Role of Endemism and Other Factors in Determining the Introduction Success of Rare and Threatened Species in Tashkent Botanical Garden
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kruckeberg, A.; Rabinowitz, D. Biological aspects of endemism in higher plants. Annu. Rev. Ecol. Syst. 1985, 16, 447–479. [Google Scholar] [CrossRef]
- Gaston, K.J. Species-range size distributions: Products of speciation, extinction and transformation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998, 353, 219–230. [Google Scholar] [CrossRef]
- McKinney, M.; Lockwood, J. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Lamoreux, J.; Morrison, J.; Ricketts, T.; Olson, D.; Dinerstein, E.; McKnight, M.; Shugart, H. Global tests of biodiversity concordance and the importance of endemism. Nature 2006, 440, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Hobohm, C. Endemism in Vascular Plants; Springer Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Guerrant, E.O.J.; Fiedler, P.L. Accounting for sample decline during ex situ storage and reintroduction. In Ex Situ Plant Conservation: Supporting Species Survival in the Wild; Guerrant, E.O.J., Havens, K., Maunder, M., Eds.; Island Press: Washington, DC, USA, 2004; pp. 365–386. [Google Scholar]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef]
- Volis, S. Conservation utility of botanic garden living collections: Setting a strategy and appropriate methodology. Plant Divers. 2017, 39, 365–372. [Google Scholar] [CrossRef]
- Abeli, T.; Dalrymple, S.; Godefroid, S.; Mondoni, A.; Müller, J.V.; Rossi, G.; Orsenigo, S. Ex situ collections and their potential for the restoration of extinct plants. Conserv. Biol. 2020, 34, 303–313. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic garden solutions to the plant extinction crisis. Plants People Planet 2021, 3, 22–32. [Google Scholar] [CrossRef]
- Wendelberger, K.S.; Fellows, M.Q.N.; Maschinski, J. Rescue and restoration: Experimental translocation of Amorpha herbacea Walter var. crenulata (Rybd.) Isley into a novel urban habitat. Restor. Ecol. 2008, 16, 542–552. [Google Scholar] [CrossRef]
- Noël, F.; Prati, D.; van Kleunen, M.; Gygax, A.; Moser, D.; Fischer, M. Establishment success of 25 rare wetland species introduced into restored habitats is best predicted by ecological distance to source habitats. Biol. Conserv. 2011, 144, 602–609. [Google Scholar] [CrossRef]
- Fotinos, T.D.; Namoff, S.; Lewis, C.; Maschinski, J.; Griffith, M.P.; von Wettberg, E.J.B. Genetic evaluation of a reintroduction of Sargent’s Cherry Palm, Pseudophoenix sargentii. J. Torrey Bot. Soc. 2015, 142, 51–62. [Google Scholar] [CrossRef]
- Fenu, G.; Cogoni, D.; Bacchetta, G. The role of fencing in the success of threatened plant species translocation. Plant Ecol. 2016, 217, 207–217. [Google Scholar] [CrossRef]
- Menges, E.S.; Smith, S.A.; Weekley, C.W. Adaptive introductions: How multiple experiments and comparisons to wild populations provide insights into requirements for long-term introduction success of an endangered shrub. Plant Divers. 2016, 38, 238–246. [Google Scholar] [CrossRef]
- Zimmer, H.C.; Offord, C.A.; Auld, T.D.; Baker, P.J. Establishing a wild, ex situ population of a critically endangered shade-tolerant rainforest conifer: A translocation experiment. PLoS ONE 2016, 11, e0157559. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C.J. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Tojibaev, K.S.; Jang, C.G.; Lazkov, G.A.; Chang, K.S.; Sitpayeva, G.T.; Safarov, N.; Beshko, N.; Muktubaeyeva, S.; Vesselova, P.; Turakulov, I.; et al. An annotated checklist of endemic vascular plants of the Tian-Shan Mountains in Central Asian countries. Phytotaxa 2020, 464, 117–158. [Google Scholar] [CrossRef]
- Volis, S. Conservation-oriented restoration and its application to Central Asia. Plant Diversity of Central Asia 2022, 1, 1–19. [Google Scholar]
- Tojibaev, K.S.; Beshko, N.Y.; Popov, V. Botanical-geographical regionalization of Uzbekistan. Botanicheskiĭ Zhurnal 2016, 101, 1105–1132. [Google Scholar]
- Khassanov, F. The Red Data Book of the Republic of Uzbekistan; Chinor ENK Press: Tashkent, Uzbekistan, 2019. [Google Scholar]
- Akopian, J. Conservation of native plant diversity at the Yerevan Botanic Garden, Armenia. Kew Bull. 2010, 65, 663–669. [Google Scholar] [CrossRef]
- Ageeva, S.; Kruglova, L.; Buganova, A.; Zholobova, O.; Safronova, G. Biodiversity conservation of rare and endangered plant species in the Volgograd Regional Botanical Garden. Immanuel Kant Balt. Fed. Univ. Vestn. Ser. 2012, 7, 103–109. [Google Scholar]
- Danilova, N. The results of the introduction of rare and endemic plants of Yakutia in the Yakutsk Botanical Garden. Arct. Subarct. Nat. Resour. 2017, 1, 97–104. [Google Scholar]
- Isaenko, T. Negative experience of introducing rare and endangered herbaceous plants in cultivation. In Proceedings of the Flora and Conservation in the Caucasus: History and Current State of Knowledge, Pyatigorsk, Russia, 22–25 May 2019; pp. 49–51. [Google Scholar]
- Belolipov, I.V. Introduction of Herbaceous Plants of the Natural Flora of Central Asia; Fan: Tashkent, Uzbekistan, 1989. [Google Scholar]
- Tursunov, T.T.; Sharipov, A.H. Introduction of rare and endangered plants of the flora of Uzbekistan. Introd. Acclim. Plants 1992, 35, 65–69. [Google Scholar]
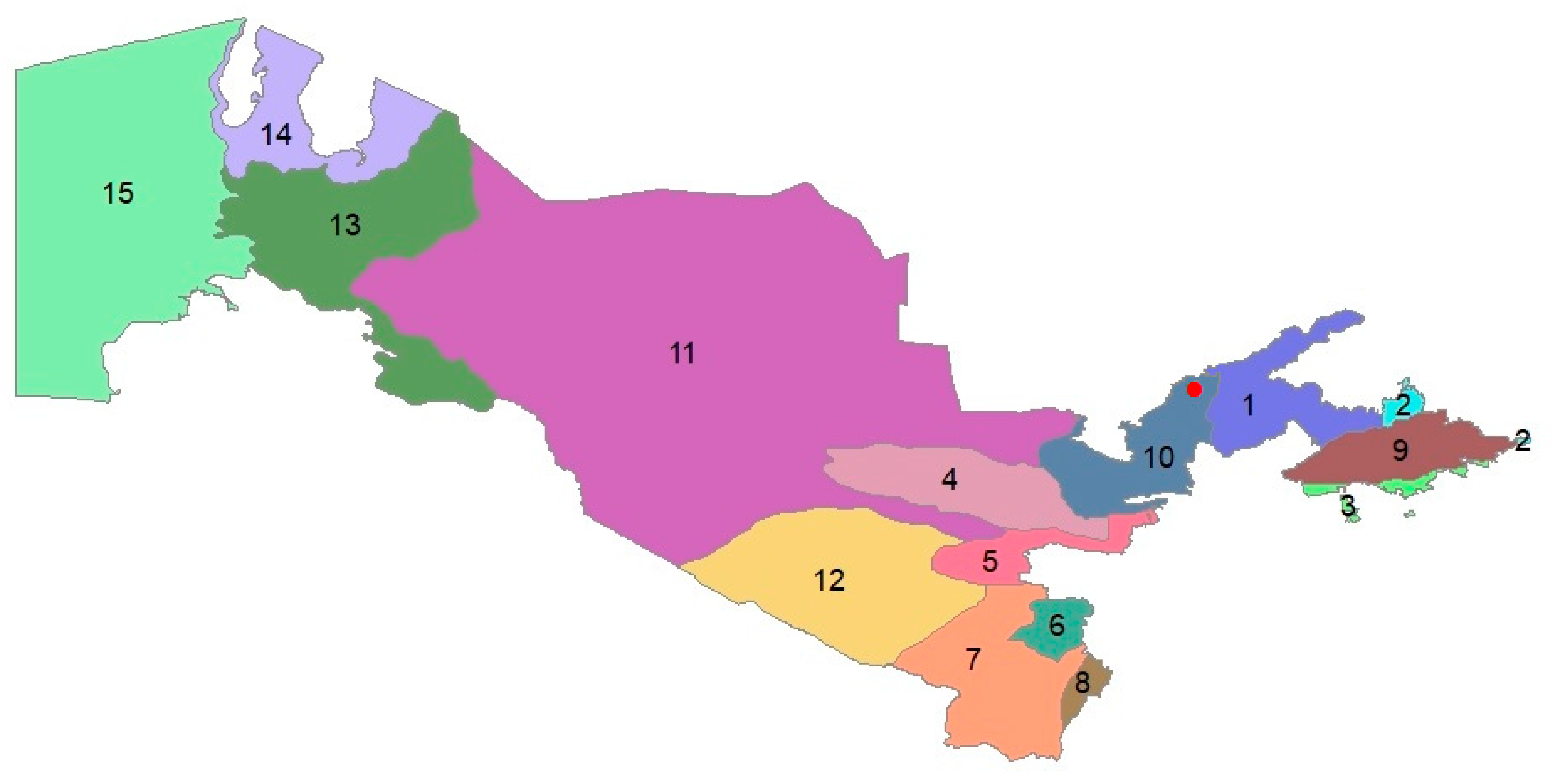
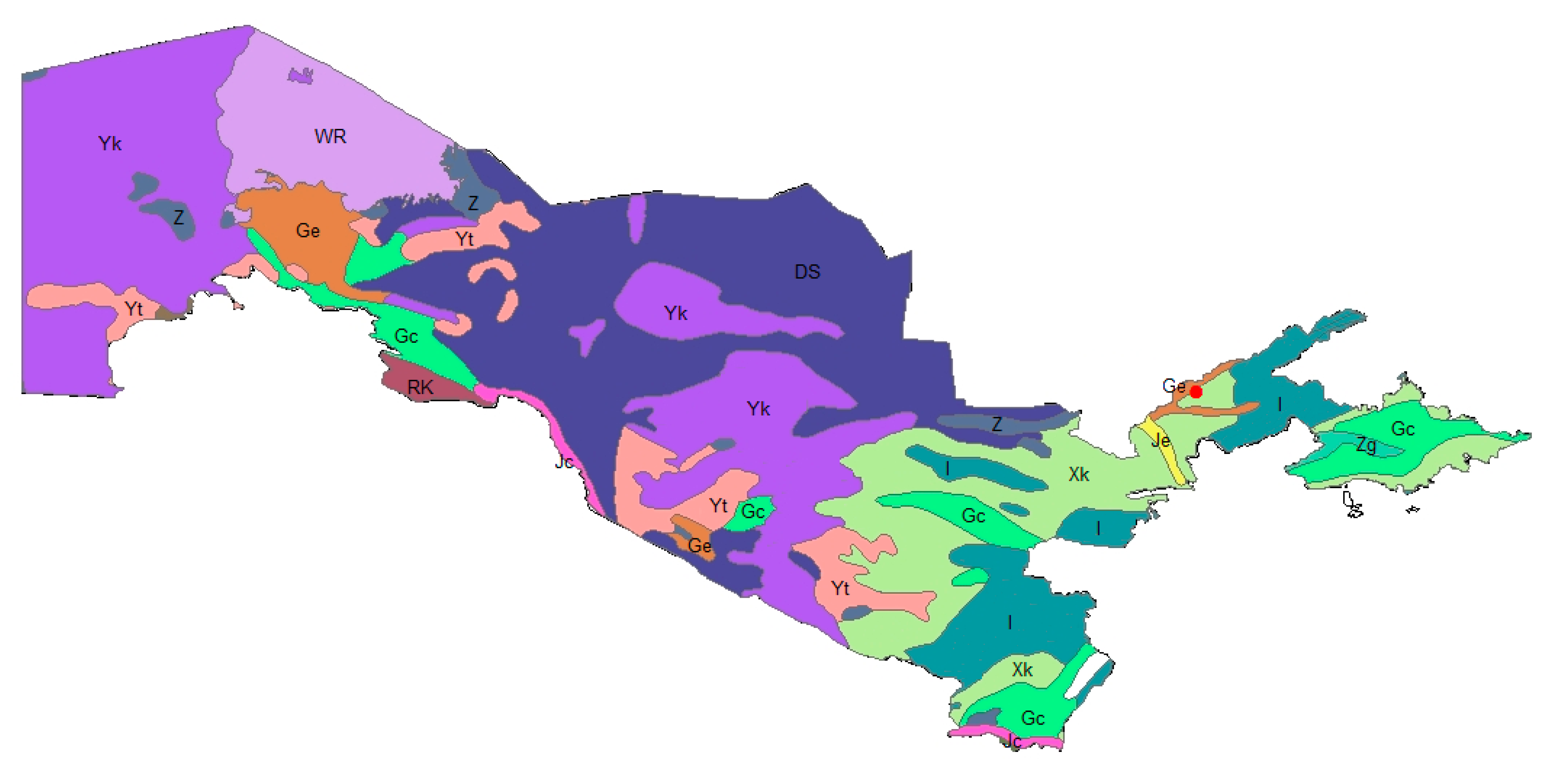
| Species | Family | Introduced Material | Introduction Success | Endemism | Life Form | Habitat Type | Soil Type | Phytogeographic Region |
|---|---|---|---|---|---|---|---|---|
| Acantholimon ekatherinae * | Plumbaginaceae | Seeds/adults | Yes | U | Subshr | A | I | 1 |
| Acantholimon margaritae * | Plumbaginaceae | Seeds/adults | Yes | U | Subshr | M | I | 1 |
| Acantholimon nuratavicum * | Plumbaginaceae | Seeds | Yes | U | Subshr | M | I | 4 |
| Acantholimon subavenaceum * | Plumbaginaceae | Seeds | Yes | U | Subshr | M | I | 4 |
| Aconitum talassicum * | Ranunculaceae | Seeds/adults | Yes | CA | Per | M, A | I, Gc | 1, 5, 7 |
| Aconitum seravschanicum | Ranunculaceae | Adults | No | CA | Per | M, A | I | 6 |
| Adonis chrysocyathus | Ranunculaceae | Adults | No | - | Per | A | I | 3 |
| Allium decoratum * | Amaryllidaceae | Seeds/adults | No | U | Per | A | I | 7 |
| Allium giganteum * | Amaryllidaceae | Seeds/adults | Yes | - | Per | F, M | Xk, Gc | 7, 8 |
| Allium praemixtum * | Amaryllidaceae | Seeds/adults | Yes | U | Per | M | I, Xk | 4 |
| Allium pskemense * | Amaryllidaceae | Seeds/adults | Yes | CA | Per | M | I | 1 |
| Alliumisakulii * | Amaryllidaceae | Seeds/adults | Yes | CA | Per | M | I, Xk | 3, 4 |
| Allium stipitatum | Amaryllidaceae | Seeds/adults | Yes | U | Per | M | I | 4 |
| Allium oshaninii | Amaryllidaceae | Adults | No | CA | Per | F, M | I | 7, 6, 8, 5, 1 |
| Acanthophyllum gypsophiloides * | Caryophyllaceae | Seeds | Yes | CA | Per | M | I | 1 |
| Acanthophyllum tadshikistanicum | Caryophyllaceae | Seeds | Yes | CA | Per | F | I | 8 |
| Andrachne vvedenskyi * | Phyllanthaceae | Seeds/adults | Yes | U | Subshr | F | I | 7 |
| Anemone baissunensis * | Ranunculaceae | Seeds/adults | Yes | CA | Per | F, M | Xk | 7 |
| Anemone bucharica * | Ranunculaceae | Seeds/adults | Yes | CA | Per | M | I, Xk | 7 |
| Anemone narcissiflora | Ranunculaceae | Seeds/adults | No | CA | Per | M, A | I | 1 |
| Astragalus belolipovii * | Fabaceae | Seeds | Yes | U | Per | M | I | 4 |
| Astragalus bucharicus * | Fabaceae | Adults | No | U | Per | M | I | 8 |
| Astragalus rhacodes * | Fabaceae | Seeds | Yes | U | Subshr | M | I | 3 |
| Astragalus terrae-rubrae * | Fabaceae | Seeds | No | U | Per | M | I | 7 |
| Astragalus willisii * | Fabaceae | Seeds | No | U | Per | M | I | 7 |
| Aulacospermum popovii * | Apiaceae | Seeds | No | U | Per | M | I | 1 |
| Bryonia melanocarpa * | Cucurbitaceae | Seeds/adults | No | CA | Per | D, P | Yk, Ds | 4, 10, 11 |
| Bunium vaginatum * | Apiaceae | Seeds/adults | Yes | CA | Per | M | I | 1 |
| Argyrolobium aegacanthoides * | Fabaceae | Adults | No | U | Subshr | M | I | 7 |
| Capparis spinosa var. herbacea * | Capparaceae | Seeds | No | U | Per | F, M | I | 8 |
| Cephalopodum hissaricum * | Apiaceae | Seeds | No | CA | Per | M | Xk | 7 |
| Cleome gordjaginii * | Cleomaceae | Seeds | Yes | CA | Ann | F | I, Xk | 7 |
| Colchicum kesselringii * | Colchicaceae | Adults | Yes | CA | Per | P, F, M, A | I | 1, 4, 5, 7 |
| Corydalis sewerzovii | Papaveraceae | Seeds/adults | Yes | U | Per | (F) M | I, Xk | 1, 7, 11 |
| Crambe gordjaginii * | Brassicaceae | Seeds | No | U | Per | F | I | 7 |
| Crocus alatavicus | Iridaceae | Adults | Yes | CA | Per | M | I, Xk | 1, 10 |
| Crocus korolkovii | Iridaceae | Seeds/adults | Yes | CA | Per | F, M | I, Xk | 7, 4, 5 |
| Dianthus uzbekistanicus * | Caryophyllaceae | Seeds | Yes | U | Per | M | I | 5, 7 |
| Dionysia hissarica * | Primulaceae | Seeds | No | U | Subshr | M | I | 6 |
| Dipcadi turkestanicum * | Asparagaceae | Adults | No | U | Per | M | Gc | 7 |
| Dorema microcarpum * | Apiaceae | Seeds | Yes | CA | Per | (F) M | I, Xk | 2, 3 |
| Eremurus aitchisonii * | Asphodelaceae | Seeds/adults | Yes | - | Per | P, A | I, Xk | 5, 6, 7, 12 |
| Eremurus alberti * | Asphodelaceae | Seeds/adults | Yes | - | Per | F | Xk, Z | 7, 8 |
| Eremurusambigens | Asphodelaceae | Seeds/adults | Yes | CA | Per | F | Xk, Gc | 7, 8 |
| Eremurus baissunensis * | Asphodelaceae | Adults | No | U | Per | F | I, Xk | 7 |
| Eremurus lactiflorus * | Asphodelaceae | Seeds/adults | Yes | CA | Per | F, M | I, Xk | 1, 4 |
| Eremuruskorolkovii * | Asphodelaceae | Adults | No | U | Per | P | Yk, Yt, Z | 11 |
| Eremurusluteus * | Asphodelaceae | Seeds/adults | Yes | - | Per | F, M | I | 7 |
| Eremurusnuratavicus * | Asphodelaceae | Seeds/adults | Yes | U | Per | F, M | I | 4 |
| Eremurusrobustus * | Asphodelaceae | Seeds/adults | Yes | CA | Per | F, M | I, Xk | 1, 4, 5, 7, 10, 12 |
| Eremurus stenophyllus | Asphodelaceae | Seeds/adults | Yes | - | Per | F, A | I, Xk, Gc | 6, 7 |
| Eremurussuworowii * | Asphodelaceae | Seeds/adults | Yes | - | Per | F, M | I, Xk | 6, 7, 8 |
| Eversmannia botschantzevii * | Fabaceae | Seeds | Yes | U | Shrub | F | I | 7 |
| Ferula gigantea | Apiaceae | Seeds | Yes | - | Per | F, M | I, Gc | 6 or 8 |
| Fritillaria eduardii * | Liliaceae | Seeds/adults | Yes | - | Per | M | I | 6 |
| Gladiolus italicus * | Iridaceae | Seeds/adults | Yes | - | Per | F, M | I | 7, 6 |
| Heliotropium bucharicum * | Heliotropiaceae | Seeds | Yes | U | Ann | M | I | 7 |
| Incarvillea olgae * | Bignoniaceae | Seeds | Yes | CA | Per | M | I | 3 |
| Iris hippolyti * | Iridaceae | Adults | No | U | Per | D, F | Yk | 4 |
| Iris magnifica * | Iridaceae | Seeds/adults | Yes | U | Per | F | Gc, I | 5 |
| Iris orchioides * | Iridaceae | Seeds/adults | Yes | CA | Per | F, M | I, Xk | 1, 10 |
| Iris svetlanae * | Iridaceae | Seeds/adults | Yes | U | Per | F, M | I, Xk | 5, 7, 12 |
| Lagochilus inebrians * | Lamiaceae | Seeds | Yes | U | Subshr | P | Xk | 4 |
| Lepidolopha fedtschenkoana * | Asteraceae | Seeds | Yes | U | Subshr | M | I | 7 |
| Lipskya insignis * | Apiaceae | Seeds | Yes | CA | Per | F | I, Xk | 7 |
| Malacocarpus crithmifolius * | Zygophyllaceae | Seeds | Yes | - | Shrub | P | Yk | 15 |
| Nanophyton botschantzevii | Amaranthaceae | Seeds | No | U | Shrub | M | I | 1 |
| Onobrychis tavernierifolia * | Fabaceae | Seeds | Yes | - | Ann | P | Yt | 11 |
| Ostrowskia magnifica * | Campanulaceae | Seeds/adults | Yes | - | Per | M | I | 6 |
| Moluccella bucharica * | Lamiaceae | Seeds/adults | No | U | Subshr | F, M | I | 7 |
| Oxytropis tachtensis | Fabaceae | Seeds | Yes | - | Per | M | I | 4, 5 |
| Oxytropis seravschanica | Fabaceae | Seeds | Yes | CA | Per | A | I | 5 |
| Paeonia intermedia * | Paeoniaceae | Seeds/adults | Yes | - | Per | M | I | 1, 6, 7 |
| Physochlaina alaica * | Solanaceae | Adults | Yes | U | Per | M | I, Xk | 3 |
| Prangos tschimganica | Apiaceae | Seeds | Yes | U | Per | M | I | 1 |
| Rhus coriaria * | Anacardiaceae | Seeds/adults | Yes | - | Shrub | F, M | I | 1, 6 |
| Rubia laevissima * | Rubiaceae | Seeds | No | CA | Subshr | M | I | 1 |
| Salvia korolkowii * | Lamiaceae | Seeds/adults | Yes | U | Subshr | F, M | I | 1 |
| Salvia lilacinocoerulea * | Lamiaceae | Seeds/adults | Yes | U | Per | M | I | 7 |
| Salvia submutica * | Lamiaceae | Seeds | Yes | U | Per | M | Xk, I | 4 |
| Spirostegia bucharica * | Plantaginaceae | Seeds | No | CA | Biann | F | I | 7 |
| Sternbergia lutea * | Amaryllidaceae | Seeds/adults | No | - | Per | M | I | 7 |
| Tulipa affinis * | Liliaceae | Seeds/adults | Yes | CA | Per | P, M | I, Xk, Yk | 4, 11, 5 |
| Tulipa bifloriformis | Liliaceae | Seeds/adults | Yes | CA | Per | P, M | I, Xk, Ge | 1, 2, 10 |
| Tulipa buhseana | Liliaceae | Seeds/adults | Yes | CA | Per | P | Xk, Yk, | 4, 10, 11, 15 |
| Tulipa carinata * | Liliaceae | Seeds/adults | Yes | U | Per | M, A | I, Xk | 6, 7 |
| Tulipa ferganica * | Liliaceae | Seeds/adults | Yes | U | Per | F | Gc, I, Xk | 2, 3, 9 |
| Tulipa fosteriana * | Liliaceae | Seeds/adults | Yes | U | Per | F, M | I | 5 |
| Tulipa greigii * | Liliaceae | Seeds/adults | Yes | CA | Per | P, M | I, Xk, Ge | 1, 10 |
| Tulipa ingens * | Liliaceae | Seeds/adults | Yes | CA | Per | M | I, Xk | 5, 6, 7 |
| Tulipa kaufmanniana * | Liliaceae | Seeds/adults | Yes | CA | Per | P, M | I, Xk, Ge | 1 |
| Tulipa korolkowii * | Liliaceae | Seeds/adults | Yes | CA | Per | P, M | I, Xk, Ge, Yk, Gc | 1, 3, 4, 5, 6, 7, 8 |
| Tulipa lanata * | Liliaceae | Seeds/adults | Yes | CA | Per | F, M | I, Xk, Gc | 6, 7,8 |
| Tulipa micheliana * | Liliaceae | Seeds/adults | Yes | CA | Per | P, F | I, Gc, Yk, Xk | 4, 5, 7, 11, 12 |
| Tulipa orythioides * | Liliaceae | Seeds/adults | Yes | U | Per | F, A | I | 6, 7 |
| Tulipa scharipovii * | Liliaceae | Seeds/adults | Yes | U | Per | F | Gc, Xk | 1, 2 |
| Tulipa tschimganica | Liliaceae | Seeds/adults | Yes | U | Per | F, M | I | 1 |
| Tulipa tubergeniana * | Liliaceae | Seeds/adults | Yes | CA | Per | F, M | I, Gc, Xk | 6, 7, 8 |
| Tulipa turkestanica | Liliaceae | Seeds/adults | Yes | CA | Per | P, M | I, Xk | 1, 3, 4, 5, 6, 7, 9, 10, 11, 12 |
| Tulipasogdiana | Liliaceae | Adults | No | CA | Per | P | Yk | 11, 12, 13, 15 |
| Tulipauzbekistanica * | Liliaceae | Seeds/adults | Yes | U | Per | M | I | 7 |
| Tulipavvedenskyi * | Liliaceae | Seeds/adults | Yes | U | Per | F, A | I | 1 |
| Zeravschania regeliana * | Apiaceae | Seeds | Yes | U | Per | M | I | 7 |
| Zygophyllum bucharicum * | Zygophyllaceae | Seeds/adults | No | CA | Shrub | F | Jc | 7 |
| Analyzed Factors | Results of Species Introduction (Number of Species] | |
|---|---|---|
| Success | Failure | |
| Endemism | ||
| Non-endemic | 15 | 2 |
| Endemic to Central Asia (Uzbekistan + other CA countries) | 31 | 9 |
| Endemic to Uzbekistan | 32 | 15 |
| Life form | ||
| Shrub | 3 | 2 |
| Subshrub | 9 | 4 |
| Perennial | 64 | 19 |
| Annual or biannual | 3 | 1 |
| Habitat type | ||
| Desert | 0 | 2 |
| Plain | 13 | 3 |
| Foothills | 34 | 9 |
| Mid mountains | 57 | 15 |
| Alpine | 9 | 4 |
| Soil type | ||
| Xk | 37 | 2 |
| Other types | 38 | 23 |
| Phytogeographic region | ||
| Middle Syrdarya | 7 | 1 |
| Other regions | 67 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volis, S.; Belolipov, I.V.; Asatulloev, T.; Turgunov, M. Role of Endemism and Other Factors in Determining the Introduction Success of Rare and Threatened Species in Tashkent Botanical Garden. J. Zool. Bot. Gard. 2023, 4, 325-334. https://doi.org/10.3390/jzbg4020027
Volis S, Belolipov IV, Asatulloev T, Turgunov M. Role of Endemism and Other Factors in Determining the Introduction Success of Rare and Threatened Species in Tashkent Botanical Garden. Journal of Zoological and Botanical Gardens. 2023; 4(2):325-334. https://doi.org/10.3390/jzbg4020027
Chicago/Turabian StyleVolis, Sergei, Igor V. Belolipov, Temur Asatulloev, and Mirabdulla Turgunov. 2023. "Role of Endemism and Other Factors in Determining the Introduction Success of Rare and Threatened Species in Tashkent Botanical Garden" Journal of Zoological and Botanical Gardens 4, no. 2: 325-334. https://doi.org/10.3390/jzbg4020027
APA StyleVolis, S., Belolipov, I. V., Asatulloev, T., & Turgunov, M. (2023). Role of Endemism and Other Factors in Determining the Introduction Success of Rare and Threatened Species in Tashkent Botanical Garden. Journal of Zoological and Botanical Gardens, 4(2), 325-334. https://doi.org/10.3390/jzbg4020027







