Abstract
Given the current and future threats to Asian elephants (Elephas maximus), maintaining a sustainable ex situ population is crucial for the longevity of the species. Using Infrared Thermography (IRT), thermoregulation of Asian elephants at low ambient temperatures was examined. Thermal images were taken at 15 min intervals over 60–90-min observation periods, once weekly, during January and February 2022. A total of 374 images were examined from 10 Asian elephants, which varied from 1 to 56 years of age. Data from thermograms of the ear and body were interpreted in view of weight, age and behavior. Variability in surface temperature was found most frequently in the ears, occasionally presenting as thermal windows—areas with dense underlying blood supply that aid in heat exchange. Thermal windows occurred most frequently in the distal, then medial, regions of the ear. The pattern of appearance of thermal windows in the ear provides support that the increase of blood flow is utilized as a method of warming. This preliminary study provides key insight into Asian elephant thermoregulation, suggesting that the species may be more well-adapted to lower ambient temperatures than previously thought.
Keywords:
infrared thermography; Elephas maximus; thermal windows; age; behavior; weight; ambient temperature 1. Introduction
Asian elephants (Elephas maximus) are threatened due to habitat loss and fragmentation, human–elephant conflict, poaching and illegal trade [1]. Disruptions in social hierarchies and decreased disease resilience, resulting from direct threats, threaten the populations of remaining wild animals [2]. Further, climate change models predict that ~42% of the habitat available to Asian elephants at present will be lost by the end of this century, due to the combined effects of climate change and human pressure [3]. As a result, the Asian elephant is classified on the International Union for the Conservation of Nature (IUCN) Red List as “Endangered” [4,5].
Human activities have resulted in altered environmental conditions that are impacting the demography and evolution of the Asian elephant, and many other species, globally [6]. In response to this crisis, an agreement was reached by the United Nations at the Kunming–Montreal biodiversity summit in December 2022, with the goal to halt and reverse global biodiversity loss by 2030. A recent publication assessing the efficacy of each target in the Kunming–Montreal biodiversity framework found that the extinction risk for over half (57%) of the world’s threatened species would not be reduced sufficiently without targeted species-specific recovery actions, including ex situ conservation [7]. According to the authors, focused interventions and increased attention on individual species is needed when crafting solutions to address global biodiversity loss. Target Four was developed to address this need, noting that the recovery and conservation of species must include the consideration of both in situ and ex situ conservation [7].
The African Lion Safari has had a program devoted to assisting in the conservation of the Asian elephant since 1987. Given the species’ current status and new threats to the wild population due to climate change, maintaining a sustainable ex situ population of Asian elephants, such as the one found at African Lion Safari, will provide an assurance population for the species’ long-term persistence [8]. In the continuing efforts to provide excellence in care and to assist in research that can benefit the conservation of Asian elephants, African Lion Safari initiated research on thermoregulation patterns in Asian elephants in 2021, using Infrared Thermography (IRT). IRT is a non-invasive tool that detects surface temperature distribution patterns [9]. It has been used to measure physiological changes in humans and other warm-blooded animals, including in diagnosing disease, gaining insight into reproductive processes, analyzing animal behavior and estimating individuals’ thermal states [10]. IRT has been used in several studies of African (Loxodonta africana) and Asian elephants. Williams [11] measured heat loss by convection, radiation and conduction on different parts of the elephant (body, legs, head, trunk, neck and ears). Phillips and Heath [12] documented that convection and radiation from the ears alone could account for 100% of the total heat loss in African elephants, with heat transfer being facilitated by “thermal windows.” As defined by Šumbera et al. [13] and Mota-Rojas et al. [14], thermal windows are body regions with a high density of blood vessels and arteriovenous anastomoses close to the body’s surface in areas devoid of fur, which permits heat exchange via vasoconstriction or vasodilation. The ears of both African and Asian elephants, while differing significantly [15], can be defined under these criteria as thermal windows [12,16]. Further work by Weissenböck et al. [16] suggested that areas of the torso and limbs may also function as thermal windows in Asian elephants. However, the work did not investigate if these areas had the prerequisite underlying vascularization comprising a thermal window.
Elephants in the wild can experience a wide range of ambient temperatures, ranging from 0 °C to 50 °C [17]. Similarly, ex situ populations are located in areas exhibiting a wide range of environmental conditions. Given the temperature variation experienced by the species in situ and ex situ, we sought to more closely examine the responses of individual Asian elephants to varying environmental conditions using IRT. We present findings on the thermoregulation of Asian elephants, in the context of the low ambient temperatures experienced in southwestern Ontario. We were particularly interested in ear surface temperature variation, imparting the sensitivity of the ear tissue in temperature extremes [12]. Our results provide insight into the relationships between age, sex, weight, and behavior, in terms of the surface body temperatures observed using IRT.
2. Materials and Methods
African Lion Safari, Cambridge, Ontario, Canada, (43.3410° N, 80.1801° W) currently maintains a herd of 17 Asian elephants. Individuals are identifiable by unique physical characteristics. A subset of the herd was monitored during a portion of their normal daily routine, during which they roam throughout a 200 acre area consisting largely of woods, with a stream, pond, and open fields. During the observation period, elephants made their own choices in terms of their location in the study area and their activity. The weight of each individual was monitored monthly. Elephant weights were obtained from each individual and averaged over the study period.
Thermal images were obtained using a FLIR T540 thermal camera (Teledyne FLIR Systems, Oregon) with a 24° lens. The camera was automatically calibrated, with an infrared resolution of 464 × 348 pixels, and a spectral range of 7.5 to 14.0 µm. Emissivity was set to 0.96, as is appropriate for this species [18]. Images were obtained weekly during January and February 2022. Thermal imaging commenced after the elephants had left their heated barns and been roaming in the 200 acre area for at least 30 min. During each observation period, three lateral-view images of each elephant were obtained in succession (10 s time period) every 15 min. Each series of three images for each individual at each interval was compared with the others, and the “best” image was chosen for analysis. The “best” image was the one that most closely provided a fully perpendicular, full-frame, lateral view, in which there were no elements obscuring the ear or body, such as the appendages of other elephants or an individual’s tail. The distance at which the image was obtained varied from 3.5 m to 10 m, depending on the size of the elephant, with the goal of obtaining an image that filled the frame. The distance remained consistent for each individual across all observational days. Images were analyzed using the FLIR Thermal Studio Pro software (Teledyne FLIR LLC, 2021 Wilsonville, OR, USA).
Ambient temperature (Ta) was recorded using a DS 1923 iButton Hygrochron Temperature/ Humidity Logger ® (Maxim Integrated, San Jose, CA, USA), programmed to record data hourly. Temperatures were recorded to the nearest tenth of a degree. Loggers were placed in an outdoor location on the property, out of direct sunlight and away from other heat sources.
The behavior of each individual was categorized at each 15 min interval, to coincide with the time at which the images were obtained. Behaviors were recorded as “eating” (individuals had been and were eating in a stationary position prior to imaging), “moving” (individuals had been moving immediately before or at the time an image was obtained) or “drinking”. Behaviors characterized as moving included walking, running and playing. All observations were recorded by the same observer to maintain consistency.
In each ‘best’ image, a polygon was drawn that encompassed the entire body or ear, but excluded the outer edge of the body, in order to avoid background interference bias (Figure 1). Within the polygon, the maximum surface temperature/pixel (TMax), the minimum surface temperature/pixel (TMin) and average surface temperature across all pixels (TAvg) were obtained. The mean ± standard error (SEM) of TAvg of the body, and ear surface temperatures for each individual were calculated for each best image obtained at each interval throughout the observation period. Images in which the difference between TMax or TMin and TAvg was ≥5 °C, indicative of a potential thermal window, were included in additional analyses. The ≥5 °C criterion was chosen, as it was the same as that used in previous studies of thermal windows in elephants [16]. As solar radiation can influence the surface temperatures obtained from thermal images [10], only images with consistent environmental conditions throughout the observation period were used in further analysis. Specifically, we utilized images that had the same environmental conditions throughout the data collection day. All images analyzed for each individual were either taken in totally overcast or totally sunny conditions, to avoid the difference in solar radiance going from overcast to full sun observations within the same observation day.
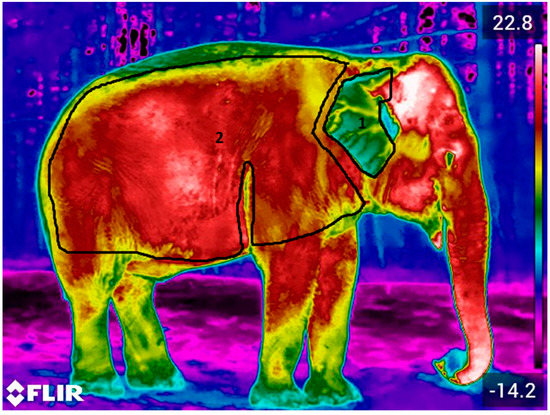
Figure 1.
Images were analyzed in terms of both the body and ear. Polygons were drawn by hand within FLIR Thermal Studio Pro software to delineate the ear (1) and body (2). TAvg, TMax and TMin were derived for each polygon.
The first step in identifying thermal windows was identifying when the ≥5 °C surface temperature difference was characterized by a discrete area of increased temperature, occurring in an area with the requisite underlying vascularization. When a potential thermal window was identified, a polygon was drawn around the area of increased temperature. The TAvg of the polygon encompassing the thermal window was obtained and then compared to the TAvg of the polygon encompassing the remaining portion of the ear or body. The percentage of the total surface area of the thermal window polygon within the polygon encompassing the entire ear or body was calculated (Figure 2). The area of occurrence of thermal windows in the ear (the only area in which we identified potential thermal windows) was characterized as occurring in the proximal, medial or distal region of the ear, based on patterns of underlying vascularization (Figure 3). A Chi-squared (λ) test was conducted to determine if the thermal windows were occurring at random, among the analyzed regions.
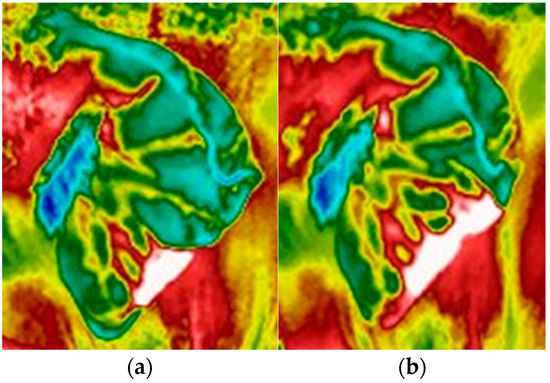
Figure 2.
Example of a thermal window in the ear of an Asian elephant J, (a) growing in size within a 15 min interval (b) on 11 January 2022. The thermal window is characterized by a temperature ≥5 °C when compared to the surrounding tissue [16]. The thermal window(s) was delineated using a polygon. The total area of the thermal window was calculated as a percentage of the ear polygon.
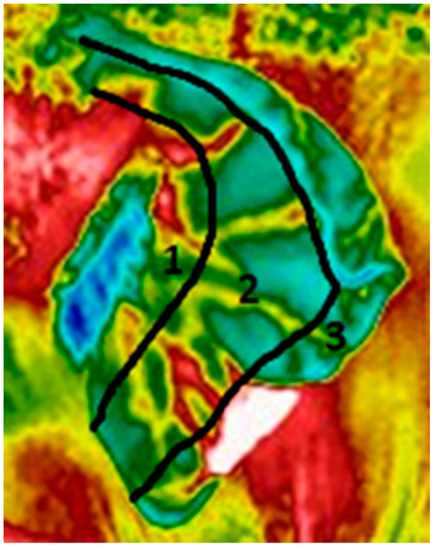
Figure 3.
Example of an ear, divided into proximal (1), medial (2) and distal (3) regions, based on the approximation of blood vessels present in the ear in each location [15,16]. In this image, a thermal window is evident in the distal portion of the ear.
The index of vasomotion (VMI), as described by Phillips and Heath [19], was calculated for each individual in our study. VMI is a measure of an animal’s ability to control their surface temperature, which was calculated using the equation VMI = 0.27717 + 0.27929log (weight in kg) [19]. Phillips and Heath [19] used the equation to describe the ability of 29 species in regulating their body surface temperatures, based on surface area to volume ratio, metabolic production and thermal thresholds. We extended the use of VMI to investigate the extent to which size variations (weight and surface area to volume ratio) could potentially affect an individual Asian elephant’s ability to regulate its body temperature, as weight and size varies by degrees of magnitude between young and adult elephants (Table 1). As such, we hypothesized that an individual’s thermoregulation would likely vary based on age, similar to the variations observed among other species varying in size.

Table 1.
Demographic details of the Asian elephants observed in our study. Index of vasomotion (VMI) values were calculated using the equation VMI = 0.27717 + 0.27929log (weight in kg) [19].
3. Results
Data were obtained from 10 of the total 17 Asian elephants within the herd, who varied from 1 to 56 years of age (Table 1), including two male and eight female elephants. Weights ranged from 397 to 4084 kg (Table 1).
A total of 1131 thermal images were obtained from seven observation periods across a six-week period (4 January 2022 to 15 February 2022). Of these, 374 images were used in analyses. Observation time varied from 60 to 90 min/day, with a total of 575 min of observation combined across all individuals. Average ambient temperature during the observation periods ranged from −9.4 to 4.0 °C (Table 2). Surface temperatures, as determined by IRT, in both the ear and body were found to be quite variable. On any one observation day, the greatest difference between TMax and TMin in a single elephant’s body varied up to 23.9 °C, and varied up to 29.4 °C within a single elephant’s ear. Average body surface temperature was higher than that of the average ear temperature among all observation periods (Figure 4). The pattern of variation between the mean surface temperature of the body versus ear was similar among the observation periods (Figure 4).

Table 2.
Summary of data collection and occurrence of thermal windows between 4 January 2022 and 15 February 2022, with ambient temperatures ranging from −9.4 to 4.0 °C.
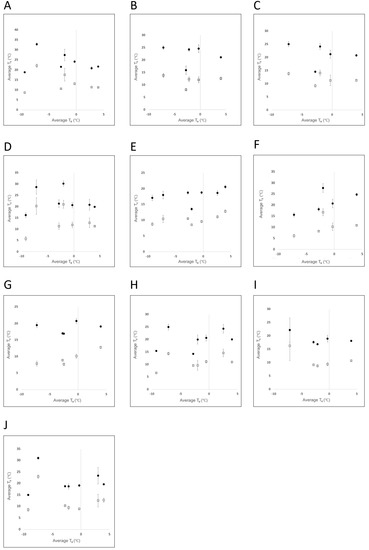
Figure 4.
Mean ± SEM surface temperature (Ts) of the body (●) and ear (□), obtained from 10 Asian elephants (A–J) across all observation days, with mean ambient temperatures (Ta) ranging from (−9.4 to 4.0 °C).
TAvg of the body surface varied by ≥5 °C from TMin in 343 (92%) and from TMax values in 231 (62%) of the images analyzed. The TAvg of the ear surface varied by ≥5 °C from TMin in 339 (91%) and from TMax in 374 (100%) of the images analyzed. However, on further examination, none of the images of the body with the ≥5 °C differential were found to represent potential thermal windows, but instead could be attributed to other causes. Conversely, several, large potential thermal windows were identified in the ear. In total, five individuals appeared to develop thermal windows in the ear, with thermal windows ranging in size from 2 to 33% of the total surface area of the ear (Table 3). Three individuals had multiple occurrences of thermal windows within a single observation day. However, only one individual developed more than one thermal window concurrently (Table 3). No pattern was apparent that suggested a relationship between the occurrence of thermal windows and ambient environmental temperature (i.e., the thermal windows did not occur only on the coldest days) (Table 3). Thermal windows developed most frequently (74%) in the distal region of the ear, and to a lesser degree (26%) in the medial region of the ear (Table 4). No thermal windows were identified in the proximal region of the ear (Table 4). A Chi-squared test revealed that the thermal windows occurred significantly more frequently in the distal portion of the ear (λ2 = 2.9 × 10−5).

Table 3.
Dates and environmental conditions on days when thermal window events were observed in January and February 2022. Thermal windows were only found in individuals A, B, G, I and J (see Table 1 for demographic information on these individuals). Thermal window events were defined as per Weissenböck et al. [16], where the temperature differential between the thermal window and surrounding tissue was ≥5 °C.

Table 4.
Location of thermal windows observed in the ears of each of the five individuals (see Table 1 for a summary of demographics of each individual). If a thermal window extended into a second region of the ear, the thermal window was counted as occurring in both locations. A Chi-squared test revealed a statistically significant pattern of occurrence ( 2 = 2.9 × 10−5), suggesting that thermal windows most frequently developed in the distal portion of the ear.
The index of vasomotion ranged from 1.00 to 1.29, with the smallest/youngest elephant (Elephant A) having the lowest VMI (1.00) and the largest (Elephant F) having the highest VMI (1.29) (Table 1). In general, VMI increased linearly as individual age and weight increased (Table 1).
Behavioral observations suggested that the younger individuals (Elephants A and B) were more frequently active, being observed to engage in play/running/walking activities during 45–56% of the monitoring intervals (Table 5). Older individuals were largely observed eating in a stationary position (Table 5).

Table 5.
Summary of the total intervals in which individual elephants engaged in varying behaviors over the study period. Behaviors were recorded every 15 min, and coincided with thermography.
4. Discussion
Research on thermoregulation in elephants has, to date, been largely focused on African elephants [11,12,16,20,21], and on the development of thermal windows at higher ambient temperatures. While no study specifically looking at the response of Asian elephants to lower ambient temperatures was found, results suggest that elephants are able to constrict blood flow to their ears as a means of conserving heat in these situations [12,16,18]. We used Infrared Thermography (IRT) to characterize and gain a better understanding of the patterns of thermoregulation at lower ambient temperatures. Our data empirically support what has been observed at African Lion Safari for nearly five decades: that Asian elephants are comfortable during the time spent outdoors in colder temperatures. Patterns of temperature variation among thermograms suggested that individuals were actively adjusting temperatures in their ears, but not their body. We believe our data suggest that an individual’s weight, age and behavior impacted the variations we noted in the appearance of thermal windows among individuals. This is the first study to reveal that vasodilation, specifically when presenting as the development of thermal windows, in Asian elephant ears in lower ambient temperatures, is similar to that documented by previous studies as a response to higher ambient temperatures; however, these responses are often accompanied by behaviours such as ear flapping [12,20,21]. Our research revealed that images should be interpreted by taking into account numerous factors. This could include the analysis of behaviors, such as ear flapping, that are important in thermoregulation, but also behaviors such as choosing to stand in the sun versus the shade, which also impacts thermoregulation. Doing so can help to gain a deeper understanding of both behavioral and physiological thermoregulatory adaptations in Asian elephants.
A five degree variability between TAvg and both TMin and TMax was observed in most of our images in both the body and ear. Behavior, solar radiation and underlying physiological processes can all create significant variations in surface temperature [10,22]. Upon further examination of the images, all the variability between TMin and TAvg within the polygons circumscribing the body was due to the presence of materials such as hay, snow or hair. However, patterns were detected that were suggestive of a causal factor for variations in TMax from the TAvg in images of both the body and ear, which could be attributed to (1) increased blood flow (i.e., lactating mammary tissue) (2) surface area to volume ratio considerations or (3) thermal windows. No discrete regions of increased temperature that were indicative of thermal windows could be diagnosed on the body, while several occurrences were found in the ear.
Physiologically, endotherms, which are animals with the ability to maintain a core body temperature [23], produce both metabolic heat and waste, as well as work heat, caused by muscle activity [23,24]. Thermoregulation occurs by both physiological and behavioral mechanisms [23]. For mega-vertebrates [18], such as elephants, their large body size creates unique thermoregulation considerations. Specifically, temperature exchange with their surroundings is hampered by a low surface area to volume ratio, making it difficult for these extremely large animals to adequately lose heat in high ambient temperatures [16]. This is exacerbated even further, as they lack sweat glands to aid in heat dissipation [25]. Conversely, their large body size is considered to be advantageous at low ambient temperatures [26].
Endotherms will employ physiological mechanisms in conjunction with behavior [14] to obtain thermal neutrality [27]. Behavioral thermoregulation is often species-specific and associated with unique morphology [14,23]. For example, both African and, to a lesser degree, Asian elephants will either fan or tuck their ears close to their body as a means of dissipating or conserving heat, respectively [12,20,21]. Additionally, it is well-known that elephants will vasoconstrict blood vessels in their ears as a primary response to lower ambient temperatures [12,15]. The differential that we observed between the body and ear surface temperatures suggests that vasoconstriction occurred frequently in the ear throughout our study period (Figure 4).
The Index of Vasomotion (VMI), as described by Phillips and Heath [19], is a measure of a species’ ability to manage heat exchange with their environment. Results of Phillips and Heath’s [19] research indicate that higher VMI values are associated with heavier individuals and a greater ability to control their surface temperatures [19]. Smaller individuals have lower VMI values and lesser control over their surface temperature. Similarly, VMI values were lowest in the younger and smaller elephants, and increased with age and size, with an overall linear relationship. Sexual size dimorphism in Asian elephants prevented a complete linear association [28]. Thus, it would be expected that smaller/younger elephants would have a greater need to employ behavioral or physiological mechanisms for thermoregulation compared to larger/older elephants. However, the degree to which specific physiological adaptations that would be employed would be impacted by behavior—more physically active individuals would develop more metabolic heat, and, thus, fewer thermal windows.
We believe that our data reveal that Asian elephants are actively thermoregulating by producing thermal windows in their ears, in order to counter the potential adverse effects of lower ambient temperatures. We found that the older/larger and younger/smaller elephants both developed thermal windows. The greatest number of thermal windows was observed in one of the oldest elephants, conversely to what was predicted by VMI. However, older elephants were more sedentary, and likely not producing the same degree of excess metabolic heat as the younger elephants, which were more active (i.e., they were most often observed to be walking, running or playing). Furthermore, it was anecdotally noted that the older elephants kept their ears close to their body, consistent with heat-conserving behavior observed in previous studies [21]. However, other physiological processes also impact blood pressure and circulation in older elephants, as in older humans [29,30], and additional research is needed to more fully understand inter-individual differences in thermoregulation in older Asian elephants.
Thermal windows were found only in the distal and medial regions of the ear. Thermal windows appeared in the distal region most often, and in some cases, they would extend into the medial region, similar to that documented by Weissenböck et al. [16], in elephants thermoregulating at warmer temperatures. The thermal windows we diagnosed that were observed at lower ambient temperatures were not seen consistently. In most cases following potential vasoconstriction, we believe the thermal windows are indicative of a physiological mechanism, in which individuals supply warm blood to their extremities as a means of warming their ears, as Phillips and Heath [12] theorized would be possible.
The development of thermal windows in the extremities of Asian elephants suggest that this occurs as an adaptation similar to ‘counter-current’ heat exchange. The historic range of the Asian elephant indicates that their habitats were once much more temperate than they are today, suggesting that, historically, they may have needed to develop this type of physiological adaptation, in order to survive in colder climates [5,26,31]. Counter-current heat exchange is a physiological adaptation that has been observed in several mammal species endemic to habitats with lower ambient temperatures, such as the beaver (Castor canadensis) [32]. This adaptation is used in addition to morphological characteristics, such as fur or a thick layer of fat, to enable an animal to survive in low ambient temperatures. For example, in addition to their renowned pelt, beavers possess a counter current heat exchange mechanism in their hind legs and tails. Arteries containing warm blood from the body core are situated in the middle of superficial veins travelling from their legs and tail [32]. The warm blood from the arteries prewarms the blood returning to the body’s core from these extremities, thus mitigating both heat loss from extremities and cooling of the core body temperature from cold blood returning to the heart [32].
The Asian elephant is the closest living relative to the woolly mammoth (Mammuthus primigenius), sharing more genetic material with it than any other living animal [33]. This relationship may also provide insight into our results. The woolly mammoth possessed several morphological traits that adapted them to life in extremely cold environments [34]. These traits included long, thick fur, a marked layer of subcutaneous fat and prevalent sebaceous glands that aided in insulation, as well as small tails and ears that helped to reduce heat loss [34]. Research to better understand the molecular basis of the phenotypic traits in woolly mammoth revealed other, less readily apparent, genetic adaptations to life in the extreme cold [33]. To the best of our knowledge an assessment of the molecular basis of thermoregulation has been restricted to extinct pachyderms. However, based on these findings, a similar study focused on Asian elephants would likely reveal unique and important insights.
Our findings have broadened the understanding of Asian elephant thermoregulation, and empirically support that the species has a physiological mechanism of warming. However, additional research is needed to fully understand both the cause and the underlying physiological basis behind the occurrence of thermal windows in low ambient temperatures, in particular, how it varies among individuals. Future studies should include efforts to quantify core body temperatures and correlate core body temperature with surface body temperature. Additional physiological factors, such as blood pressure, should also be assessed, as well as environmental factors, in particular relative humidity and solar radiation. Our results suggest that studies should also include animals varying in age and size. While our methodology is broadly used, future studies should also consider alternative analytical methods to identify deviations in minimum and maximum temperature from the average in thermograms.
5. Conclusions
Our results suggest that the Asian elephant may be more well-adapted to lower ambient temperatures than previously thought. Given the species history, it is plausible that thermal windows are a physiological adaptation that was developed historically to survive in colder climates, but which may also still remain relevant today, given the temperature extremes found in situ. Given African Lion Safari’s large and diverse herd, we were able to comment on the impact that age, size and behavior may have on the development of thermal windows at lower ambient temperatures. Results suggest that larger or more active elephants have a lesser need to utilize thermal windows as a warming mechanism. Our data on age indicates that sex-specific differences in the size of adult elephants prevents a strictly linear relationship in VMI, which can be used as a measure of an individual animal’s ability to control their surface temperature. Our results can assist with Asian elephant conservation both in situ, in light of climate change, and also ex situ, as regards populations managed across different temperature gradients.
Author Contributions
Conceptualization, J.L, C.G. and T.P.; Methodology, J.L. and A.C.; Validation, J.L. and T.P.; Formal Analysis, J.L. and A.C; Investigation, J.L., C.G. and T.P; Resources, J.L., C.G. and T.P.; Data Curation J.L.; Writing—Original Draft Preparation, J.L.; Writing—Review & Editing, J.L., C.G., T.P. and A.C.; Visualization, J.L.; Supervision, J.L. and A.C. Project Administration, J.L.; Funding Acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by African Lion Safari. Lefebvre also received partial funding from EcoCanada as part of a wage subsided program, which allowed her to lead this research project from October 2021–October 2022.
Institutional Review Board Statement
The work was reviewed and approved by the Animal Care Committee at African Lion Safari (ERC-22-JK, December 2021).
Data Availability Statement
Due to institutional policy, data is not publicly available. Data is available upon request from the corresponding author.
Acknowledgments
We would like to thank the entire African Lion Safari elephant team for training elephants and monitoring behavior: Cassandra De Boer, Jonathan Dawson, Ben Scott and Piotr Wisniewski. We would also like to thank the reviewers for their input on our manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The research reported was part of an undertaking to integrate and to better understand potential applications of IRT in the zoological industry.
References
- Menon, V.; Tiwari, S.K.R. Population status of Asian elephants Elephas maximus and key threats. Int. Zoo Yearb. 2019, 53, 17–30. [Google Scholar] [CrossRef]
- Vijayakrishnan, S.; Kumar, M.A.; Umapathy, G.; Kumar, V.; Sinha, A. Physiological stress responses in wild Asian elephants Elephas maximus in a human-dominated landscape in the Western Ghats, southern India. Gen. Comp. Endocrinol. 2018, 266, 150–156. [Google Scholar] [CrossRef]
- Kanagaraj, R.; Araujo, M.B.; Barman, R.; Davidar, P.; De, R.; Digal, D.K.; Gopi, G.V.; Johnsingh, A.J.T.; Kakati, K.; Kramer-Schadt, S.; et al. Predicting range shifts of Asian elephants under global change. Divers. Distrib. 2019, 25, 822–838. [Google Scholar] [CrossRef]
- Choudhury, A.; Lahiri Choudhury, D.K.L.; Desai, A.; Duckworth, J.W.; Easa, P.S.; Johnsingh, A.J.T.; Fernando, P.; Hedges, S.; Gunawardena, M.; Kurt, F.; et al. Elephas maximus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland; Cambridge, UK, 2008; p. e.T7140A12828813. Available online: https://democracy.blackpool.gov.uk/documents/s12685/The%20IUCN%20Red%20List%20of%20Threatened%20Species.pdf (accessed on 22 February 2023).
- Williams, C.; Tiwari, S.K.; Goswami, V.R.; de Silva, S.; Kumar, A.; Baskaran, N.; Yoganand, K.; Menon, V. Elephas maximus. In The IUCN Red List of Threatened Species; IUCN Red List: Gland, Switzerland, 2020; p. e.T7140A45818198. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Bolam, F.C.; Ahumada, J.; Akçakaya, H.R.; Brooks, T.M.; Elliott, W.; Hoban, S.; Mair, L.; Mallon, D.; McGowan, P.J.; Raimondo, D.; et al. Over half of threatened species require targeted recovery actions to avert human-induced extinction. Front. Ecol. Environ. 2022, 14, e12762. [Google Scholar] [CrossRef]
- Riddle, H.S.; Rasmussen, B.; Schmitt, D.L. Are captive elephants important to conservation. Gajah 2003, 22, 57–61. [Google Scholar]
- McCafferty, D.J. The value of infrared thermography for research on mammals: Previous applications and future directions. Mammal Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Hilsberg-Merz, S. Infrared Thermography in Zoo and Wild Animals. In Zoo and Wild Animal Medicine: Current Therapy; Fowler, M.E., Eric Miller, R., Eds.; Saunders Elsevier: Amsterdam, The Netherlands, 2008; p. 12. [Google Scholar]
- Williams, T.M. Heat transfer in elephants: Thermal partitioning based on skin temperature profiles. J. Zool. 1990, 222, 235–245. [Google Scholar] [CrossRef]
- Phillips, P.K.; Heath, J.E. Heat exchange by the pinna of the African elephant (Loxodonta africana). Comp. Biochem. Physiol. Part A Physiol. 1992, 101, 693–699. [Google Scholar] [CrossRef]
- Šumbera, R.; Zelová, J.; Kunc, P.; Knížková, I.; Burda, H. Patterns of surface temperatures in two mole-rats (Bathyergidae) with different social systems as revealed by IR-thermography. Physiol. Behav. 2007, 92, 526–532. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Ghezzi, M.D.; Mora-Medina, P.; Hernández-Ávalos, I.; Jacome, J.; Castellón, A.; Falcón, I.; Reséndiz, F.; Romero, N.; Ponce, R.; et al. Anatomical, physiological, and behavioral mechanisms of thermoregulation in elephants. J. Anim. Behav. Biometeorol. 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Weissenböck, N.M.; Weiss, C.M.; Schwammer, H.M.; Kratochvil, H. Thermal windows on the body surface of African elephants (Loxodonta Africana) studied by infrared thermography. J. Therm. Biol. 2010, 35, 182–188. [Google Scholar] [CrossRef]
- Kinahan, A.A.; Pimm, S.L.; van Aarde, R.J. Ambient temperature as a determinant of landscape use in the savanna elephant, Loxodonta Africana. J. Therm. Biol. 2007, 32, 47–58. [Google Scholar] [CrossRef]
- Rowe, M.F.; Bakken, G.S.; Ratliff, J.J.; Langman, V.A. Heat storage in Asian elephants during submaximal exercise: Behavioral regulation of thermoregulatory constraints on activity in endothermic gigantotherms. J. Exp. Biol. 2013, 216, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.K.; Heath, J.E. Dependency of surface temperature regulation on body size in terrestrial mammals. J. Therm. Biol. 1995, 20, 281–289. [Google Scholar] [CrossRef]
- Wright, P.G. Why do elephants flap their ears? South Afr. J. Zool. 1984, 19, 266–269. [Google Scholar] [CrossRef]
- Buss, I.O.; Estes, J.A. The Functional Significance of Movements and Positions of the Pinnae of the African Elephant, Loxodonta africana. J. Mammal. 1971, 52, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mole, M.A.; Rodrigues DÁraujo, S.; van Aarde, R.J.; Mitchell, D.; Fuller, A. Coping with heat: Behavioural and physiological responses of savanna elephants in their natural habitat. Conserv. Physiol. 2016, 4, cow044. [Google Scholar] [CrossRef]
- McCafferty, D.J.; Pandraud, G.; Gilles, J.; Fabra-Puchol, M.; Henry, P.-Y. Animal thermoregulation: A review of insulation, physiology and behaviour relevant to temperature control in buildings. Bioinspiration Biomim. 2017, 13, 011001. [Google Scholar] [CrossRef]
- Soroko, M.; Górniak, W.; Howell, K.; Zielińska, P.; Dudek, K.; Eberhardt, M.; Kalak, P.; Korczyński, M. Changes in Body Surface Temperature Associated with High-Speed Treadmill Exercise in Beagle Dogs Measured by Infrared Thermography. Animals 2021, 11, 2982. [Google Scholar] [CrossRef]
- Lamps, L.W.; Smoller, B.R.; Rasmussen LE, L.; Slade, B.E.; Fritsch, G.; Goodwin, T.E. Characterization of interdigital glands in the Asian elephant (Elephas maximus). Res. Vet. Sci. 2001, 71, 197–200. [Google Scholar] [CrossRef]
- Weissenböck, N. Thermoregulation of African (Loxodonta Africana) and Asian (Elephas Maximus) Elephants: Heterothermy as an Adaptation of Living in Hot Climates. Ph.D. Thesis, University of Vienna, Wien, Austria, 2010; pp. 1–73. [Google Scholar]
- Mota-Rojas, D.; Pereira AM, F.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Mora-Medina, P.; Casas-Alvarado, A.; Rios-Sandoval, J.; de Mira Geraldo, A.; Wang, D. Thermal Imaging to Assess the Health Status in Wildlife Animals under Human Care: Limitations and Perspectives. Animals 2022, 12, 3558. [Google Scholar] [CrossRef] [PubMed]
- Mumby, H.S.; Chapman, S.N.; Crawley JA, H.; Mar, K.U.; Htut, W.; Soe, A.T.; Aung, H.H.; Lummaa, V. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Ecol. Evol. 2015, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.; O’Rourke, M.F.; Frohlich, E.D. Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Maksuti, E.; Westerhof, N.; Westerhof, B.E.; Broomé, M.; Stergiopulos, N. Contribution of the Arterial System and the Heart to Blood Pressure during Normal Aging—A Simulation Study. PLoS ONE 2016, 11, e0157493. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A. Status and conservation of the Asian Elephant Elephas maximus in north-eastern India. Mammal Rev. 1999, 29, 141–174. [Google Scholar] [CrossRef]
- Cutright, W.J.; McKean, T. Countercurrent blood vessel arrangement in beaver (Castor canadensis). J. Morphol. 1979, 161, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lynch Vincent, J.; Bedoya-Reina Oscar, C.; Ratan, A.; Sulak, M.; Drautz-Moses Daniela, I.; Perry George, H.; Miller, W.; Schuster Stephan, C. Elephantid Genomes Reveal the Molecular Bases of Woolly Mammoth Adaptations to the Arctic. Cell Rep. 2015, 12, 217–228. [Google Scholar] [CrossRef]
- Repin, V.E.; Taranov, O.S.; Ryabchikova, E.I.; Tikhonov, A.N.; Pugachev, V.G. Sebaceous Glands of the Woolly Mammoth, Mammothus primigenius Blum.: Histological Evidence. Dokl. Biol. Sci. 2004, 398, 382–384. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).