangered deer. Diversity 2021, 13, 92. [Google Scholar] [CrossRef]
- Pinzari, C.; Peck, R.; Zinn, T.; Gross, D.; Montoya-Aiona, K.; Brinck, K.; Gorresen, M.; Bonaccorso, F. Hawaiian Hoary Bat (Lasiurus cinereus semotus): Activity, Diet and Prey Availability at the Waihou Mitigation Area, Maui. Tech. Rep. 2019, HCSU-090, 1–60. [Google Scholar]
- Romo, H.; García-Barros, E.; Lobo, J.M. Identifying recorder-induced geographic bias in an Iberian butterfly database. Ecography 2006, 29, 873–885. [Google Scholar] [CrossRef]
- Grant, P.B.C.; Samways, M.J. Micro-hotspot determination and buffer zone value for Odonata in a globally significant biosphere reserve. Biol. Conserv. 2011, 144, 772–781. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2023. Available online: https://www.iucnredlist.org (accessed on 17 January 2023).
- Román-Palacios, C.; Moraga-López, D.; Wiens, J.J. The origins of global biodiversity on land, sea and freshwater. Ecol. Lett. 2022, 25, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.G. Ecoregions: The Geography of Oceans and Continents; Springer: New York, NY, USA, 1998; ISBN 0-387-98311-2. [Google Scholar]
- Wiens, J.J. Faster diversification on land than sea helps explain global biodiversity patterns among habitats and animal phyla. Ecol. Lett. 2015, 18, 1234–1241. [Google Scholar] [CrossRef]
- Thode, V.A.; Inacio, C.D.; Eggers, L.; Reginato, M.; Souza-Chies, T.T. Spatial-temporal evolution and diversification in Sisyrinchium (Iridaceae) with emphasis on abiotic drivers. Bot. J. Linn. Soc. 2021, 99, 93–108. [Google Scholar] [CrossRef]
- Teske, P.R.; Sandoval-Castillo, J.; Waters, J.; Beheregaray, L.B. An overview of Australia’s temperate marine phylogeography, with new evidence from high-dispersal gastropods. J. Biogeogr. 2017, 44, 217–229. [Google Scholar] [CrossRef]
- Murali, G.; Gumbs, R.; Meiri, S.; Roll, U. Global determinants and conservation of evolutionary and geographic rarity in land vertebrates. Sci. Adv. 2021, 7, eabe5582. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, R. How many species of seed plants are there? Taxon 2001, 50, 1085–1090. [Google Scholar] [CrossRef]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The global decline of nonmarine mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Paton, A.J.; Brummit, N.; Govaerts, R.; Harman, K.; Hinchcliffe, S.; Allkin, B.; Lughadha, N. Towards target 1 of the global strategy for plant conservation: A working list of all known plant species—Progress and prospects. Taxon 2008, 57, 602–611. [Google Scholar]
- Chapman, A.D. Numbers of Living Species in Australia and the World, 2nd ed.; Department of Climate Change, Energy, the Environment and Water: Canberra, Australia, 2009; ISBN 978-0-642-56860-1. [Google Scholar]
- Barrowclough, G.F.; Cracraft, J.; Klicka, J.; Zink, R.M. How many kinds of birds are there and why does it matter? PLoS ONE 2016, 11, e0166307. [Google Scholar] [CrossRef]
- Groombridge, B.; Jenkins, D. World Atlas of Biodiversity; University of California Press: Berkeley, CA, USA, 2002; ISBN 0-520-23668-8. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007; ISBN 9780198566120. [Google Scholar]
- Rabitsch, W.; Essl, F. (Eds.) Endemiten. Kostbarkeiten in Österreichs Pflanzen- und Tierwelt; Umweltbundesamt: Vienna, Austria, 2009; ISBN 978-3-85328-049-2. [Google Scholar]
- Hobohm, C.; Janišová, M.; Jansen, J.; Bruchmann, I.; Deppe, U. Biogeography of endemic vascular plants—Overview. In Endemism in Vascular Plants; Hobohm, C., Ed.; Plant and Vegetation; Springer: Dordrecht, The Netherlands, 2014; Volume 9, pp. 85–163. [Google Scholar]
- Rangel, F.C.S.; Gomes, S.R.; Canuto, T.; Rodrigues, P.S.; Theingo, S. Diversity of non-marine gastropods of the Fiocruz Atlantic Forest Biological Station and adjacents urban areas, Rio de Janeiro, RJ, Brasil. An. Acad. Bras. Cienc. 2021, 93, e20190691. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.S.; Gillett, H.J. (Eds.) IUCN Red List of Threatened Plants; The World Conservation Union: Gland, Switzerland; Cambridge, UK, 1998; ISBN 2-8317-0328-X. [Google Scholar]
- Vanderplank, S.E.; Moreira-Muñoz, A.; Hobohm, C.; Pils, G.; Noroozi, J.; Clark, V.R.; Barker, N.P.; Yang, W.; Huang, J.; Ma, K.; et al. Endemism in mainland regions: Case studies. Plant Veg. 2014, 9, 205–308. [Google Scholar]
- Zhang, Z.; Yan, Y.; Tian, Y.; He, J.-S.; Tang, Z. Distribution and conservation of orchid species richness in China. Biol. Cons. 2015, 181, 64–72. [Google Scholar] [CrossRef]
- Gleich, M.; Maxeiner, D.; Miersch, M.; Nicolay, F. Life Counts: Eine Globale Bilanz des Lebens; Berlin Verlag: Berlin, Germany, 2000. [Google Scholar]
- Barrientos, Z. Estado actual del conocimiento y la conservacíon de los moluscos continentales de Costa Rica. Rev. Biol. Trop. 2003, 51, 285–292. [Google Scholar]
- Yeung, N.W.; Hayes, K.A. Biodiversity and extinction of Hawaiian land snails: How many are left now and what must we do to conserve them—A reply to Solem (1990). Integr. Comp. Biol. 2018, 58, 1157–1169. [Google Scholar] [CrossRef]
- Zumbado-Ulate, H.; Nelson, K.N.; García-Rodríguez, A.; Chaves, G.; Arias, E.; Bolaños, F.; Whitfield, M.M.; Catherine, L.; Searle, C.L. Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level. Diversity 2019, 11, 129. [Google Scholar] [CrossRef]
- Forman, R.T. Land Mosaics: The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, UK, 1995; ISBN 978-1-59726-646-8. [Google Scholar]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Olofsson, J.; de Mazancourt, C.; Crawley, M.J. Spatial heterogeneity and plant species richness at different spatial scales under rabbit grazing. Oecologia 2008, 156, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Megías, A.G.; Gómez, J.M.; Sánchez-Pinero, F. Spatio-temporal change in the relationship between habitat heterogeneity and species diversity. Acta Oecol. 2011, 37, 179–186. [Google Scholar] [CrossRef]
- Chen, J.; Shiyomi, M.; Wei, Z. Determining spatial heterogeneity in species richness of plant community. Grassl. Sci. 2015, 61, 56–60. [Google Scholar] [CrossRef]
- Huston, M.A. Biological Diversity: The Coexistence of Species on Changing Landscapes; Cambridge University Press: Cambridge, UK, 1994; ISBN 0-521-36093-5. [Google Scholar]
- Fortin, M.-J.; Dale, M.R.T. Spatial Analysis: A Guide for Ecologists; Cambridge University Press: Cambridge, UK, 2005; ISBN 0521143500. [Google Scholar]
- Pugh, P.J.A.; Convey, P. Surviving out in the cold: Antarctic endemic invertebrates and their refugia. J. Biogeogr. 2008, 35, 2176–2186. [Google Scholar] [CrossRef]
- Huang, J.-H.; Chen, B.; Ying, J.-S.; Ma, K. Features and distribution patterns of Chinese endemic seed plant species. J. Syst. Evol. 2011, 49, 81–94. [Google Scholar] [CrossRef]
- Loiseau, N.; Mouquet, N.; Casajus, N.; Grenié, M.; Guéguen, M.; Maitner, B.; Mouillot, D.; Ostling, A.; Renaud, J.; Tucker, C.; et al. Global distribution and conservation status of ecologically rare mammal and bird species. Nat. Commun. 2020, 11, 5071. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, B. Der Endemismus in der Flora der Alpen, der Karpaten und der Balkanischen Gebirge im Verhältnis zu den Pflanzengesellschaften. Mitt. Der Ostalpin-Dinarischen Pflanzensoziol. Arb. 1969, 9, 167–178. [Google Scholar]
- Talbot, S.S.; Yurtsev, B.A.; Murray, D.F.; Argus, G.W.; Bay, C.; Elvebakk, A. Atlas of rare endemic vascular plants in the Arctic. Conservation of Arctic flora and fauna (CAFF). Tech. Rep. 1999, 3, 1–73. [Google Scholar]
- Beard, J.S.; Chapman, A.R.; Gioia, P. Species richness and endemism in the Western Australian flora. J. Biogeogr. 2000, 27, 1257–1268. [Google Scholar] [CrossRef]
- Jonsell, B.; Karlsson, T. Endemic vascular plants in Norden. In Flora Nordica. General Volume; Jonsell, B., Ed.; Bergius Foundation: Stockholm, Sweden, 2004; pp. 139–159. [Google Scholar]
- Wollenberg, K.C.; Vieites, D.R.; van der Meijden, A.; Glaw, F.; Cannatella, D.C.; Vences, M. Patterns of endemism and species richness in Malagasy cophyline frogs support a key role of mountainous areas for speciation. Evolution 2008, 62, 1890–1907. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
- McCain, C.M. The mid-domain effect applied to elevational gradients: Species richness of small mammals in Costa Rica. J. Biogeogr. 2004, 31, 19–31. [Google Scholar] [CrossRef]
- McCain, C.M. Elevational gradients in diversity of small mammals. Ecology 2005, 86, 366–372. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.-A. Elevational Gradients in Species Richness. In Encyclopedia of Life Sciences (ELS); Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Storch, D.; Keil, P.; Jetz, W. Universal species-area and endemics-area relationships at continental scales. Nature 2012, 488, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Varzinczak, L.H.; Zanata, T.B.; Moura, M.O.; Passos, F.C. Geographical patterns and current and short-term historical correlates of phylogenetic diversity and endemism for New World primates. J. Biogeogr. 2019, 47, 890–902. [Google Scholar] [CrossRef]
- Orme, C.; Davies, R.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.-S.; Rasmussen, P.C.; Ridgely, R.S.; et al. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef]
- Wikelski, M.; Wilcove, D.S. Endemic species in the narrowest niches. In The Atlas of Global Conservation; Molnar, J.L., Ed.; University of California Press: Berkeley, CA, USA, 2010; p. 60. ISBN 978-0-520-26256-0. [Google Scholar]
- Cronk, Q.C.; Percy, D.M. Endemism. In Encyclopedia of Islands; Gillespie, R.G., Clague, D.A., Eds.; University of California Press: Berkeley, CA, USA, 2009. [Google Scholar]
- Hobohm, C. Plant species diversity and endemism on islands and archipelagos—With special reference to the Macaronesian Islands. Flora 2000, 195/1, 9–24. [Google Scholar] [CrossRef]
- Hobohm, C.; Müller-Benedict, V. Vergleich der Biodiversität insularer und kontinentaler Regionen unter besonderer Berücksichtigung der Endemitenvielfalt. Ber. RTG 2018, 30, 57–71. [Google Scholar]
- Stuessy, T.F.; Grau, J.; Zizka, G. Diversidad de plantas en las Islas Robinson Crusoe. In Flora Silvestre de Chile; Grau, J., Zizka, G., Eds.; Stadt: Frankfurt am Main, Germany, 1992; Volume 19, pp. 54–66, Palmengarten Sonderh. [Google Scholar]
- Tryon, R. Biogeography of the Antillean fern flora. In Plants and Islands; Bramwell, D., Ed.; Academic Press: London, UK, 1979; pp. 55–68. [Google Scholar]
- Balslev, H.; Valencia, R.; Paz y Miño, G.; Christensen, H.; Nielsen, I. Species count of vascular plants in one hectare of humid lowland forest in Amazonian Ecuador. In Forest Biodiversity in North, Central and South America, and the Caribbean: Research and Monitoring; Dallmeier, F., Comiskey, J.A., Eds.; UNESCO: Paris, France, 1998; pp. 585–594. [Google Scholar]
- Rolecek, J.; Drevojan, P.; Hajkova, P.; Goia, I.; Hajek, M. Update on maxima of fine-scale vascular plant species richness in a Transsylvanian steppe meadow. Tuexenia 2021, 41, 459–466. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Ann. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Piedade, M.T.F.; Müller, E.; Long, S.P.; Junk, W.J.; Jones, M.B. Very high productivity of the C4 aquatic grass Echinochloa polystachya in the Amazon floodplain confirmed by net ecosystem CO2 flux measurements. Oecologia 2000, 125, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Piedade, M.T.F.; Long, S.P.; Junk, W.J. Leaf and canopy photosysthesis CO2 uptake of a stand of Echinochloa polystachya on the Amazon floodplain. Funct. Ecol. 1994, 11, 60–65. [Google Scholar] [CrossRef]
- Stuessy, T.F.; Ono, M. (Eds.) Evolution and Speciation of Island Plants; Cambridge University Press: Cambridge, UK, 1998; ISBN 0-521-49653-5. [Google Scholar]
- Roberts, B.A.; Proctor, J. (Eds.) The Ecology of Areas with Serpentinized Rocks: A World View; Kluwer Academic: Dordrecht, The Netherlands, 1992; ISBN 978-94-011-3722-5. [Google Scholar]
- Stevanovic, V.; Tan, K.; Iatrou, G. Distribution of the endemic Balkan flora on serpentine I: Obligate serpentine endemics. Plant Syst. Evol. 2003, 242, 149–170. [Google Scholar] [CrossRef]
- Hobohm, C.; Moro-Richter, M.; Beierkuhnlein, C. Distribution and Habitat Affinity of Endemic and Threatened Species—Global Assessment. In Perspectives for Biodiversity and Ecosystems; Environmental Challenges and Solutions; Springer: Cham, Switzerland, 2021; pp. 233–277. [Google Scholar]
- MacArthur, R.; Wilson, E.O. Island Biogeography; Princeton University Press: Princeton, UK, 1967; ISBN 0-691-08836-5. [Google Scholar]
- Givnish, T.J. Adaptive radiation, dispersal, and diversification of the Hawaiian Lobeliads. In The Biology of Biodiversity; Kato, M., Ed.; Springer: Tokyo, Japan, 2000; pp. 67–90. [Google Scholar]
- Humphries, C.J. Endemism and evolution in Macaronesia. In Plants and Islands; Bramwell, D., Ed.; Academic Press: London, UK, 1979. [Google Scholar]
- Mucina, L.; Wardell-Johnson, G. Landscape age and soil fertility, climatic stability, and fire regime predictability: Beyond the OCBIL framework. Plant Soil 2011, 341, 1–23. [Google Scholar] [CrossRef]
- Rosauer, D.; Jetz, W. Phylogenetic endemism in terrestrial mammals. Glob. Ecol. Biogeogr. 2014, 24, 168–179. [Google Scholar] [CrossRef]
- Médail, F.; Verlaque, R. Ecological characteristics and rarity of endemic vascular plants from Southeastern France and Corsica: Implications for biodiversity conservation. Biol. Cons. 1997, 80, 269–281. [Google Scholar] [CrossRef]
- Pärtel, M. Local plant diversity patterns and evolutionary history at local scale. Ecology 2002, 83, 2361–2366. [Google Scholar] [CrossRef]
- Price, M.R.; Hadfield, M.G. Population genetics and the effects of a severe bottleneck in an ex situ population of critically endangered hawaiian tree snails. PLoS ONE 2014, 9, e114377. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A.; Elórtegui Francioli, S.; Hobohm, C.; Sequeira, M. Endemism on islands: Case Studies. Plant Veg. 2014, 9, 165–204. [Google Scholar]
- Linder, H.P.; Barker, N.P. Does polyploidy facilitate long-distance dispersal? Ann. Bot. 2014, 113, 1175–1183. [Google Scholar] [CrossRef]
- Meudt, H.M.; Albach, D.C.; Tanentzap, A.J.; Igea, J.; Newmarch, S.C.; Brandt, A.J.; Lee, W.G.; Tate, J.A. Polyploidy on islands: Its emergence and importance for diversification. Front. Plant Sci. 2021, 12, 637214. [Google Scholar] [CrossRef]
- Reiss, M.J. Optimization theory in behavioural ecology. J. Biol. Educ. 1987, 21, 241–247. [Google Scholar] [CrossRef]
- Parker, G.A.; Maynard Smith, J. Optimality theory in evolutionary biology. Nature 1990, 348, 27–33. [Google Scholar] [CrossRef]
- Richardson, R.C. Optimization in evolutionary ecology. Proc. Bienn. Meet. Philos. Sci. Found. 1994, 1, 13–21. [Google Scholar] [CrossRef]
- Fukami, T.; Morin, P.J. Productivity-species diversity relationships depend on the history of community assembly. Nature 2003, 424, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.K.; Tan, K.C. Evolutionary Multi-Objective Optimization in Uncertain Environments: Issues and Algorithms; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-95975-5. [Google Scholar]
- Reluga, T.C.; Shaw, A.K. Resource distribution drives the adaption of migratory, partially migratory, or residential strategies. Theor. Ecol. 2015, 8, 437–447. [Google Scholar] [CrossRef]
- McGlone, M.S.; Duncan, R.P.; Heenan, P.B. Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. J. Biogeogr. 2001, 28, 199–216. [Google Scholar] [CrossRef]
- López-Aguirre, C.; Hand, S.J.; Laffan, S.W.; Archer, M. Zoogeographical regions and geospatial patterns of phylogenetic diversity and endemism of New World bats. Ecography 2019, 6, 1188–1199. [Google Scholar] [CrossRef]
- Hobohm, C.; Vanderplank, S.E. Resources for Humans, Plants and Animals: Who is the Ruler of the Driver? And: Can Resource Use Explain Everything? In Perspectives for Biodiversity and Ecosystems; Environmental Challenges and Solutions; Springer: Cham, Switzerland, 2021; pp. 79–106. [Google Scholar]
- Beierkuhnlein, C.; Walentowitz, A.; Welss, W. FloCan—A Revised Checklist for the Flora of the Canary Islands. Diversity 2021, 13, 480. [Google Scholar] [CrossRef]
- Dynesius, M.; Jansson, R. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl. Acad. Sci. USA 2000, 97, 9115–9120. [Google Scholar] [CrossRef]
- Jansson, R.; Dynesius, M. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 2002, 33, 741–777. [Google Scholar] [CrossRef]
- Magellan, K.; Weyl, O.L.F.; Booth, A.J. Preference for artificial refugia over natural refugia in an endangered fish. Diversity 2021, 13, 635. [Google Scholar] [CrossRef]
- Jansson, R. Global patterns in endemism explained by past climatic change. Proc. R. Soc. Lond. B 2003, 270, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Magoulick, D.D.; Kobza, R.M. The role of refugia for fishes during drought: A review and synthesis. Freshw. Biol. 2003, 48, 1186–1198. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.; Zappa, E.; Mariotti, M.G.; Medail, F.; Minuto, L. Ecological and historical factors affecting distribution pattern and richness of endemic plant species: The case of the maritime and Ligurian Alps hotspot. Divers. Distrib. 2008, 14, 47–58. [Google Scholar] [CrossRef]
- López-Aguirre, C.; Hand, S.J.; Laffan, S.W.; Archer, M. Phylogenetic diversity, types of endemism and the evolutionary history of New World bats. Ecography 2018, 41, 12. [Google Scholar] [CrossRef]
- Sampaio, M.B.; Schiel, N.; da Silva Souto, A. From exploitation to conservation: A historical analysis of zoos and their functions in human societies. Ethnobiol. Conserv. 2020, 9. [Google Scholar] [CrossRef]
- Marešová, J.; Frynta, D. Noah’s Ark is full of common species attractive to humans: The case of bold snakes in zoos. Ecol. Econ. 2008, 64, 554–558. [Google Scholar] [CrossRef]
- Bowkett, A.E. Recent captive-breeding proposals and the return of the ark concept to global species conservation. Conserv. Biol. 2009, 23, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Gardner, R.; Kraaijeveld, A.R.; Riordan, P. Contributions of zoos and aquariums to reintroductions: Historical reintroduction efforts in the context of changing conservation perspectives. Int. Zoo Yearb. 2017, 51, 15–31. [Google Scholar] [CrossRef]
- Fraser, J.; Wharton, D. The future of zoos: A new model for cultural institutions. Curator Mus. J. 2007, 50, 41–54. [Google Scholar] [CrossRef]
- Palmer, C.; Kasperbauer, T.J.; Sandøe, P. Bears or butterflies? How should zoos make value-driven decisions about their collections? In The Ark and Beyond: The Evolution of Zoo and Aquarium Conservation; Minteer, B.A., Maienschein, J., Collins, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2018; pp. 179–191. ISBN 978-0-226-538446-4. [Google Scholar]
- Brereton, J.; Brereton, S. Sixty years of collection planning: What species do zoos and aquariums keep? Int. Zoo Yearb. 2020, 54, 131–145. [Google Scholar] [CrossRef]
- Martin, T.E.; Lurbiecki, H.; Joy, J.B.; Mooers, A.O. Mammal and bird species held in zoos are less endemic and less threatened than their close relatives not held in zoos. Anim. Conserv. 2014, 17, 89–96. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Dapporto, L.; Davini, G.; Di Biase, L. Using Botanical Gardens as Butterfly Gardens: Insights from a Pilot Project in the Gran Sasso and Monti Della Laga National Park (Italy). Conservation 2023, 3, 109–126. [Google Scholar] [CrossRef]
- Swanson, W.F.; Johnson, W.E.; Cambre, R.C.; Citino, S.B.; Quigley, K.B.; Brousset, D.M.; Morais, R.N.; Moreira, N.; O’Brien, S.J.; Wildt, D.E. Reproductive status of endemic felid species in Latin American zoos and implications for ex situ conservation. Zoo Biol. 2003, 22, 421–441. [Google Scholar] [CrossRef]
- Barthlott, W.; Rauer, G.; Ibisch, P.L.; von den Driesch, M.; Lobin, W. Biodiversität und Botanische Gärten. In Botanische Gärten und Biodiversität: Erhaltung Biologischer Vielfalt durch Botanische Gärten und die Rolle des Übereinkommens über die Biologische Vielfalt (Rio de Janeiro, 1992); Bundesamt für Naturschutz, Ed.; Landwirtschaftsverlag: Münster, Germany, 1999; pp. 1–24. ISBN 978-3-89624-615-8. [Google Scholar]
- Price, M.R.S.; Maunder, M.; Soorae, P.S.; Guerrant, E.O.; Havens, K. Ex situ support to the conservation of wild populations and habitats: Lessons from zoos and opportunities for botanic gardens. Ex Situ Plant Conserv. Support. Species Surviv. Wild 2004, 3, 84. [Google Scholar]
- Conde, D.; Colchero, F.; Gusset, M.; Pearce-Kelly, P.; Byers, O.; Flesness, N.; Browne, R.; Jones, O. Zoos through the lens of the IUCN Red List: A global metapopulation approach to support conservation breeding programs. PLoS ONE 2013, 8, e80311. [Google Scholar] [CrossRef]
- Lammers, R.; Scholten, C.; Marcordes, B.; Pagel, T.; Rödder, D.; Ziegler, T. Malagasy birds in zoological gardens–an analysis of zoo databases as basis for improved ex situ conservation measures. Madagassische Vögel in Zoologischen Gärten—Zoodatenbankanalysen als Grundlage für verbesserten ex- situ-Artenschutz. Der Zool. Gart. 2022, 90, 121–150. [Google Scholar] [CrossRef]
- Carr, N. Star attractions and damp squibs at the zoo: A study of visitor attention and animal attractiveness. Tour. Recreat. Res. 2016, 41, 326–338. [Google Scholar] [CrossRef]
- Biega, A.; Lamont, M.; Mooers, A.; Bowkett, A.; Martin, T. Guiding the prioritization of the most endangered and evolutionary distinct birds for new zoo conservation programs. Zoo Biol. 2019, 38, 305–315. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef]
- Browne, R.K.; Wolfram, K.; García, G.; Bagaturov, M.F.; Pereboom, Z.J.J.M. Zoo-based amphibian research and conservation breeding programs. Amphib. Reptile Conserv. 2011, 5, 1–14. [Google Scholar]
- Biega, A.; Martin, T.E. Do amphibian conservation breeding programmes target species of immediate and future conservation concern? Oryx 2018, 52, 723–729. [Google Scholar] [CrossRef]
- Biega, A.; Greenberg, D.A.; Mooers, A.O.; Jones, O.R.; Martin, T.E. Global representation of threatened amphibians ex situ is bolstered by non-traditional institutions, but gaps remain. Anim. Conserv. 2017, 20, 113–119. [Google Scholar] [CrossRef]
- Ziegler, T.; Kamphausen, J.; Glaw, F.; Crottini, A.; Garcia, G.; Rödder, D.; Rauhaus, A.; Stenger, L.; Wahle, A. Threatened Malagasy amphibians and reptiles in zoos–a call for enhanced implementation of the IUCN’s One Plan Approach. Der Zool. Gart. 2022, 90, 21–69. [Google Scholar]
- Jenkins, R.K.; Tognelli, M.F.; Bowles, P.; Cox, N.; Brown, J.L.; Chan, L.; Andreone, F.; Andriamazava, A.; Andriantsimanarilafy, R.R.; Anjeriniaina, M.; et al. Extinction risks and the conservation of Madagascar’s reptiles. PLoS ONE 2014, 9, e100173. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgen, N.J.; Wilson, J.R.U.; Elith, J.; Wintle, B.A.; Richardson, D.M. Alien invaders and reptile traders: What drives the live animal trade in South Africa? Anim. Conserv. 2010, 13, 24–32. [Google Scholar] [CrossRef]
- Brereton, J.; Brereton, S. Short Communication: Examining taxa representation in Asian zoos and aquaria using historic records. Biodivers. J. Biol. Divers. 2021, 22, 2870–2875. [Google Scholar] [CrossRef]
- Carrizo, S.F.; Smith, K.G.; Darwall, W.R.T. Progress towards a global assessment of the status of freshwater fishes (Pisces) for the IUCN Red List: Application to conservation programmes in zoos and aquariums. Int. Zoo Yearb. 2013, 47, 46–64. [Google Scholar] [CrossRef]
- Valdez, J.W.; Mandrekar, K. Assessing the species in the CARES preservation program and the role of aquarium hobbyists in freshwater fish conservation. Fishes 2019, 4, 49. [Google Scholar] [CrossRef]
- Raghavan, R.; Dahanukar, N.; Tlusty, M.F.; Rhyne, A.L.; Kumar, K.K.; Molur, S.; Rosser, A.M. Uncovering an obscure trade: Threatened freshwater fishes and the aquarium pet markets. Biol. Conserv. 2013, 164, 158–169. [Google Scholar] [CrossRef]
- Leiss, L.; Rauhaus, A.; Rakotoarison, A.; Fusari, C.; Vences, M.; Ziegler, T. Review of threatened Malagasy freshwater fishes in zoos and aquaria: The necessity of an ex situ conservation network—A call for action. Zoo Biol. 2022, 41, 244–262. [Google Scholar] [CrossRef]
- Rose, P.E.; Brereton, J.E.; Rowden, L.J.; de Figueiredo, R.L.; Riley, L.M. What’s new from the zoo? An analysis of ten years of zoo-themed research output. Palgrave Commun. 2019, 5, 128. [Google Scholar] [CrossRef]
- Hughes, D.G.; Bennett, P.M. Captive breeding and the conservation of invertebrates. Int. Zoo Yearb. 1991, 30, 45–51. [Google Scholar] [CrossRef]
- Pearce-Kelly, P. Invertebrate propagation and re-establishment programmes: The conservation and education potential for zoos and related institutions. In Creative Conservation Interactive Management of Wild and Captive Animals; Springer: Dordrecht, The Netherlands, 1994; pp. 329–337. [Google Scholar]
- Pearce-Kelly, P.; Jones, R.; Clarke, D.; Walker, C.; Atkin, P.; Cunningham, A.A. The captive rearing of threatened Orthoptera: A comparison of the conservation potential and practical considerations of two species’ breeding programmes at the Zoological Society of London. J. Insect Conserv. 1998, 2, 201–210. [Google Scholar] [CrossRef]
- Saul-Gershenz, L. Insect Zoos. In Encyclopedia of Insects; Academic Press: London, UK, 2009; pp. 516–523. ISBN 978-0-12-374144-8.00147-8. [Google Scholar]
- Boppré, M.; Vane-Wright, R.I. The butterfly house industry: Conservation risks and education opportunities. Conserv. Soc. 2012, 10, 285–303. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic garden solutions to the plant extinction crisis. Plants People Planet 2021, 3, 22–32. [Google Scholar] [CrossRef]
- Fara, P. Sex, Botany and Empire: The Story of Carl Linnaeus and Joseph Banks; Icon Books: London, UK, 2004; ISBN 9780231134262. [Google Scholar]
- Hobhouse, H. Seeds of Change: Six Plants That Transformed Mankind; Papermac, Macmillan Publishers Ltd.: New York, NY, USA, 1999; ISBN 9780333736289. [Google Scholar]
- Oldfield, S.F. Botanic gardens and the conservation of tree species. Trends Plant Sci. 2009, 14, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.S. Botanic gardens science for conservation and global change. Trends Plant Sci. 2009, 14, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Novy, A.; Glover, J.; Kellogg, E.A.; Maul, J.E.; Raven, P.; Jackson, P.W. Expanding the role of botanical gardens in the future of food. Nat. Plants 2015, 1, 15078. [Google Scholar] [CrossRef]
- Krishnan, S.; Moreau, T.; Kuehny, J.; Novy, A.; Greene, S.L.; Khoury, C.K. Resetting the table for people and plants: Botanic gardens and research organizations collaborate to address food and agricultural plant blindness. Plants People Planet 2019, 1, 157–163. [Google Scholar] [CrossRef]
- Cheek, D.; Procheş, S. The value of arboreta in South Africa. S. Afr. J. Sci. 2022, 118, 1–4. [Google Scholar] [CrossRef]
- Sharrock, S. Botanic gardens and food security–The results of BGCI’s survey. BGJournal 2013, 10, 3–7. [Google Scholar]
- Maunder, M. Botanic gardens: Future challenges and responsibilities. Biodivers. Conserv. 1994, 3, 97–103. [Google Scholar] [CrossRef]
- Smith, P. Building a global system for the conservation of all plant diversity: A vision for botanic gardens and Botanic Gardens Conservation International. Sibbaldia Int. J. Bot. Gard. Hortic. 2016, 14, 5–13. [Google Scholar] [CrossRef]
- Smith, P. The challenge for botanic garden science. Plants People Planet 2019, 1, 38–43. [Google Scholar] [CrossRef]
- Maunder, M.; Higgens, S.; Culham, A. The effectiveness of botanic garden collections in supporting plant conservation: A European case study. Biodivers. Conserv. 2001, 10, 383–401. [Google Scholar] [CrossRef]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef]
- Volis, S. Complementarities of two existing intermediate conservation approaches. Plant Divers. 2017, 39, 379–382. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sharrock, S. The contribution of botanic gardens to ex situ conservation through seed banking. Plant Divers. 2017, 39, 373–378. [Google Scholar] [CrossRef]
- Van der Werff, H.; Consiglio, T. Distribution and conservation significance of endemic species of flowering plants in Peru. Biodiv. Cons. 2004, 13, 1699–1713. [Google Scholar] [CrossRef]
- Mackay, R. The Atlas of Endangered Species: Threatened Plants and Animals of the World; Earthscan: London, UK, 2002; ISBN 1853838748. [Google Scholar]
- Harding, J. Historic deforestation and the fate of endemic invertebrate species in streams. N. Z. J. Mar. Freshw. Res. 2003, 37, 333–345. [Google Scholar] [CrossRef]
- Pearson, R. Climate Change, Biodiversity and Extinction Risk; Sterling: New York, NY, USA, 2011. [Google Scholar]
- Hobohm, C.; Vanderplank, S.E. Change: Risks and Predictability. In Perspectives for Biodiversity and Ecosystems; Environmental Challenges and Solutions; Springer: Cham, Switzerland, 2021; pp. 181–193. [Google Scholar] [CrossRef]
- Fordham, D.A.; Akçakaya, H.R.; Araújo, M.B.; Keith, D.A.; Brook, B.W. Tools for integrating range change, extinction risk and climate change information into conservation management. Ecography 2001, 36, 956–964. [Google Scholar] [CrossRef]
- Gerlach, J. Climate change, species extinctions and ecosystem collaps. Phelsuma 2010, 17A, 13–31. [Google Scholar]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Platts, P.J.; Gereau, R.E.; Burgess, N.D.; Marchant, R. Spatial heterogeneity of climate change in an Afromontane centre of endemism. Ecography 2013, 36, 518–530. [Google Scholar] [CrossRef]
- Li, T.; Luo, P.; Xiong, Q.; Yang, H.; Gu, X.; Qiu, Y.; Lin, B.; Liu, Y.; La, C. Spatial heterogeneity of tree diversity response to climate warming in montane forests. Ecol. Evol. 2021, 11, 931–941. [Google Scholar] [CrossRef]
- Hoekstra, J.M.; Molnar, J.L.; Jennings, M.; Revenga, C.; Spalding, M.D.; Boucher, T.M.; Robertson, J.C.; Heibel, T.J. The Atlas of Gobal Conservation; University of California Press: Berkeley, CA, USA, 2010; ISBN 9780520262560. [Google Scholar]
- Hobohm, C. (Ed.) Perspectives for Biodiversity and Ecosystems; Environmental Challenges and Solutions; Springer: Cham, Switzerland, 2021; pp. 1–483. [Google Scholar]
- Poulin, R. Parasite biodiversity revisited: Frontiers and constraints. Int. J. Parasitol. 2014, 44, 581–589. [Google Scholar] [CrossRef] [PubMed]
- McCallum, H.I. Lose biodiversity, gain disease. Proc. Natl. Acad. Sci. USA 2015, 112, 8523–8524. [Google Scholar] [CrossRef]
- Aerts, R.; Honnay, O.; Van Nieuwenhuyse, A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med. Bull. 2018, 127, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Tribot, A.-S.; Deter, J.; Mouquet, N. Integrating the aesthetic value of landscapes and biological diversity. Proc. R. Soc. B 2018, 285, 20180971. [Google Scholar] [CrossRef]
- Giacinto, J.J.; Fricker, G.A.; Ritter, M.; Yost, J.; Doremus, J. Urban forest biodiversity and cardiovascular disease: Potential health benefits from California’s street trees. PLoS ONE 2021, 16, e0254973. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, W. The role of botanical gardens in scientific research, conservation, and citizen science. Plant Divers. 2018, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Asase, A.; Mzumara-Gawa, T.I.; Owino, J.O.; Peterson, A.T.; Saupe, E. Replacing “parachute science” with “global science” in ecology and conservation biology. Conserv. Sci. Pract. 2022, 4, e517. [Google Scholar] [CrossRef]
| Maxima | Mode of Calculation | Country/Region | Ecosystem (Dominant) | Climate (Dominant) | |
|---|---|---|---|---|---|
| Endemism in mammals and birds plus reptiles [131] | >70 | Numbers of endemics by terrestrial ecoregion | Eastern Madagascar | Rainforest | Wet tropical and subtropical |
| Endemism in birds [132] | 92 | E/S as percentage value | Hawaiian Islands | diverse | Humid tropical and subtropical oceanic |
| Endemism in freshwater animals (vertebrates and invertebrates) [97] | 54 | E/S as percentage value | Lake Baikal, Russia | Freshwater lake | Temperate continental |
| Endemism in fish, freshwater turtles, and crocodiles plus amphibians [131] | >150 | Numbers of endemics by freshwater ecoregion | High Andes, western India, East African Rift Valley lakes | Wetlands and freshwater ecosystems | Tropical and subtropical |
| Endemism in cichlid fishes [97] | Up to 99 | E/S as percentage value | Tectonic Lakes Tanganyika, Malawi, Victoria, Africa | Freshwater lake | Tropical |
| Endemism in land snails [98] | c. 100 | E/S as percentage value (rounded) | Hawaiian Islands | Diverse | Humid tropical and subtropical oceanic |
| Endemism in vascular plants [99,100,133] | >80 | E/S as percentage value | New Caledonia, Hawaiian Islands, Madagascar, St. Helena, New Zealand | Diverse | Subtropical and tropical oceanic |
| Endemism in vascular plants [134,135] | 4.7–5.1 and 4–4.4 | Relative distance of residual to regression (Res. E) | Mas a Tierra, Chile, and St. Helena | Forest | Subtropical oceanic |
| Endemism in pteridophytes [136] | 37/31.7 | Percentage of endemism/index of insularity | Easter Island | Reeds and grasslands replace the original tropical forest | Tropical oceanic/subtropical humid |
| Species richness in vascular plants [137] | 942 | No. of species per 10,000 m2 | Ecuador | Lowland rainforest | Humid tropical |
| Species richness in vascular plants [138] | 115 | No. of species per 10 m2 | Romania | Steppe meadow (currently grazed) | Temperate |
| Biomass [139,140] | 1819 or 2844 | tC ha-1 (above-ground biomass) or tC ha-1 (total biomass) | SE Australia | Eucalyptus regnans forest | Warm temperate |
| Productivity [141,142] | 8.93–9.93 | kg m-2 year-1 (dry matter) | Amazon | Swamps dominated by C4 grass Echinochloa polystachya | Wet tropical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobohm, C.; Barker, N. Centers of Endemism and The Potential of Zoos and Botanical Gardens in Conservation of Endemics. J. Zool. Bot. Gard. 2023, 4, 527-548. https://doi.org/10.3390/jzbg4030038
Hobohm C, Barker N. Centers of Endemism and The Potential of Zoos and Botanical Gardens in Conservation of Endemics. Journal of Zoological and Botanical Gardens. 2023; 4(3):527-548. https://doi.org/10.3390/jzbg4030038
Chicago/Turabian StyleHobohm, Carsten, and Nigel Barker. 2023. "Centers of Endemism and The Potential of Zoos and Botanical Gardens in Conservation of Endemics" Journal of Zoological and Botanical Gardens 4, no. 3: 527-548. https://doi.org/10.3390/jzbg4030038
APA StyleHobohm, C., & Barker, N. (2023). Centers of Endemism and The Potential of Zoos and Botanical Gardens in Conservation of Endemics. Journal of Zoological and Botanical Gardens, 4(3), 527-548. https://doi.org/10.3390/jzbg4030038








