Behavioural Responses to Temporary Separation of a Captive Herd of African Elephants (Loxodonta africana)
Abstract
1. Introduction
1.1. Elephants in Captivity
1.2. Focal Herd
1.3. Aims
2. Materials and Methods
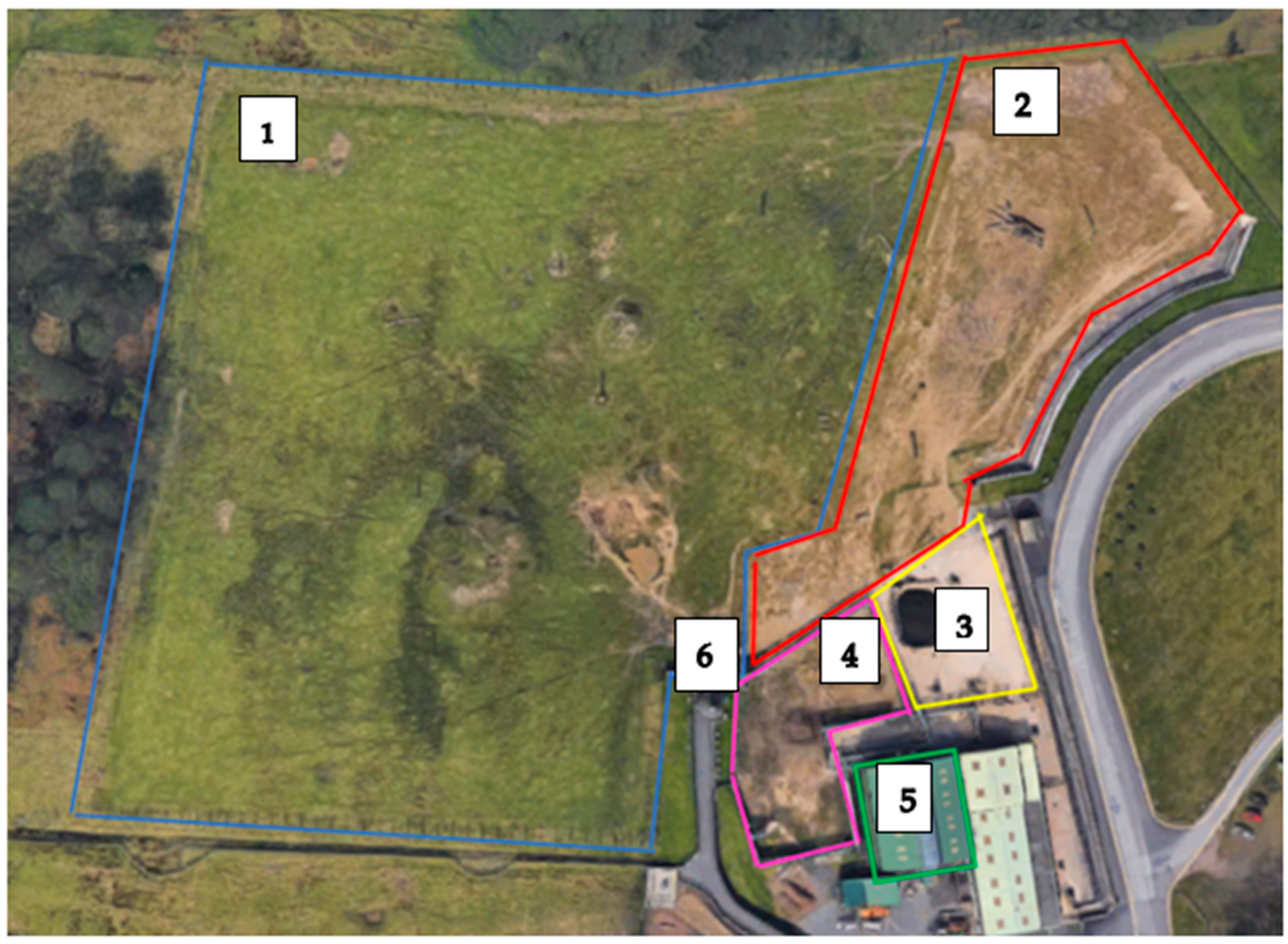
2.1. Study Site
2.2. Data Collection
2.2.1. Social Behaviour
2.2.2. Individual Behaviour
2.3. Ethical Consideration
3. Results
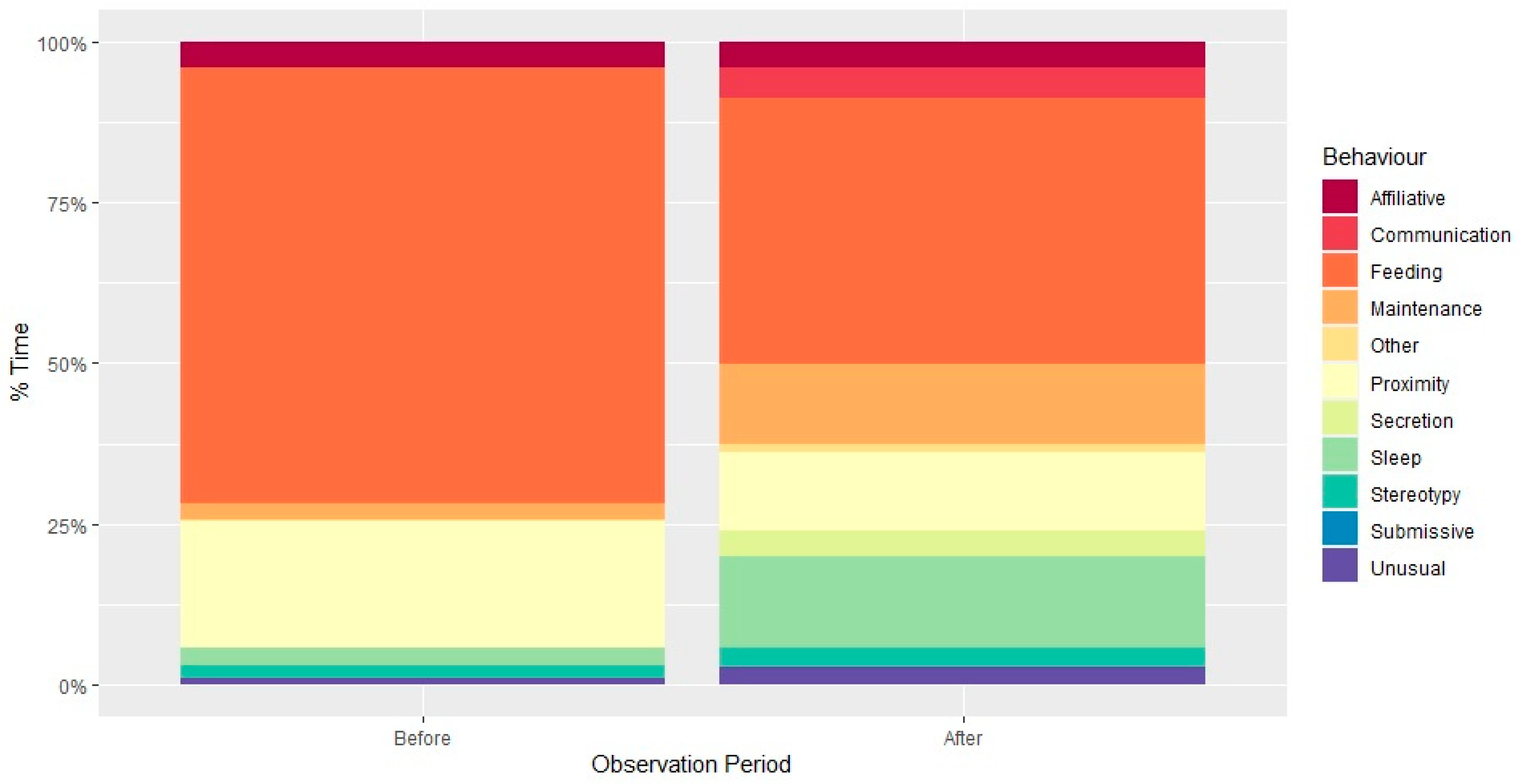
3.1. Social Behaviour
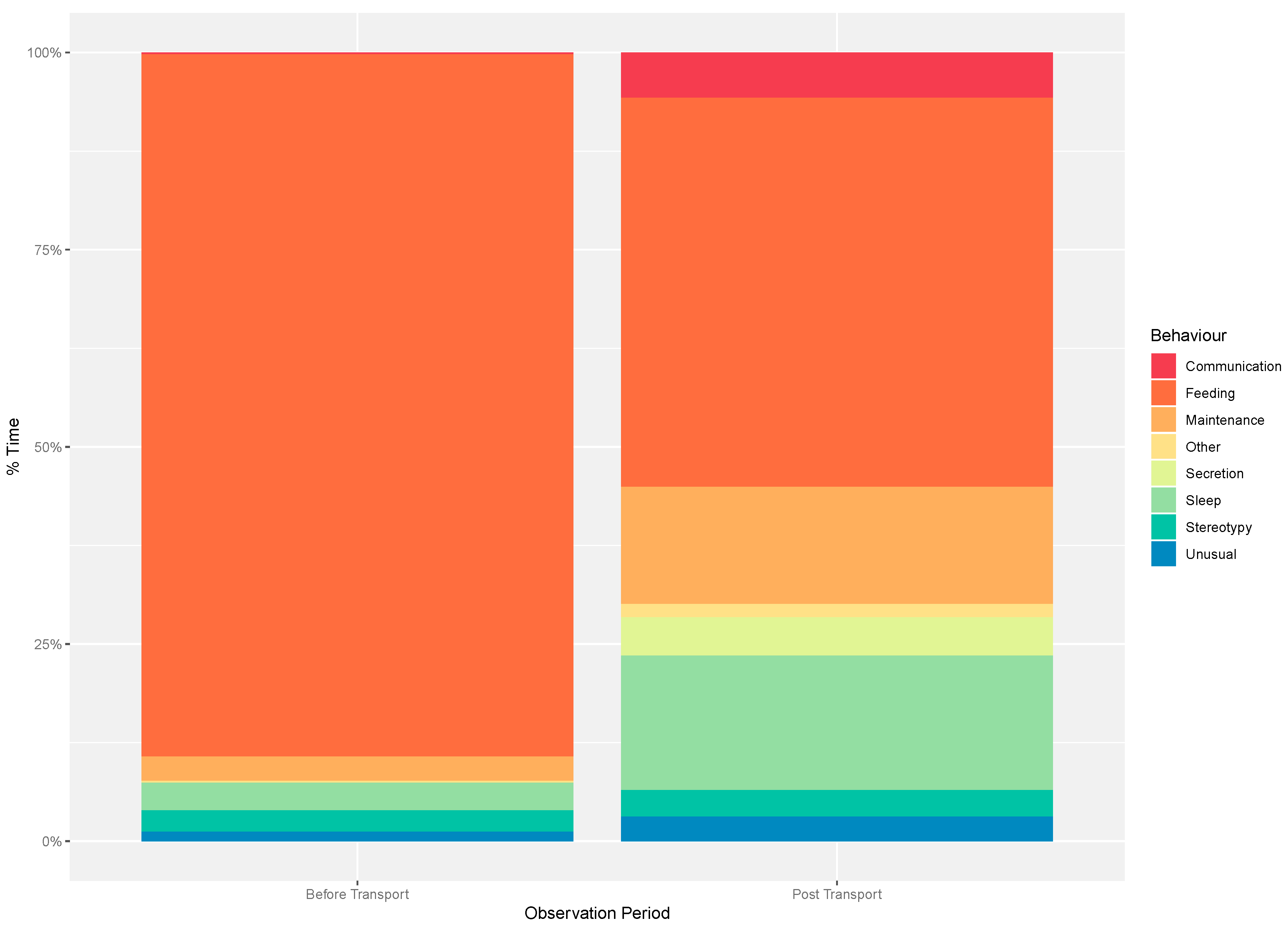
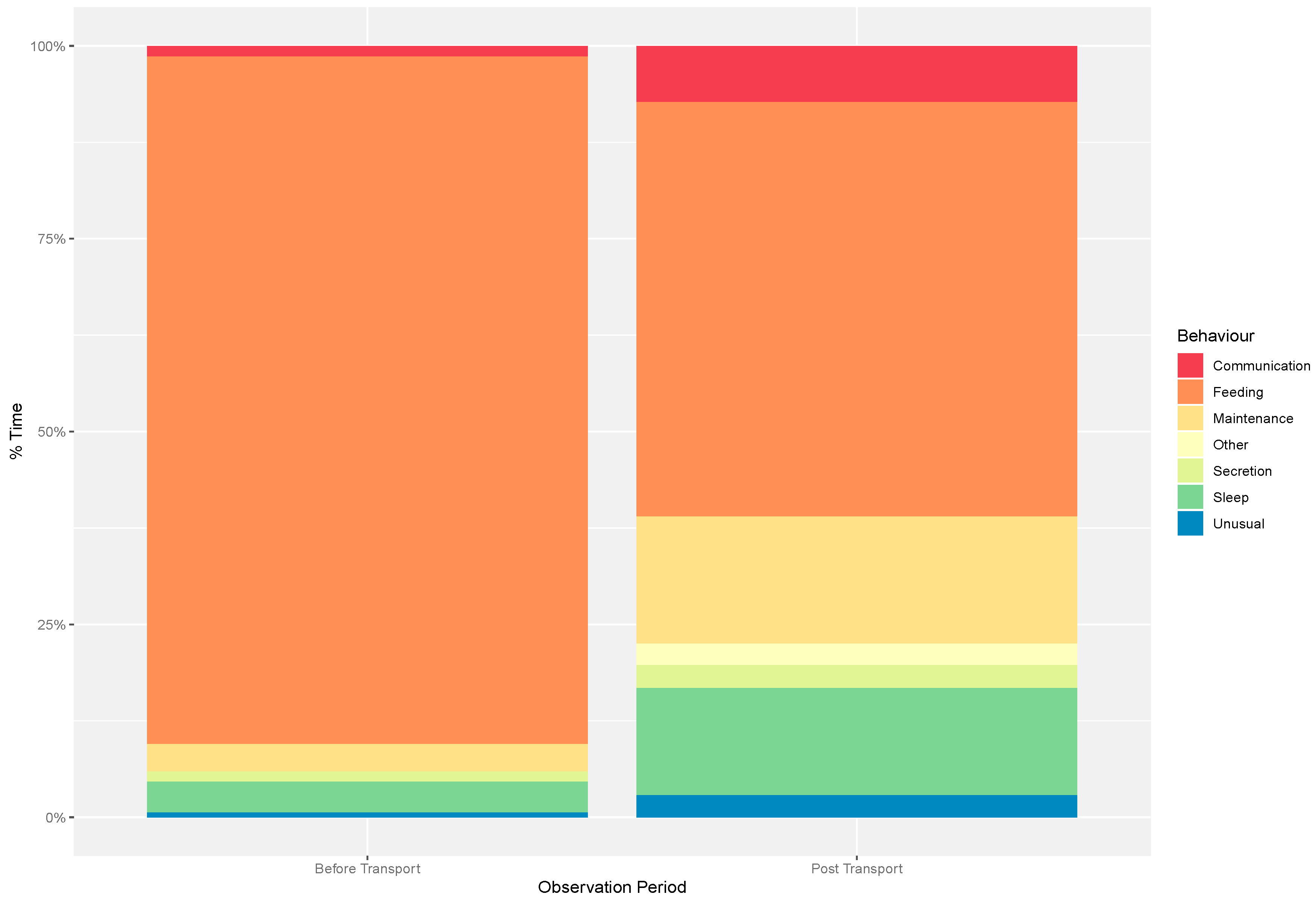
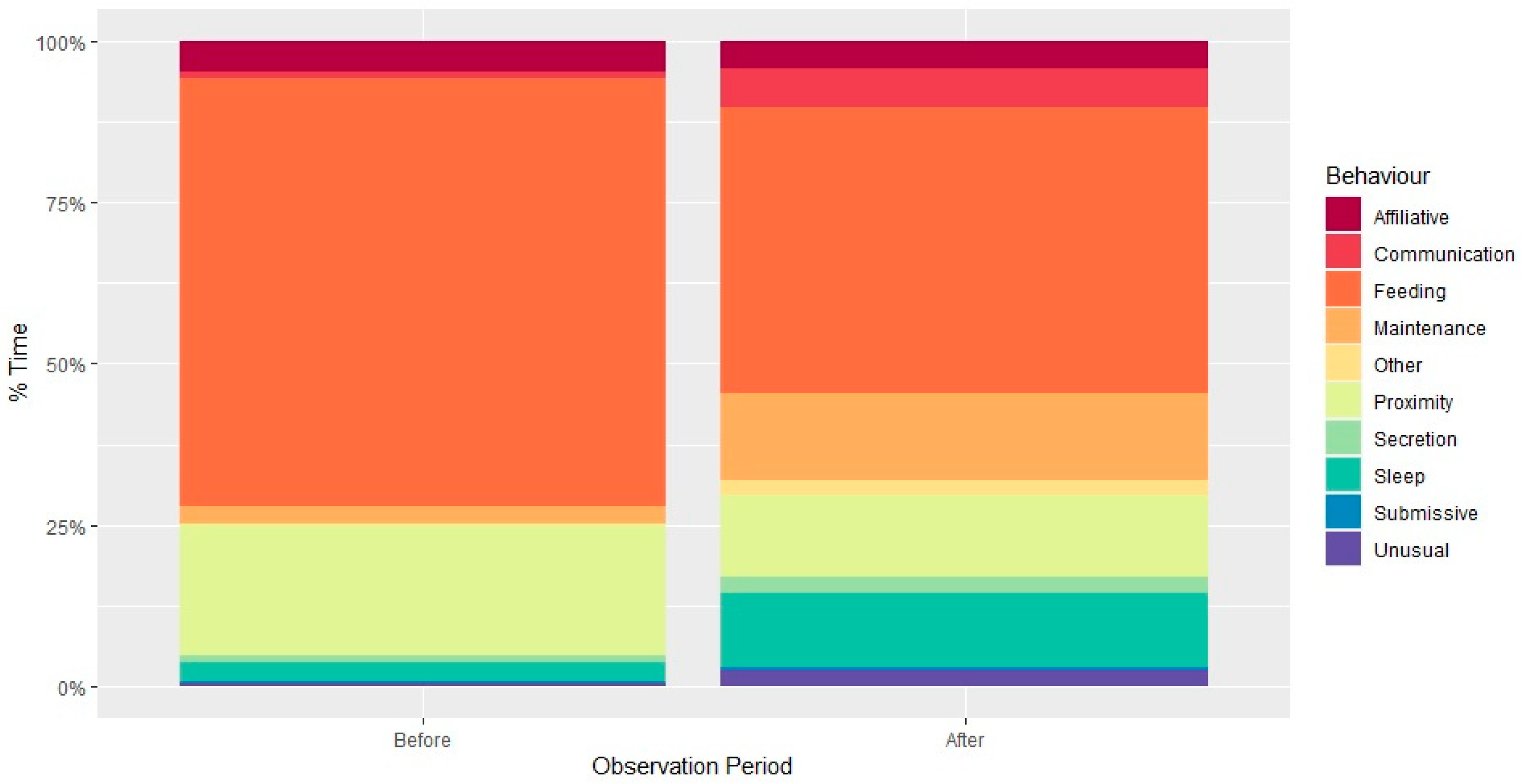
3.2. Individual Behaviour
4. Discussion
4.1. Social Behaviour
4.2. Individual Behaviour
4.3. Limitations
5. Conclusions
- Even when housed in a nontypical grouping, captive African elephants exhibit similar responses to separation and uncertainty to wild elephants. Although there is correctly a focus on high relatability in captive elephant herds, not all institutions have this, and the same allowances for herds with low or no relatability need to be made when collection planning.
- Reduced social capacity and play opportunities, combined with increases in stereotypy and temporal gland secretion, all indicate reduced welfare.
- Temporary separation in this case was necessary due to logistics and safety; however, when arranging transports for any animal, the psychological repercussions of separations, be they temporary or permanent, must be taken into account, and alternative methods should be explored for all social species.
- In the future, when transports or major events changing social structure or individual behavioural opportunities occur, where possible, there should also be a focus on behavioural and physical data collection prior to, during, and after. The subsequent analysis, publication, and incorporation into actual procedure and protocol are vital to continue improving captive welfare.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, C.J.; Poole, J.H. Relationship and social structure in African Elephants. In Primate Social Relationships: An Integrated Approach; Hinde, R.A., Ed.; Blackwell Scientific: Oxford, UK, 1983; pp. 315–325. [Google Scholar]
- Hoare, R. African elephants and humans in conflict: The outlook for co-existence. Oryx 2000, 34, 34–38. [Google Scholar] [CrossRef]
- Cameron, E.; Ryan, S. Welfare at Multiple Scales: Importance of Zoo Elephant Population Welfare in a World of Declining Wild Populations. PLoS ONE 2016, 11, e0158701. [Google Scholar] [CrossRef]
- Poulsen, J.R.; Koerner, S.E.; Moore, S.; Medjibe, V.P.; Blake, S.; Clark, C.J.; Akou, M.E.; Fay, M.; Meier, A.; Okouyi, J.; et al. Poaching empties critical Central African wilderness of forest elephants. Curr. Biol. 2017, 27, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Payne, K.; Langbauer, W.R.; Moss, C. The social contexts of some very low frequency calls of African Elephants. Behav. Ecol. Sociobiol. 1988, 22, 385–392. [Google Scholar] [CrossRef]
- Lee, P.; Moss, C. African Elephant Play, Competence and Social Complexity. Anim. Behav. Cogn. 2016, 1, 144–156. [Google Scholar] [CrossRef]
- Moss, C.J.; Croze, H.; Lee, P.C. (Eds.) The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; University of Chicago Press: Chicago, IL, USA, 2011. [Google Scholar]
- Lucas, C.; Stanyon, B. Improving the welfare of African elephants Loxodonta africana in zoological institutions through enclosure design and husbandry management: An example from Blair Drummond Safari and Adventure Park. Int. Zoo Yearb. 2017, 51, 248–257. [Google Scholar] [CrossRef]
- Harris, M.; Sherwin, C.; Harris, S. The Welfare, Housing and Husbandry of Elephants in UK Zoos. Report. Available online: http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&ProjectID=13192&FromSearch=Y&Publisher=1&SearchText=wc05007&SortString=ProjectCode&SortOrder=Asc&Paging=10 (accessed on 5 January 2019).
- Stevenson, M.; Walter, O.; British & Irish Association of Zoos & Aquariums. Management Guidelines for the Welfare of Zoo Animals: Elephants, Loxodonta africana and Elephas Maximus; British & Irish Association of Zoos & Aquariums: London, UK, 2006. [Google Scholar]
- Williams, E.; Chadwick, C.; Yon, L.; Asher, L. A review of current indicators of welfare in captive elephants (Loxodonta africana and Elephas maximus). Anim. Welf. 2018, 27, 235–249. [Google Scholar] [CrossRef]
- Garstang, M. Elephant Sense and Sensibility; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Barkai, R. Elephants are people, people are elephants: Human–proboscideans similarities as a case for cross cultural animal humanization in recent and Paleolithic times. Quat. Int. 2016, 406, 239–245. [Google Scholar]
- Elephant Welfare Group; British & Irish Association of Zoos & Aquariums Elephant Welfare Group. BIAZA Website 2016. Available online: https://biaza.org.uk/elephant-care-management (accessed on 5 January 2019).
- Chadwick, C.; Williams, E.; Asher, L.; Yon, L. Incorporating stakeholder perspectives into the assessment and provision of captive elephant welfare. Anim. Welf. 2017, 26, 461–472. [Google Scholar] [CrossRef]
- Whittaker, M.; Laule, G. Protected contact and elephant welfare. In An Elephant in the Room: The Science and Well-Being of Elephants in Captivity; Tufts Center for Animals and Public Policy: Medford, MA, USA, 2009. [Google Scholar]
- Wilson, M.; Perdue, B.; Bloomsmith, M.; Maple, T. Rates of reinforcement and measures of compliance in free and protected contact elephant management systems. Zoo Biol. 2015, 34, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Csuti, B. Elephants in captivity. In Biology, Medicine, and Surgery of Elephants, 1st ed.; Fowler, M., Mikota, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 15–22. [Google Scholar]
- Stoinski, T.; Daniel, E.; Maple, T. A preliminary study of the behavioral effects of feeding enrichment on African elephants. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 2000, 19, 485–493. [Google Scholar] [CrossRef]
- Laws, N.; Ganswindt, A.; Ganswindt, A.; Heistermann, M.; Harris, S.; Sherwin, C. A Case Study: Fecal Corticosteroid and Behavior as Indicators of Welfare During Relocation of an Asian Elephant. J. Appl. Anim. Welf. Sci. 2007, 10, 349–358. [Google Scholar] [CrossRef]
- Bateson, M.; Martin, P. Measuring Behavior: An Introductory Guide, 4th ed.; Cambridge University Press: Cambridge, UK, 2021; pp. 105–106. [Google Scholar]
- Bagley, K.; Goodwin, T.; Rasmussen, L.; Schulte, B. Male African elephants, Loxodonta africana, can distinguish oestrous status via urinary signals. Anim. Behav. 2006, 71, 439–1445. [Google Scholar] [CrossRef]
- Gruber, T.; Friend, T.; Gardner, J.; Packard, J.; Beaver, B.; Bushong, D. Variation in stereotypic behavior related to restraint in circus elephants. Zoo Biol. 2000, 19, 209–221. [Google Scholar] [CrossRef]
- Langbauer, W. Elephant communication. Zoo Biol. 2000, 19, 425–445. [Google Scholar] [CrossRef]
- Olson, D. (Ed.) Ethogram of elephant behaviors. In Elephant Husbandry Resource Guide; Allen Press: Lawrence, KS, USA, 2004; pp. 103–121. [Google Scholar]
- Goldenberg, S.; Douglas-Hamilton, I.; Wittemyer, G. Vertical transmission of social roles drives resilience to poaching in elephant networks. Curr. Biol. 2016, 26, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J. Concepts in the care and welfare of captive elephants. Int. Zoo Yearb. 2006, 40, 63–79. [Google Scholar] [CrossRef]
- Held, S.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Buss, I. Some observations of food habits and behaviour of African Elephants. J. Wildl. Manag. 1961, 25, 131–148. [Google Scholar] [CrossRef]
- Vicino, G.; Marcacci, E. Intensity of play behavior as a potential measure of welfare: A novel method for quantifying the integrated intensity of behavior in African elephants. Zoo Biol. 2015, 34, 492–496. [Google Scholar] [CrossRef]
- Leighty, K.A.; Soltis, J.; Wesolek, C.M.; Savage, A. Rumble vocalizations mediate interpartner distance in African elephants, Loxodonta africana. Anim. Behav. 2008, 76, 1601–1608. [Google Scholar] [CrossRef]
- Poole, J. Elephant Trunk Calls. Swara 1988, 6, 29–31. [Google Scholar]
- Brown, J.L.; Carlstead, K.; Bray, J.D.; Dickey, D.; Farin, C.; Ange-van Heugten, K. Individual and environmental risk factors associated with fecal glucocorticoid metabolite concentrations in zoo-housed Asian and African elephants. PLoS ONE 2019, 14, e0217326. [Google Scholar] [CrossRef] [PubMed]
- Kioko, J.; Taylor, K.; Milne, H.; Hayes, K.; Kiffner, C. Temporal gland secretion in African elephants (Loxodonta africana). Mamm. Biol. 2017, 82, 34–40. [Google Scholar] [CrossRef]
- Poole, J.; Granli, P. Mind and movement: Meeting the interests of elephants. In An Elephant in the Room: The Science and Well Being of Elephants in Captivity; Forthman, D.L., Kane, F.L., Hancocks, D., Waldau, P.F., Eds.; Center for Animals and Public Policy, Cummings School of Veterinary Medicine, Tufts University: Medford, MA, USA, 2009. [Google Scholar]
- O’Connell-Rodwell, C. Keeping an “ear” to the ground: Seismic communication in elephants. Physiology 2007, 22, 287–294. [Google Scholar] [CrossRef]
- Buss, I.; Estes, J. The functional significance of movements and positions of the pinnae of the African elephant, Loxodonta africana. J. Mammal. 1971, 52, 21–27. [Google Scholar] [CrossRef]
- Langbauer, W.R.; Payne, K.B.; Charif, R.A.; Rapaport, L.; Osborn, F. African elephants respond to distant playbacks of low frequency conspecific calls. J. Exp. Biol. 1991, 157, 35–46. [Google Scholar] [CrossRef]
- McComb, K. Studying vocal communication in elephants. In Studying Elephants; African Wildlife Foundation: Nairobi, Kenya, 1996; pp. 112–119. [Google Scholar]
- McComb, K.; Reby, D.; Baker, L.; Moss, C.; Sayialel, S. Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 2003, 65, 317–329. [Google Scholar] [CrossRef]
- Mason, G.J.; Latham, N. Can’t stop, won’t stop: Is stereotypy a reliable animal welfare indicator? Anim. Welf. 2004, 13, 57–69. [Google Scholar]
- Greco, B.; Meehan, C.; Hogan, J.; Leighty, K.; Mellen, J.; Mason, G.; Mench, J. The days and nights of zoo elephants: Using epidemiology to better understand stereotypic behavior of African elephants (Loxodonta africana) and Asian elephants (Elephas maximus) in North American zoos. PLoS ONE 2016, 11, e0144276. [Google Scholar]
- Powell, D.; Baskir, E. A matter of time: Comparing observation methods. In Exploring Animal Behavior in Laboratory and Field; Academic Press: Cambridge, MA, USA, 2021; pp. 49–61. [Google Scholar]
- Margulis, S.; Westhus, E. Evaluation of different observational sampling regimes for use in zoological parks. Appl. Anim. Behav. Sci. 2008, 110, 363–376. [Google Scholar] [CrossRef]
| Name | Sex | Origin | Age | Arrival KS | Relatedness | Dominance | Transport |
|---|---|---|---|---|---|---|---|
| Juba | F | w.1998 Zimbabwe | ~31 | 1993 | Unrelated | 1 | 1 |
| Tana | F | w.1988 Zimbabwe | ~30 | 1993 | Mother of Nala | 2 | 2 |
| Ashanti | F | c. KS | 14 | b. 10 January 2003 | ½ sister of Nala | 4 | 1 |
| Nala | F | c. KS | 13 | b. 5 April 2003 | Daughter of Tana | 3 | 2 |
| Behaviour | Description | Function/Category |
|---|---|---|
| Approach | Individual moves to within three body lengths of another | Proximity |
| In proximity | Individual is within three body lengths of another (recorded in seconds) | Proximity |
| Leave | Individual moves out of proximity (further than three body lengths away) of another | Proximity |
| Trunk towards | Extension of the trunk in the direction of another individual in proximity | Affiliative/associative |
| Trunk to mouth | Trunk makes contact with the mouth of another individual | Affiliative/associative |
| Trunk to gland | Trunk makes contact with the area between the eye and ear of another individual | Affiliative/associative |
| Trunk to eye | Trunk makes contact with the eye of another individual | Affiliative/associative |
| Trunk to trunk | The trunks of two individuals touch and/or intertwine | Affiliative/associative |
| Trunk to body | Trunk makes contact with any other area of the body not specified | Affiliative/associative |
| Head to head | Direct contact of two individuals’ heads, resting together for 2 s or more | Affiliative/associative |
| Body contact | Nonaggressive body contact of any kind not specified | Affiliative/associative |
| Play fighting | Two or more individuals pushing one another non-aggressively | Affiliative/associative |
| Back into | One individual walks backwards towards another and contact is made | Affiliative/associative |
| Follow | One individual leaves with another following within 5 s in the same direction | Affiliative/associative |
| Trunk to genitals | Trunk touches the area around the genitals/anus | Investigatory |
| Flehmen | Trunk touches the genital area, faeces, or urine of another, then touches individuals own mouth | Investigatory |
| Trunk slap | Aggressive swing of the trunk making contact with any area of the body on another individual | Dominance/aggression |
| Tusk stab/blow | Using the tusk to stab any area of another individual’s body with force | Dominance/aggression |
| Pushing | Using the head, shoulders or side, or backside to nudge/push another, forcing them to take a minimum of two steps | Dominance/aggression |
| Displacement | One individual approaches another, with the focal individual leaving within 5 s | Submission |
| Low posturing | Ears are held out 90 degrees/perpendicular to the head with their head bowed towards another for more than 2 s | Submission |
| Turn around | One individual approaches another, with the other turning 90 degrees away within 5 s | Submission |
| Back towards | One individual walks backwards towards another when in proximity | Submission |
| Other | Any other social behaviour performed between twoindividuals not described | Other |
| Out of sight | Observed individual is out of sight/in house | Not visible |
| Behaviour | Description | Function/Category |
|---|---|---|
| Stereotypic | Individual stereotypy (rocking, bobbing, swaying, air blowing, pacing), time in seconds | Stereotypy |
| Drinking | Ingestion of water | Maintenance |
| Feeding | Ingestion of feed | Feeding |
| Elimination | Urination/defecation | Maintenance |
| Dusting | With mud, sand, etc. | Maintenance |
| Bathing | Splashing with water/swimming/mud wallowing | Maintenance |
| Ear flap | Using repetitive ear movements to cool | Maintenance |
| Sleep/rest | Standing/lying/leaning, time in seconds | Maintenance |
| Social vocalisation | Vocalisation directed at another elephant as a greeting or for an affiliative purpose | Communication |
| Trumpet | Taking note of potential cause (other elephant, environmental stimulus) | Communication |
| Purr/rumble | Taking note of potential cause (other elephant, environmental stimulus) | Communication |
| Other vocalisation | Any other audible vocalisation, taking note of potential cause | Communication |
| Draining | Visible drainage/squirting secretion from temporal gland | Communication |
| Unusual | Any behaviour not classed as stereotypical that is rarely seen, with given description/duration | |
| Other | Any other behaviour thought to be worth noting, with given description/duration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armstrong, S.R.; Johnson, B. Behavioural Responses to Temporary Separation of a Captive Herd of African Elephants (Loxodonta africana). J. Zool. Bot. Gard. 2021, 2, 487-501. https://doi.org/10.3390/jzbg2030035
Armstrong SR, Johnson B. Behavioural Responses to Temporary Separation of a Captive Herd of African Elephants (Loxodonta africana). Journal of Zoological and Botanical Gardens. 2021; 2(3):487-501. https://doi.org/10.3390/jzbg2030035
Chicago/Turabian StyleArmstrong, Sarah R., and Bridget Johnson. 2021. "Behavioural Responses to Temporary Separation of a Captive Herd of African Elephants (Loxodonta africana)" Journal of Zoological and Botanical Gardens 2, no. 3: 487-501. https://doi.org/10.3390/jzbg2030035
APA StyleArmstrong, S. R., & Johnson, B. (2021). Behavioural Responses to Temporary Separation of a Captive Herd of African Elephants (Loxodonta africana). Journal of Zoological and Botanical Gardens, 2(3), 487-501. https://doi.org/10.3390/jzbg2030035







