Soot Particle Emissions: Formation and Suppression Mechanisms in Gas Turbines
Abstract
1. Introduction
2. Technical Reminders
2.1. Gas Turbine (GT) Technology Aspects [2]
2.2. Particulate Matter (PM) and Filterable Particles (FPs)
2.3. Metrological Aspects of Particle Emission Measurements
2.3.1. Particle Emissions on Natural Gas
2.3.2. Particle Emissions on Liquid Fuels
2.3.3. Overview of Particle Abatement Technologies
3. Summary of the Four Field Tests
3.1. Field Test Program
3.2. Gas Turbine Burning Natural Gas
3.3. Gas Turbines Burning Liquid Fuels
- -
- A No. 2 distillate oil or “No. 2 DO (i.e., a light, ashless fuel).
- -
- A heavy crude oil or “HCO” (a viscous but low-ash fuel).
- -
- A heavy fuel oil or “HFO” (a viscous, ash-forming fuel).
3.3.1. Gas Turbine Running on No. 2 Distillate Oil (“No. 2 DO”)
3.3.2. Gas Turbine Running on Heavy Crude Oil
3.3.3. Gas Turbine Running on Heavy Fuel Oil
A—Particular Observations: “Memory Effect”
- Event A—When starting the additive injection (Run 1), the opacity signal initially showed a rapid drop followed by a long decrease (shown by the dotted purple square in Figure 9) and then stabilized after about half an hour elapsed.
- Event B—Once the injection was stopped, opacity did not immediately return to its initial value but instead did so gradually (dotted blue square); this is not consistent with the fact that the residence time of the combustion gas inside the complete GT-HRSG path was only approximately 1 min.
- Event C—During the second injection run, “Event A” was no longer observed, which meant that it was not due to the slow response time of the opacimeter. However, “Event B” happened again.
B—Measurement Results
4. Discussion
4.1. Bibliographic Data
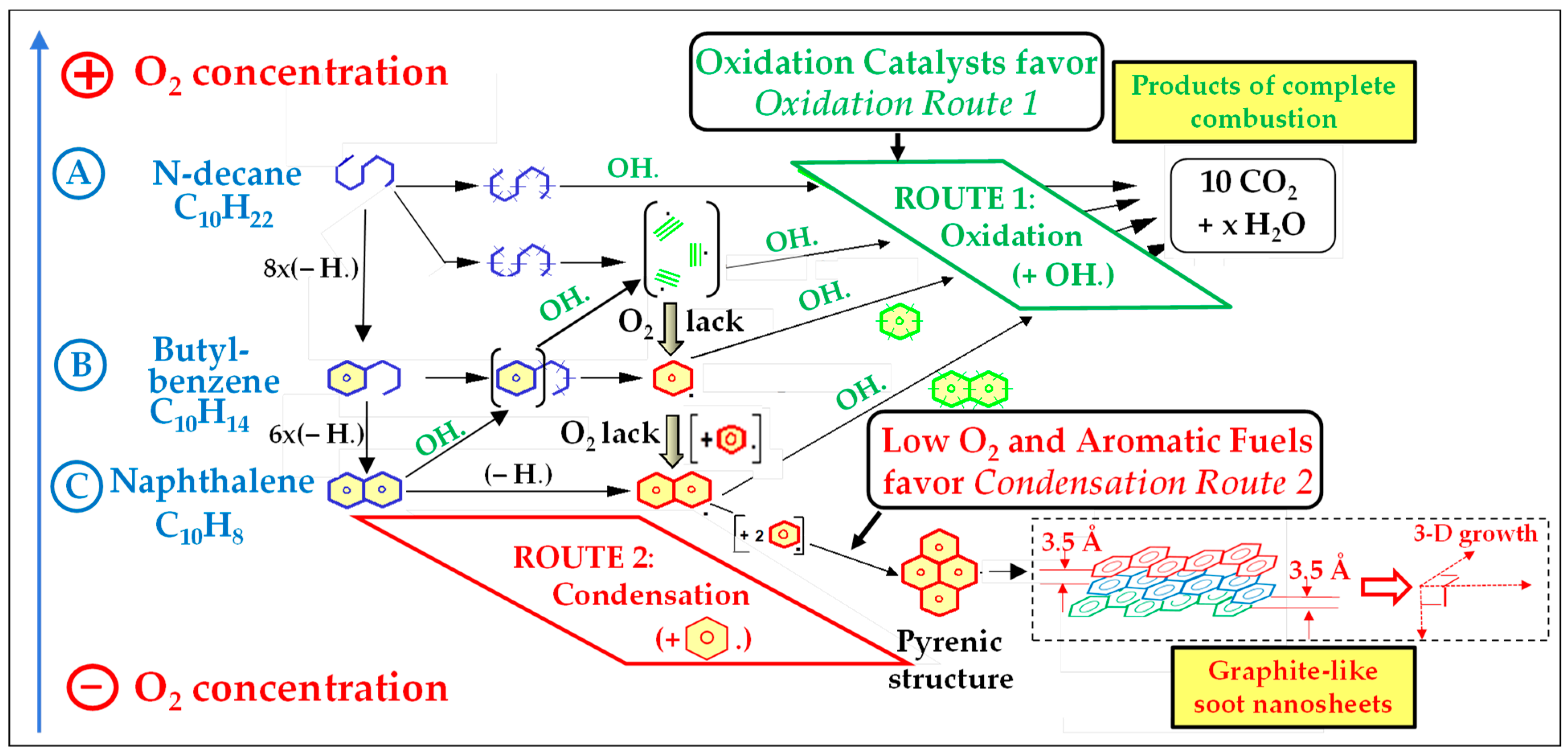
4.2. Processes Underlying Soot Formation
Individual PAH Molecules Are Also Emitted
4.3. Mechanisms of Soot Suppression Using Fuel Additives
4.3.1. Summary of the Three Field Tests
- The ranking of additives in terms of effectiveness is as follows: Ce(III) > Ce(IV) >> Fe(III).
- The ranking of fuels in terms of “ease” of soot reduction is as follows: No. 2 distillate oil >> heavy crude oil (HCO) > heavy fuel oil (HFO).
4.3.2. Methodology Used to Interpret Results
4.3.3. Soot Suppression by Iron
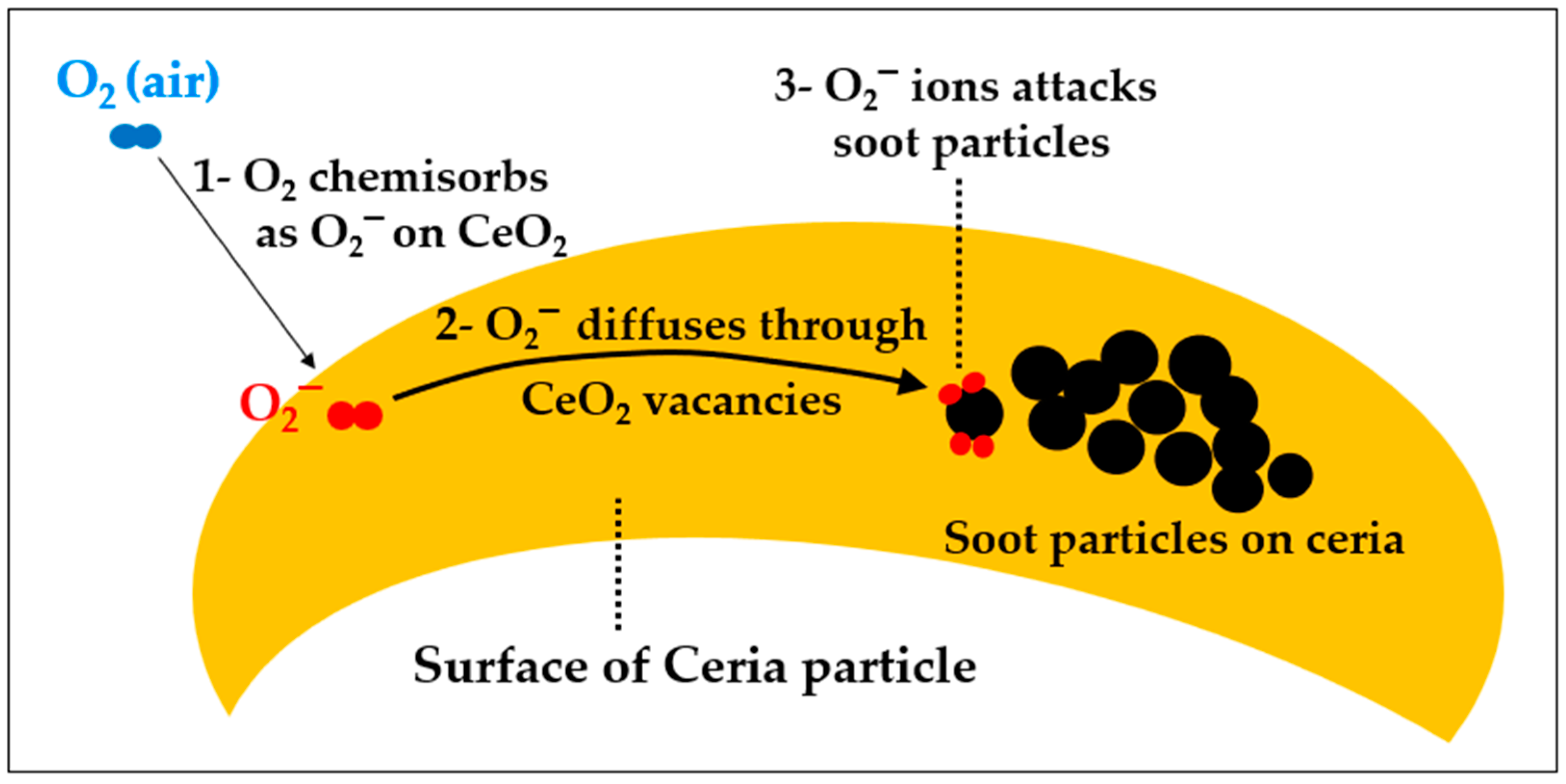
4.3.4. Soot Suppression by Cerium
A—Catalytic Effect of Cerium at High Temperature
B—Catalytic Effect of Cerium at Medium Temperature
4.3.5. Interpretation of the Difference in Effectiveness Between Cerium(III) and Cerium(IV)
4.3.6. Difference in Additive Dosages Between Jet Engines and Stationary Turbines
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Disclaimer
Appendix A. Exponential Decrease in Soot Concentration Versus Cerium Dosage
References
- Best Available Techniques (BAT). Applicable to Industrial Emissions. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1503383091262&uri=CELEX:32017D1442 (accessed on 10 July 2024).
- Shiozaki, S.; Fujii, T.; Takenaga, K.; Ozawa, M.; Yamada, A. Advances, in Power Boilers. In JSME Series in Thermal and Nuclear Power Generation, 1st ed.; Chapter 6—Gas Turbine Combined Cycle; Koizumi, Y., Okawa, T., Mori, S., Eds.; Elsevier: New York, NY, USA, 2021; Volume 2, pp. 305–344. [Google Scholar]
- Sarkar, D.K. (Ed.) Chapter 7—Gas Turbine and Heat Recovery Steam Generator. In Thermal Power Plant, Design and Operation; Elsevier: New York, NY, USA, 2015; pp. 239–283. [Google Scholar]
- Boyce, M.P. Combustors, Chapter 10. In Gas Turbine Engineering Handbook, 4th ed.; Elsevier: New York, NY, USA, 2012; pp. 427–490. [Google Scholar]
- Goldmeer, D.J. Fuel Flexible Gas Turbines as Enablers for a Low or Reduced Carbon Energy Ecosystem, GEA33861, Electrify Europe Whitepaper, Electrify Europe 2018 Congress, Vienna, Austria. 2018. Available online: https://www.gevernova.com/content/dam/gepower/global/en_US/documents/fuel-flexibility/GEA33861%20-%20Fuel%20Flexible%20Gas%20Turbines%20as%20Enablers%20for%20a%20Low%20Carbon%20Energy%20Ecosystem.pdf (accessed on 10 July 2024).
- Molière, M. The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook. Energies 2023, 16, 3962. [Google Scholar] [CrossRef]
- Gülen, S.C. Gas Turbines for Electric Power Generation; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Particulate Matter (PM) Basics. USEPA Publication. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 10 July 2024).
- What Is Particle Pollution? USEPA Publication. Available online: https://www.epa.gov/pmcourse/what-particle-pollution (accessed on 10 July 2024).
- Cernuschi, S.; Giugliano, M.; Consonni, S. Emissions of Fine and Ultrafine Particles from Stationary Combustion Plants, Final Summary; Published 26 October 2010. Available online: https://www.cewep.eu/wp-content/uploads/2017/10/905_emissioni_di_polveri_fini_e_ultrafini_english_2012-04-16.pdf (accessed on 10 July 2024).
- Bessagnet, B.; Allemand, N.; Putaud, J.-P.; Couvidat, F.; André, J.-M.; Simpson, D.; Pisoni, E.; Murphy, B.N.; Thunis, P. Emissions of Carbonaceous Particulate Matter and Ultrafine Particles from Vehicles—A Scientific Review in a Cross-Cutting Context of Air Pollution and Climate Change. Appl. Sci. 2022, 12, 3623. [Google Scholar] [CrossRef] [PubMed]
- EU Air Quality Standards. EU Publication. Available online: https://environment.ec.europa.eu/topics/air/air-quality/eu-air-quality-standards_en (accessed on 10 July 2024).
- Yang, H.H.; Lee, K.T.; Hsieh, Y.S.; Luo, S.W.; Li, M.S. Filterable and Condensable Fine Particulate Emissions from Stationary Sources. Aerosol Air Qual. Res. 2014, 14, 2010–2016. [Google Scholar] [CrossRef]
- Cipriano, M.; Couchot, M.; Poulleau, J.; Jund, L.; Moliere, M. Joint investigation of particle emission at the exhaust of a gas turbine. In Proceedings of the CEM Conference, Zurich, Switzerland, 5–7 October 2007. [Google Scholar]
- Cipriano, D.; Vierling, M.; Moliere, M.; Poulleau, J.; Le Louër, P.; Jund, L.; Fiani, E. Balance of micropollutants emitted by gas fired combustion turbines. In Proceedings of the CEM Conference, Stresa, Italy, 5–7 May 2009. [Google Scholar]
- Cipriano, D.; Vierling, M.; Moliere, M.; Poulleau, J.; Le Louër, P.; Jund, L.; Fiani, E. Emission of micropollutants from industrial combustion plants: Effect of fuel type, plant configuration and site typology. In Proceedings of the CEM Conference, Prague, Czech Republic, 5–7 October 2011. [Google Scholar]
- Method 5—Particulate Matter (PM). USEPA Publication. Available online: https://www.epa.gov/emc/method-5-particulate-matter-pm (accessed on 10 July 2024).
- EN 13284-1:2017; Stationary Source Emissions—Determination of Low Range Mass Concentration of Dust—Part 1: Manual Gravimetric Method. European Standardization Organization: Brussels, Belgium, 2017. Available online: https://standards.iteh.ai/catalog/standards/cen/39eb0f35-645e-460b-993c-6ff2a69af16c/en-13284-1-2017 (accessed on 10 July 2024).
- Veranth, J.M.; Fletcher, T.H.; Pershing, D.W.; Sarofim, A.F. Measurement of soot and char in pulverized coal fly ash. Fuel 2000, 79, 1067–1075. [Google Scholar] [CrossRef]
- Appleyard, D. Power Plant Filtration Technology to Achieve Environmental Performance. Available online: https://www.powerengineeringint.com/coal-fired/filtering-out-power-plant-emissions (accessed on 10 July 2024).
- Zhao, X.; Tan, H.; Yang, F.; Deng, S. Field Studies on the Removal Characteristics of Particulate Matter and SOx in Ultra-Low Emission Coal-Fired Power Plant. Energy Eng. 2022, 119, 49–62. [Google Scholar] [CrossRef]
- Miyamoto, N.; Hou, Z.; Harada, A.; Ogawa, H.; Murayama, T. Characteristics of Diesel Soot Suppression with Soluble Fuel Additives. SAE Trans. Reciprocation Engines Spark Ignition Diesel 1987, 96, 792–798. [Google Scholar]
- Banús, E.D.; Ulla, M.A.; Miró, E.E.; Milt, V.G. Chapter 5: Structured Catalysts for Soot Combustion for Diesel Engines. In Diesel Engine—Combustion, Emissions and Condition Monitoring; Bari, S., Ed.; Intechopen: London, UK, 2013; Available online: https://www.intechopen.com/books/2432 (accessed on 10 July 2024).
- Meloni, E.; Palma, V. Most Recent Advances in Diesel Engine Catalytic Soot Abatement: Structured Catalysts and Alternative Approaches. Catalysts 2020, 10, 745. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Boehman, A.L. The role of fuel-borne catalyst in diesel particulate oxidation behavior. Combust. Flame 2006, 146, 73–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z.; Fang, L. Effect of catalyzed diesel particulate filter and its catalyst loading on emission characteristics of a non-road diesel engine. J. Environ. Sci. 2023, 126, 794–805. [Google Scholar] [CrossRef]
- Liu, J.; Wu, P.; Sun, P.; Ji, Q.; Zhang, Q.; Wang, P. Effects of iron-based fuel borne catalyst addition on combustion, in-cylinder soot distribution and exhaust emission characteristics in a common-rail diesel engine. Fuel 2021, 290, 120096. [Google Scholar] [CrossRef]
- Smooke, M.D.; Long, M.B.; Connelly, B.C.; Colket, M.B.; Hall, R.J. Soot formation in laminar diffusion flames. Combust. Flame 2005, 143, 613–628. [Google Scholar] [CrossRef]
- Saito, K.; Gordon, A.S.; Williams, F.A.; Stickle, W.F. A Study of the Early History of Soot Formation in Various Hydrocarbon Diffusion Flames. Combust. Sci. Technol. 1991, 80, 103–119. [Google Scholar] [CrossRef]
- Wagner, H.G. Soot Formation in Combustion. Int. Symp. Combust. 1979, 17, 3–19. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Brilhac, J.F.; Gilot, P. The oxidation of soot: A review of experiments, mechanisms and models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Xi, J.; Yang, G.; Cai, J.; Gu, Z. A Review of Recent Research Results on Soot: The Formation of a Kind of Carbon-Based Material in Flames. Front. Mater. 2021, 8, 695485. [Google Scholar] [CrossRef]
- Michelsen, H.A.; Colket, M.B.; Bengtsson, P.E.; D’Anna, A.; Desgroux, P.; Haynes, B.S.; Miller, J.H.; Nathan, G.J.; Pitsch, H.; Wang, H. A Review of Terminology Used to Describe Soot Formation and Evolution under Combustion and Pyrolytic Conditions. ACS Nano 2020, 14, 12470–12490. [Google Scholar] [CrossRef]
- Frenklach, M.; Mebel, A.M. On the mechanism of soot nucleation. Phys. Chem. Chem. Phys. 2020, 22, 5314–5331. [Google Scholar] [CrossRef]
- Park, S.H. Simulation of the Formation and Growth of Soot Aerosol Particles in a Premixed Combustion Process Using a Soot Aerosol Dynamics Model. Atmosphere 2022, 13, 847. [Google Scholar] [CrossRef]
- Curinao, J.; Cepeda, F.; Escudero, F.; Dworkin, S.B.; Demarco, R. Understanding soot formation: A comprehensive analysis using reactive models in Inverse Non-Premixed Flames. Combust. Flame 2024, 267, 113569. [Google Scholar] [CrossRef]
- Kim, D.C.; Song, K.C.; Kaushik, R.D. Fuel Additives for Particulate Matter/Dust Reduction. Asian J. Chem. 2008, 20, 5797–5817. [Google Scholar]
- Howard, J.B.; Kausch, W.J. Soot control by fuel additives. Prog. Energy Combust. Sci. 1980, 6, 263–276. [Google Scholar] [CrossRef]
- Bramer, R. An Investigation of the Effectiveness of Smoke Suppressant Fuel Additives for Turbojet Engines. Ph.D. Thesis, Naval Postgraduate School, Monterey, CA, USA, October 1982. [Google Scholar]
- Krugs, A.C. An Experimental Investigation of Soot Behavior in a Gas Turbine Combustor. Ph.D. Thesis, Naval postgraduate School of Monterey, Monterey, CA, USA, June 1983. [Google Scholar]
- Geiger, F.; Molière, M. Gas Turbines in Alternative Fuel Applications: The Utilization of Highly Aromatic Fuels in Power Generation. In Proceedings of the ASME Turbo Expo Conference, Paper GT2007-53272. Vienna, Austria, 14–17 June 2004. [Google Scholar]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds, 2nd ed.; Willey-VCH: Hannover, Germany, 2002. [Google Scholar]
- Sata, T.; Yoshimura, M.J. Some Material Properties of Cerium Sesquioxide. Ceram. Assoc. Jpn. 1968, 76, 116–122. [Google Scholar] [CrossRef]
- Perrichon, V.; Laachir, A.; Abouarnadasse, S.; Touret, O.; Blanchard, C. Thermal stability of a high surface area ceria under reducing atmosphere. Appl. Catal. A Gen. 1995, 129, 69–82. [Google Scholar] [CrossRef]
- Muroyama, H.; Asajima, H.; Hano, S.; Matsui, T.; Eguchi, K. Effect of an additive in a CeO2-based oxide on catalytic soot combustion. Appl. Catal. A Gen. 2015, 489, 235–240. [Google Scholar] [CrossRef]
- Retailleau, L.; Vonarb, R.; Perrichon, V.; Jean, E.; Bianchi, D. Catalytic Oxidation of a Diesel Soot Formed in the Presence of a Cerium Additive. I. Characterization of the Cerium Fraction Using Magnetic Susceptibility and Temperature-Programmed Desorption. Energy Fuels 2004, 18, 872–882. [Google Scholar] [CrossRef]
- Poston, J.A.; Siriwardane, B.V.; Fisher, E.P.; Miltz, A.L. Thermal decomposition of the rare earth sulfates of cerium(III), cerium(IV), lanthanum(III) and samarium(III). Appl. Surf. Sci. 2003, 214, 83–102. [Google Scholar] [CrossRef]
- Tagawa, H.H. Thermal decomposition temperatures of metal sulfates. Thermochim. Acta 1984, 80, 23–33. [Google Scholar] [CrossRef]
- Gross, M.S.; Ulla, M.A.; Querini, C.A. Diesel particulate matter combustion with CeO2 as catalyst. Part I: System characterization and reaction mechanism. J. Mol. Catal. A Chem. 2012, 352, 86–94. [Google Scholar] [CrossRef]
- Krishna, K.; Bueno-López, A.; Makkee, M.; Moulijn, J.A. Potential rare earth modified CeO2 catalysts for soot oxidation: I. Characterisation and catalytic activity with O2. J. Catal. 2005, 230, 237–248. [Google Scholar] [CrossRef]
- van Setten, B.A.; Makkee, M.; Moulijn, J.A. Science and Technology of Catalytic Diesel Particulate Filter. Catal. Rev. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Kim, H.D.; Wi, S.I.; Stalder, J.P. Daesan Combined Cycle Power Plant: Successful Operating Experience on Low Sulphur Waxy Residual Fuel Oil. In Proceedings of the ASME Turbo Expo Conference, Paper 2001-GT-0074. New Orleans, LA, USA, 4–7 June 2001. [Google Scholar]
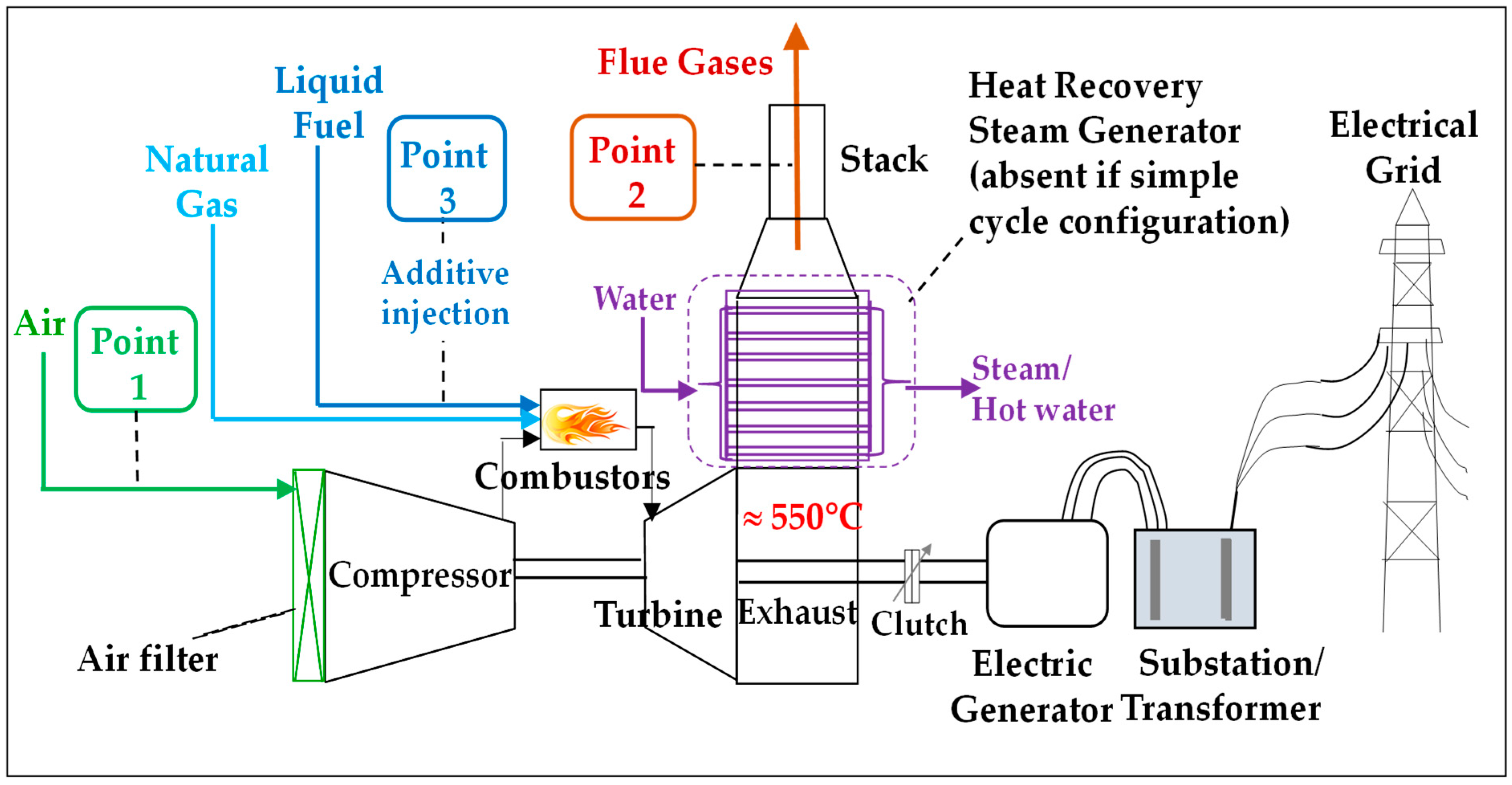
















| Site Typology: Urban, Industrialized Area | Filterable Particles (FPs) Averages of Several Measurements | |
|---|---|---|
| Point 1: Ambient air | Weekly average Daily average | 107 µg/Nm3 150 µg/Nm3 |
| Point 2: GT exhaust | 12 h per measurement | 23 to 48 µg/Nm3 |
| Fuel Type/Additive | For Cerium(III) | For Cerium(IV) | For Iron(III) |
|---|---|---|---|
| No. 2 distillate oil | FP0 = 4; k = −0.171 | FP0 = 4; k = −0.102 | FP0 = 4; k = −0.014 |
| Heavy crude oil | FP0 = 147; k = −0.037 | FP0 = 147; k = −0.031 | FP0 = 147; k = −0.015 |
| Heavy fuel oil | FP0 = 42; k = −0.036 | FP0 = 42; k = −0.020 | FP0 = 42; k = −0.007 |
| (1) | 4 Fe3O4 + O2 → 6Fe2O3 | Oxidation of Fe(II,III) oxide to Fe(III) oxide |
| (2) | 3 Fe2O3 + C → 2 Fe3O4 + CO | Reduction of Fe(III) oxide to Fe(II,III) oxide by soot particles |
| (3) | 2 CO + O2 → 2 CO2 | Oxidation of carbon monoxide downstream of flame front |
| (4) | 2 Ce2O3 + O2 → 4 CeO2 | Oxidation of Ce(III) oxide to Ce(IV) oxide (ceria) |
| (5) | 2 CeO2 + C → Ce2O3 + CO | Reduction of Ce(IV) oxide to Ce(III) oxide by soot particles |
| (3) | 2 CO + O2 → 2 CO2 | Oxidation of carbon monoxide downstream the flame front |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vierling, M.; Aboujaib, M.; Denolle, R.; Brilhac, J.-F.; Molière, M. Soot Particle Emissions: Formation and Suppression Mechanisms in Gas Turbines. Gases 2024, 4, 446-467. https://doi.org/10.3390/gases4040023
Vierling M, Aboujaib M, Denolle R, Brilhac J-F, Molière M. Soot Particle Emissions: Formation and Suppression Mechanisms in Gas Turbines. Gases. 2024; 4(4):446-467. https://doi.org/10.3390/gases4040023
Chicago/Turabian StyleVierling, Matthieu, Maher Aboujaib, Richard Denolle, Jean-François Brilhac, and Michel Molière. 2024. "Soot Particle Emissions: Formation and Suppression Mechanisms in Gas Turbines" Gases 4, no. 4: 446-467. https://doi.org/10.3390/gases4040023
APA StyleVierling, M., Aboujaib, M., Denolle, R., Brilhac, J.-F., & Molière, M. (2024). Soot Particle Emissions: Formation and Suppression Mechanisms in Gas Turbines. Gases, 4(4), 446-467. https://doi.org/10.3390/gases4040023





