A Review of Algae-Based Carbon Capture, Utilization, and Storage (Algae-Based CCUS)
Abstract
1. Introduction
2. Data Collection and Methodology
3. Overview of Algae
3.1. Classification of Algae
3.2. Advantages of Algae in CCUS
4. Algae-Based Carbon Capture
4.1. Factors Influencing Algae-Based Carbon Capture
4.2. Advancements in Algae-Based Carbon Capture
4.2.1. Enhancement of Algal Traits
4.2.2. Advances in Algal Cultivation Techniques
4.2.3. Improvement in Algal Cultivation Systems
4.2.4. Refinement of Carbon Sources
5. Algae-Based Carbon Utilization
5.1. Solid Algae-Based Biofuels
5.1.1. Algae-Based Solid Biomass Pellets
5.1.2. Algae-Based Biocoal
5.2. Liquid Algae-Based Biofuels
5.2.1. Algae-Based Biodiesel
5.2.2. Algae-Based Bioethanol
5.2.3. Integrated Production of Algae-Based Biodiesel and Bioethanol
5.3. Gaseous Algae-Based Biofuels
5.3.1. Algae-Based Biohydrogen
5.3.2. Algae-Based Biogas
5.3.3. Algae-Based Syngas
6. Algae-Based Carbon Storage
6.1. MICP and Photosynthetic MICP for Carbon Storage
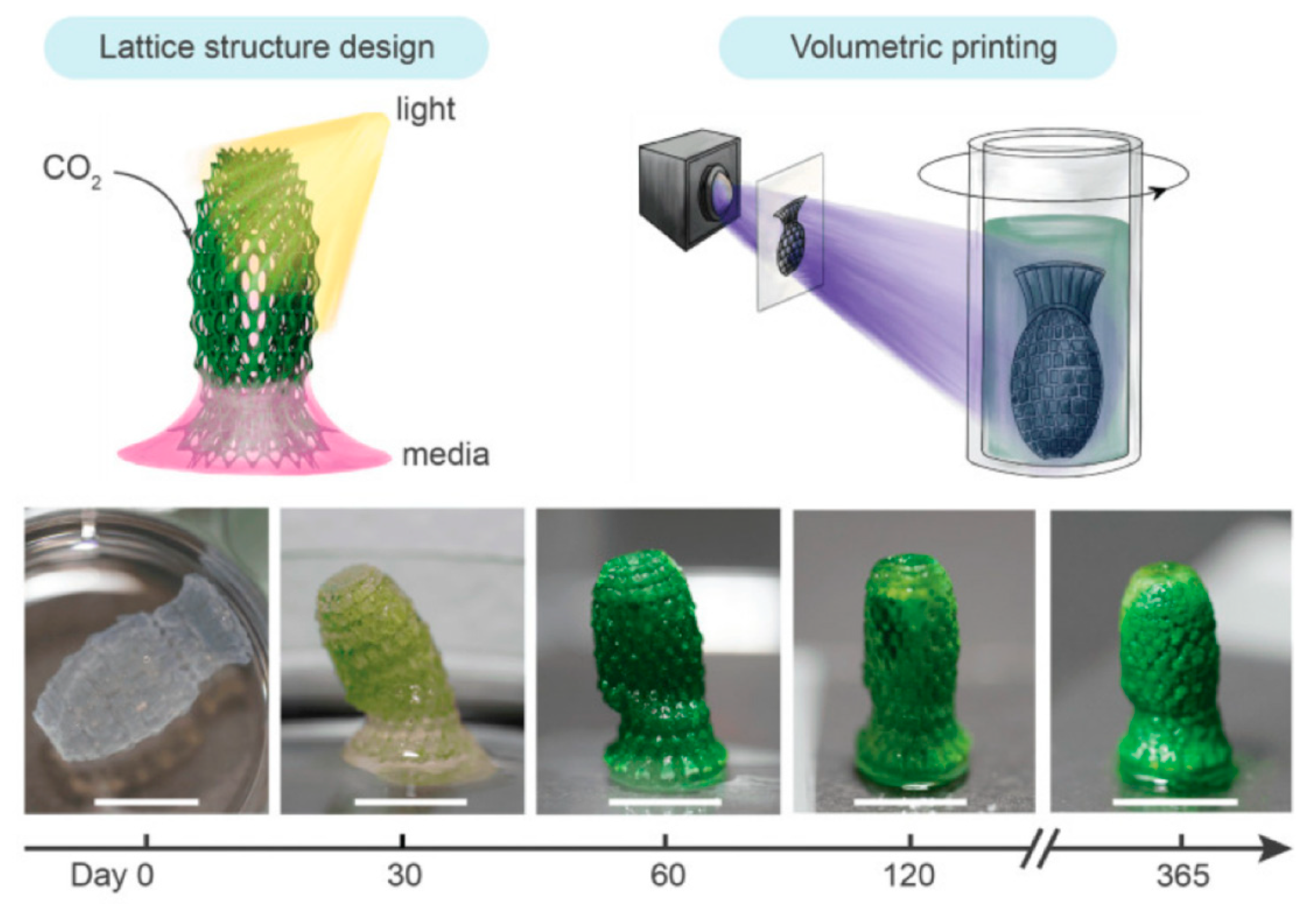
6.2. Dual Carbon Storage Using 3D-Printed Structures Embedded with Living Algae
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. In Advances in Carbon Capture; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- Hickmann, T.; Widerberg, O.; Lederer, M.; Pattberg, P. The United Nations Framework Convention on Climate Change Secretariat as an Orchestrator in Global Climate Policymaking. Int. Rev. Adm. Sci. 2021, 87, 21–38. [Google Scholar] [CrossRef]
- Falkner, R. The Paris Agreement and the New Logic of International Climate Politics. Int. Aff. 2016, 92, 1107–1125. [Google Scholar] [CrossRef]
- Schleussner, C.-F.; Rogelj, J.; Schaeffer, M.; Lissner, T.; Licker, R.; Fischer, E.M.; Knutti, R.; Levermann, A.; Frieler, K.; Hare, W. Science and Policy Characteristics of the Paris Agreement Temperature Goal. Nat. Clim. Change 2016, 6, 827–835. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from Solar Energy, a Clean Energy Carrier from a Sustainable Source of Energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Li, G.; Yao, J. A Review of In Situ Leaching (ISL) for Uranium Mining. Mining 2024, 4, 120–148. [Google Scholar] [CrossRef]
- Li, G.; Yao, J.; Song, Y.; Tang, J.; Han, H.; Cui, X. A Review of the Metallogenic Mechanisms of Sandstone-Type Uranium Deposits in Hydrocarbon-Bearing Basins in China. Eng 2023, 4, 1723–1741. [Google Scholar] [CrossRef]
- Khan, J.; Arsalan, M.H. Solar Power Technologies for Sustainable Electricity Generation—A Review. Renew. Sustain. Energy Rev. 2016, 55, 414–425. [Google Scholar] [CrossRef]
- Baharoon, D.A.; Rahman, H.A.; Omar, W.Z.W.; Fadhl, S.O. Historical Development of Concentrating Solar Power Technologies to Generate Clean Electricity Efficiently—A Review. Renew. Sustain. Energy Rev. 2015, 41, 996–1027. [Google Scholar] [CrossRef]
- Yao, J.; Li, G.; Wu, J. Application of In-Situ Combustion for Heavy Oil Production in China: A Review. J. Oil Gas Petrochem. Sci. 2018, 1, 69–72. [Google Scholar] [CrossRef]
- Yao, J.; Song, Y. Dynamic Analysis Approach to Evaluate In-Situ Combustion Performance for Heavy Oil Production. J. Oil Gas Petrochem. Sci. 2019, 2, 42–47. [Google Scholar] [CrossRef]
- Bai, X.; Yan, G.; Kong, S.; Yao, J.; Wen, P.; Li, G.; Li, J.; Zhang, J. Suppression of Anthracite Dust by a Composite of Oppositely-Charged Ionic Surfactants with Ultra-High Surface Activity: Theoretical Calculation and Experiments. Fuel 2023, 344, 128075. [Google Scholar] [CrossRef]
- Bai, X.; Yan, G.; Kong, S.; Yang, T.; Yao, J.; Wen, P.; Li, G. Study on the Mechanism of the Influence of Surfactant Alkyl Chain Length on the Wettability of Anthracite Dust Based on EDLVO Theory and Inverse Gas Chromatography. Fuel 2023, 353, 129187. [Google Scholar] [CrossRef]
- Menyah, K.; Wolde-Rufael, Y. CO2 Emissions, Nuclear Energy, Renewable Energy and Economic Growth in the US. Energy Policy 2010, 38, 2911–2915. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Liu, Y.; Rui, Z. A Storage-Driven CO2 EOR for a Net-Zero Emission Target. Engineering 2022, 18, 79–87. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.A.; Okuno, R. Measurements and Modeling of Interfacial Tension for CO2/CH4/Brine Systems under Reservoir Conditions. Ind. Eng. Chem. Res. 2016, 55, 12358–12375. [Google Scholar] [CrossRef]
- Kong, S.; Feng, G.; Liu, Y.; Li, K. Potential of Dimethyl Ether as an Additive in CO2 for Shale Oil Recovery. Fuel 2021, 296, 120643. [Google Scholar] [CrossRef]
- Liu, Y.; Rui, Z.; Yang, T.; Dindoruk, B. Using Propanol as an Additive to CO2 for Improving CO2 Utilization and Storage in Oil Reservoirs. Appl. Energy 2022, 311, 118640. [Google Scholar] [CrossRef]
- Gilmour, J. 45Q: Toward a Stronger Federal Carbon Capture Tax Credit. Environ. Claims J. 2023, 35, 235–253. [Google Scholar] [CrossRef]
- Ren, B.; Male, F.; Duncan, I.J. Economic Analysis of CCUS: Accelerated Development for CO2 EOR and Storage in Residual Oil Zones under the Context of 45Q Tax Credit. Appl. Energy 2022, 321, 119393. [Google Scholar] [CrossRef]
- IEA Energy Act of 2020. Available online: https://www.iea.org/policies/13192-energy-act-of-2020-ccus-provisions (accessed on 9 March 2022).
- IEA Infrastructure and Jobs Act: Carbon Capture, Utilization and Storage Investment. Available online: https://www.iea.org/policies/14982-infrastructure-and-jobs-act-carbon-capture-utilization-and-storage-investment (accessed on 24 May 2023).
- IEA The Utilizing Significant Emissions with Innovative Technologies (USE IT) Act. Available online: https://www.iea.org/policies/11669-the-utilizing-significant-emissions-with-innovative-technologies-use-it-act (accessed on 21 September 2024).
- Prajapati, M.; Thesia, D.; Thesia, V.; Rakholia, R.; Tailor, J.; Patel, A.; Pardiwala, J.M.; Shah, M. Carbon Capture, Utilization, and Storage (CCUS): A Critical Review towards Carbon Neutrality in India. Case Stud. Chem. Environ. Eng. 2024, 10, 100770. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sharma, M. Prospect of Intended Nationally Determined Contribution Target Achievement by Indian Power Sector. Clean. Technol. Environ. Policy 2017, 19, 1679–1692. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Malyan, A. Implications of a Net-Zero Target for India’s Sectoral Energy Transitions and Climate Policy. Oxf. Open Clim. Change 2022, 2, kgac001. [Google Scholar] [CrossRef]
- Singh, U.; Vishal, V.; Garg, A. CCUS in India: Bridging the Gap between Action and Ambition. Prog. Energy 2024, 6, 023004. [Google Scholar] [CrossRef]
- Patidar, A.K.; Singh, R.K.; Choudhury, T. The Prominence of Carbon Capture, Utilization and Storage Technique, a Special Consideration on India. Gas Sci. Eng. 2023, 115, 204999. [Google Scholar] [CrossRef]
- Gupta, N.C.; Tanwar, R.; Dipesh; Kaushik, A.; Singh, R.; Patra, A.K.; Sar, P.; Khakharia, P. Perspectives on CCUS Deployment on Large Scale in India: Insights for Low Carbon Pathways. Carbon Capture Sci. Technol. 2024, 12, 100195. [Google Scholar] [CrossRef]
- IEA Going Carbon Negative: What Are the Technology Options? Available online: https://www.iea.org/commentaries/going-carbon-negative-what-are-the-technology-options (accessed on 30 January 2020).
- Li, G.; Yao, J. Direct Air Capture (DAC) for Achieving Net-Zero CO2 Emissions: Advances, Applications, and Challenges. Eng 2024, 5, 1298–1336. [Google Scholar] [CrossRef]
- Lackner, K.S.; Azarabadi, H. Buying down the Cost of Direct Air Capture. Ind. Eng. Chem. Res. 2021, 60, 8196–8208. [Google Scholar] [CrossRef]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and Feed Products from Micro-Algae: Market Opportunities and Challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the Potential of Algal Biomass Opportunities for Bioenergy Industry: A Review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, Properties and Challenges of Algae Biomass for Biofuel Application: An Overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Ranjith Kumar, R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Praveen Kumar, R.; Palani, S. Aquatic Biomass (Algae) as a Future Feed Stock for Bio-Refineries: A Review on Cultivation, Processing and Products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Chen-Glasser, M.; McMillan, J.D. A Perspective on Renewable Bioenergy from Photosynthetic Algae as Feedstock for Biofuels and Bioproducts. Algal Res. 2017, 24, 261–264. [Google Scholar] [CrossRef]
- Srivastava, S.; Omar, P.J.; Shekhar, S.; Gupta, S. Study of Acidic Air Pollutant (SO2 and NO2) Tolerance of Microalgae with Sodium Bicarbonate as Growth Stimulant. AQUA—Water Infrastruct. Ecosyst. Soc. 2023, 72, 739–749. [Google Scholar] [CrossRef]
- Qie, F.; Zhu, J.; Rong, J.; Zong, B. Biological Removal of Nitrogen Oxides by Microalgae, a Promising Strategy from Nitrogen Oxides to Protein Production. Bioresour. Technol. 2019, 292, 122037. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Farghl, A.A.; Galal, H.R.; Bayoumi, H.S. Bioremediation of Different Types of Polluted Water Using Microalgae. Rend. Lincei 2016, 27, 401–410. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R. Effect of Algal Cells on Water Pollution Control. Curr. Pollut. Rep. 2021, 7, 213–226. [Google Scholar] [CrossRef]
- Chen, Z.; Osman, A.I.; Rooney, D.W.; Oh, W.-D.; Yap, P.-S. Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives. Sustainability 2023, 15, 5128. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the Recent Structural Advances in Open and Closed Systems for Carbon Capture through Algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, T.; Cui, X.; Peng, M.; Wang, X.; Mao, H.; Cui, M. New Insights into the Carbon Neutrality of Microalgae from Culture to Utilization: A Critical Review on the Algae-Based Solid Biofuels. Biomass Bioenergy 2022, 166, 106599. [Google Scholar] [CrossRef]
- Singh, A.; Bin Abu Sofian, A.D.A.; Chan, Y.J.; Chakrabarty, A.; Selvarajoo, A.; Abakr, Y.A.; Show, P.L. Hydrothermal Carbonization: Sustainable Pathways for Waste-to-energy Conversion and Biocoal Production. GCB Bioenergy 2024, 16, e13150. [Google Scholar] [CrossRef]
- Bibi, R.; Ahmad, Z.; Imran, M.; Hussain, S.; Ditta, A.; Mahmood, S.; Khalid, A. Algal Bioethanol Production Technology: A Trend towards Sustainable Development. Renew. Sustain. Energy Rev. 2017, 71, 976–985. [Google Scholar] [CrossRef]
- Pathy, A.; Nageshwari, K.; Ramaraj, R.; Pragas Maniam, G.; Govindan, N.; Balasubramanian, P. Biohydrogen Production Using Algae: Potentiality, Economics and Challenges. Bioresour. Technol. 2022, 360, 127514. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of Art Review on Conventional and Advanced Pyrolysis of Macroalgae and Microalgae for Biochar, Bio-Oil and Bio-Syngas Production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Nematian, T.; Barati, M. Nanobiocatalytic Processes for Producing Biodiesel from Algae. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–326. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, A.; Agrawal, V.; Yadav, N. Algal Biomass. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Wiley: Hoboken, NJ, USA, 2021; pp. 303–334. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N. Industrial and Biotechnological Applications of Algae: A Review. J. Adv. Plant Biol. 2017, 1, 1–25. [Google Scholar] [CrossRef]
- Özçimen, D.; Koçer, A.T.; İnan, B.; Özer, T. Bioethanol Production from Microalgae. In Handbook of Microalgae-Based Processes and Products; Academic Press: Cambridge, MA, USA, 2020; pp. 373–389. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as Promising Feedstocks for Fermentative Biohydrogen Production According to a Biorefinery Approach: A Comprehensive Review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Saifuddin, N.; Priatharsini, P. Developments in Bio-Hydrogen Production from Algae: A Review. Res. J. Appl. Sci. Eng. Technol. 2016, 12, 968–982. [Google Scholar] [CrossRef]
- Spalding, H.L.; Amado-Filho, G.M.; Bahia, R.G.; Ballantine, D.L.; Fredericq, S.; Leichter, J.J.; Nelson, W.A.; Slattery, M.; Tsuda, R.T. Macroalgae. In Mesphotic Coral Ecosystems; Springer: Cham, Switzerland, 2019; pp. 507–536. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Jarvis, J.; Trevathan-Tackett, S.M.; Bellgrove, A. Seagrasses and Macroalgae: Importance, Vulnerability and Impacts. In Climate Change Impacts on Fisheries and Aquaculture; Wiley: Hoboken, NJ, USA, 2017; pp. 729–770. [Google Scholar] [CrossRef]
- Zafar, A.M.; Javed, M.A.; Aly Hassan, A.; Mehmood, K.; Sahle-Demessie, E. Recent Updates on Ions and Nutrients Uptake by Halotolerant Freshwater and Marine Microalgae in Conditions of High Salinity. J. Water Process Eng. 2021, 44, 102382. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating Microalgae in Wastewater for Biomass Production, Pollutant Removal, and Atmospheric Carbon Mitigation; a Review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Nojima, D.; Yue, L.; Kanehara, H.; Naruse, H.; Ujiro, A.; Yoshino, T.; Matsunaga, T.; Tanaka, T. Potential of Water Surface-Floating Microalgae for Biodiesel Production: Floating-Biomass and Lipid Productivities. J. Biosci. Bioeng. 2017, 123, 314–318. [Google Scholar] [CrossRef]
- Nojima, D.; Ishizuka, Y.; Muto, M.; Ujiro, A.; Kodama, F.; Yoshino, T.; Maeda, Y.; Matsunaga, T.; Tanaka, T. Enhancement of Biomass and Lipid Productivities of Water Surface-Floating Microalgae by Chemical Mutagenesis. Mar. Drugs 2017, 15, 151. [Google Scholar] [CrossRef]
- Najiha Badar, S.; Mohammad, M.; Emdadi, Z.; Yaakob, Z. Algae and Their Growth Requirements for Bioenergy: A Review. Biofuels 2021, 12, 307–325. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Li, M.; Hu, C. Algal Biomass Valorisation to High-Value Chemicals and Bioproducts: Recent Advances, Opportunities and Challenges. Bioresour. Technol. 2022, 344, 126371. [Google Scholar] [CrossRef]
- Mehta, P.; Singh, D.; Saxena, R.; Rani, R.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. High-Value Coproducts from Algae—An Innovational Way to Deal with Advance Algal Industry. In Waste to Wealth; Springer: Singapore, 2018; pp. 343–363. [Google Scholar] [CrossRef]
- Verawaty, M.; Melwita, E.; Apsari, P.; Wiyahsari, M. Cultivation Strategy for Freshwater Macro- and Micro-Algae as Biomass Stock for Lipid Production. J. Eng. Technol. Sci. 2017, 49, 261–274. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient Removal and Biogas Upgrading by Integrating Freshwater Algae Cultivation with Piggery Anaerobic Digestate Liquid Treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of Water Culture Medium, Cultivation Systems and Growth Modes for Microalgae Cultivation: A Review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Knoshaug, E.P.; Dong, T.; Spiller, R.; Nagle, N.; Pienkos, P.T. Pretreatment and Fermentation of Salt-Water Grown Algal Biomass as a Feedstock for Biofuels and High-Value Biochemicals. Algal Res. 2018, 36, 239–248. [Google Scholar] [CrossRef]
- Jepsen, P.M.; Thoisen, C.V.; Carron-Cabaret, T.; Pinyol-Gallemí, A.; Nielsen, S.L.; Hansen, B.W. Effects of Salinity, Commercial Salts, and Water Type on Cultivation of the Cryptophyte Microalgae Rhodomonas salina and the Calanoid Copepod Acartia tonsa. J. World Aquac. Soc. 2019, 50, 104–118. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Mu, J.; Liu, M.; Cui, W. Removal of Nutrients, Organic Matter, and Metal from Domestic Secondary Effluent through Microalgae Cultivation in a Membrane Photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 2713–2719. [Google Scholar] [CrossRef]
- Yousefi, Y.; Hanachi, P.; Samadi, M.; Khoshnamvand, M. Heavy Metals (Copper and Iron) and Nutrients (Nitrate and Phosphate) Removal from Aqueous Medium by Microalgae Chlorella Vulgaris and Scendesmus Obliquus, and Their Biofilms. Mar. Environ. Res. 2023, 188, 105989. [Google Scholar] [CrossRef]
- Asaad, A.A.; Amer, A.S. Evaluation of Chlorella Vulgaris Biosorption Capacity for Phosphate and Nitrate Removal from Wastewater. Sci. Rep. 2024, 14, 884. [Google Scholar] [CrossRef]
- He, Z.; Fan, X.; Qu, L.; Zhou, X.; Jin, W.; Hatshan, M.R.; Li, X.; Liu, H.; Jiang, G.; Wang, Q. Cultivation of Chlorella Pyrenoidosa and Scenedesmus Obliquus in Swine Wastewater: Nitrogen and Phosphorus Removal and Microalgal Growth. Process Saf. Environ. Prot. 2023, 179, 887–895. [Google Scholar] [CrossRef]
- Diaconu, M.; Soreanu, G.; Balan, C.D.; Buciscanu, I.I.; Maier, V.; Cretescu, I. Study of Spirulina Platensis (Arthrospira) Development under the Heavy Metals Influence, as a Potential Promoter of Wastewater Remediation. Water 2023, 15, 3962. [Google Scholar] [CrossRef]
- Fu, W.; Gudmundsson, O.; Feist, A.M.; Herjolfsson, G.; Brynjolfsson, S.; Palsson, B.Ø. Maximizing Biomass Productivity and Cell Density of Chlorella Vulgaris by Using Light-Emitting Diode-Based Photobioreactor. J. Biotechnol. 2012, 161, 242–249. [Google Scholar] [CrossRef]
- Carl, C.; Magnusson, M.; Paul, N.A.; de Nys, R. The Yield and Quality of Multiple Harvests of Filamentous Ulva Tepida. J. Appl. Phycol. 2016, 28, 2865–2873. [Google Scholar] [CrossRef]
- Wilson, M.H.; Groppo, J.; Placido, A.; Graham, S.; Morton, S.A.; Santillan-Jimenez, E.; Shea, A.; Crocker, M.; Crofcheck, C.; Andrews, R. CO2 Recycling Using Microalgae for the Production of Fuels. Appl. Petrochem. Res. 2014, 4, 41–53. [Google Scholar] [CrossRef]
- Walsh, M.J.; Van Doren, L.G.; Shete, N.; Prakash, A.; Salim, U. Financial Tradeoffs of Energy and Food Uses of Algal Biomass under Stochastic Conditions. Appl. Energy 2018, 210, 591–603. [Google Scholar] [CrossRef]
- Lerat, Y.; Cornish, M.L.; Critchley, A.T. Applications of Algal Biomass in Global Food and Feed Markets: From Traditional Usage to the Potential for Functional Products. In Blue Biotechnology; Wiley: Hoboken, NJ, USA, 2018; pp. 143–189. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Ali, M.; Mujahid, M. A Critical Review of Algal Biomass: A Versatile Platform of Bio-Based Polyesters from Renewable Resources. Int. J. Biol. Macromol. 2016, 86, 937–949. [Google Scholar] [CrossRef]
- Nakhate, P.; van der Meer, Y. A Systematic Review on Seaweed Functionality: A Sustainable Bio-Based Material. Sustainability 2021, 13, 6174. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Karp, S.G.; Manzoki, M.C.; Medeiros, A.B.P.; Rodrigues, C.; Scapini, T.; Vandenberghe, L.P.d.S.; Vieira, S.; et al. Agro-Industrial Wastewaters for Algal Biomass Production, Bio-Based Products, and Biofuels in a Circular Bioeconomy. Fermentation 2022, 8, 728. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Nejat Veziroglu, T.; Allakhverdiev, S.I. Review: Biofuel Production from Plant and Algal Biomass. Int. J. Hydrogen Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Shahi Khalaf Ansar, B.; Kavusi, E.; Dehghanian, Z.; Pandey, J.; Asgari Lajayer, B.; Price, G.W.; Astatkie, T. Removal of Organic and Inorganic Contaminants from the Air, Soil, and Water by Algae. Environ. Sci. Pollut. Res. 2022, 30, 116538–116566. [Google Scholar] [CrossRef]
- Mondal, M.; Khanra, S.; Tiwari, O.N.; Gayen, K.; Halder, G.N. Role of Carbonic Anhydrase on the Way to Biological Carbon Capture through Microalgae—A Mini Review. Environ. Prog. Sustain. Energy 2016, 35, 1605–1615. [Google Scholar] [CrossRef]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall’Osto, L. Potential and Challenges of Improving Photosynthesis in Algae. Plants 2020, 9, 67. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Kookal, S.K.; Ray, J.G. Biomass Yield and Biochemical Profile of Fourteen Species of Fast-Growing Green Algae from Eutrophic Bloomed Freshwaters of Kerala, South India. Biomass Bioenergy 2018, 119, 155–165. [Google Scholar] [CrossRef]
- Shah, S.; Li, X.; Jiang, Z.; Fahad, S.; Hassan, S. Exploration of the Phytohormone Regulation of Energy Storage Compound Accumulation in Microalgae. Food Energy Secur. 2022, 11, e418. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of Temperature and Light on the Growth of Algae Species: A Review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Fuente, D.; Keller, J.; Conejero, J.A.; Rögner, M.; Rexroth, S.; Urchueguía, J.F. Light Distribution and Spectral Composition within Cultures of Micro-Algae: Quantitative Modelling of the Light Field in Photobioreactors. Algal Res. 2017, 23, 166–177. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Liu, T. The Difference in Effective Light Penetration May Explain the Superiority in Photosynthetic Efficiency of Attached Cultivation over the Conventional Open Pond for Microalgae. Biotechnol. Biofuels 2015, 8, 49. [Google Scholar] [CrossRef]
- Wang, S.; Stiles, A.R.; Guo, C.; Liu, C. Microalgae Cultivation in Photobioreactors: An Overview of Light Characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light Requirements in Microalgal Photobioreactors: An Overview of Biophotonic Aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Guardini, Z.; Gomez, R.L.; Dall’Osto, L. Improving Light Harvesting. In Photosynthesis in Action; Academic Press: Cambridge, MA, USA, 2022; pp. 135–159. [Google Scholar] [CrossRef]
- Li, G. 3D Printed Microfluidic Devices for Controlled Biomaterial Fabrication; University of Wyoming: Laramie, WY, USA, 2020. [Google Scholar]
- Liu, J.; Enloe, C.; Li-Oakey, K.D.; Oakey, J. Optimizing Immunofunctionalization and Cell Capture on Micromolded Hydrogels via Controlled Oxygen-Inhibited Photopolymerization. ACS Appl. Bio Mater. 2022, 5, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Gimmler, H. Acidophilic and Acidotolerant Algae. In Algal Adaptation to Environmental Stresses; Springer: Berlin/Heidelberg, Germany, 2001; pp. 259–290. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and Use of Bicarbonate in Plants: Current Knowledge and Challenges Ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Hlavova, M.; Turoczy, Z.; Bisova, K. Improving Microalgae for Biotechnology—From Genetics to Synthetic Biology. Biotechnol. Adv. 2015, 33, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Takouridis, S.J.; Tribe, D.E.; Gras, S.L.; Martin, G.J.O. The Selective Breeding of the Freshwater Microalga Chlamydomonas reinhardtii for Growth in Salinity. Bioresour. Technol. 2015, 184, 18–22. [Google Scholar] [CrossRef]
- Jebali, A.; Sanchez, M.R.; Hanschen, E.R.; Starkenburg, S.R.; Corcoran, A.A. Trait Drift in Microalgae and Applications for Strain Improvement. Biotechnol. Adv. 2022, 60, 108034. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, S.; Toppo, K.; Verma, S. Adaptation in Algae to Environmental Stress and Ecological Conditions. In Plant Adaptation Strategies in Changing Environment; Springer: Singapore, 2017; pp. 103–115. [Google Scholar] [CrossRef]
- Cho, C.H.; Park, S.I.; Huang, T.-Y.; Lee, Y.; Ciniglia, C.; Yadavalli, H.C.; Yang, S.W.; Bhattacharya, D.; Yoon, H.S. Genome-Wide Signatures of Adaptation to Extreme Environments in Red Algae. Nat. Commun. 2023, 14, 10. [Google Scholar] [CrossRef]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.-S.; Chang, J.-S. Genetic Engineering of Microalgae for Enhanced Biorefinery Capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing Production of Microalgal Biopigments through Metabolic and Genetic Engineering. Crit. Rev. Food Sci. Nutr. 2020, 60, 391–405. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent Developments in Synthetic Biology and Metabolic Engineering in Microalgae towards Biofuel Production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Ma, X.; Guo, B.; Liu, B.; Wu, T.; Jiang, Y.; Chen, F. Genetic Engineering of the Calvin Cycle toward Enhanced Photosynthetic CO2 Fixation in Microalgae. Biotechnol. Biofuels 2017, 10, 229. [Google Scholar] [CrossRef]
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Rahimian Koloor, S.S.; Petrů, M.; Lau, W.J.; Ismail, A.F. Fourth Generation Biofuel from Genetically Modified Algal Biomass: Challenges and Future Directions. Chemosphere 2021, 285, 131535. [Google Scholar] [CrossRef]
- Barati, B.; Zeng, K.; Baeyens, J.; Wang, S.; Addy, M.; Gan, S.-Y.; El-Fatah Abomohra, A. Recent Progress in Genetically Modified Microalgae for Enhanced Carbon Dioxide Sequestration. Biomass Bioenergy 2021, 145, 105927. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the Genetic and Metabolic Engineering Approaches to Enhance the Competence of Microalgae as Biofuel Resource: A Review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Das, A.; Kumari, R.; Kajla, S. Recent Progress and Challenges in CRISPR-Cas9 Engineered Algae and Cyanobacteria. Algal Res. 2023, 71, 103068. [Google Scholar] [CrossRef]
- Park, R.V.; Asbury, H.; Miller, S.M. Modification of a Chlamydomonas reinhardtii CRISPR/Cas9 Transformation Protocol for Use with Widely Available Electroporation Equipment. MethodsX 2020, 7, 100855. [Google Scholar] [CrossRef] [PubMed]
- Picariello, T.; Hou, Y.; Kubo, T.; McNeill, N.A.; Yanagisawa, H.; Oda, T.; Witman, G.B. TIM, a Targeted Insertional Mutagenesis Method Utilizing CRISPR/Cas9 in Chlamydomonas reinhardtii. PLoS ONE 2020, 15, e0232594. [Google Scholar] [CrossRef]
- Kim, J.C.; Zuzarte, P.C.; Murphy, T.; Chan-Seng-Yue, M.; Brown, A.M.K.; Krzyzanowski, P.M.; Smith, A.C.; Notta, F.; Minden, M.D.; McPherson, J.D. Cryptic Genomic Lesions in Adverse-Risk Acute Myeloid Leukemia Identified by Integrated Whole Genome and Transcriptome Sequencing. Leukemia 2020, 34, 306–311. [Google Scholar] [CrossRef]
- Kaczmarzyk, D.; Cengic, I.; Yao, L.; Hudson, E.P. Diversion of the Long-Chain Acyl-ACP Pool in Synechocystis to Fatty Alcohols through CRISPRi Repression of the Essential Phosphate Acyltransferase PlsX. Metab. Eng. 2018, 45, 59–66. [Google Scholar] [CrossRef]
- Kirtania, P.; Hódi, B.; Mallick, I.; Vass, I.Z.; Fehér, T.; Vass, I.; Kós, P.B. A Single Plasmid Based CRISPR Interference in Synechocystis 6803—A Proof of Concept. PLoS ONE 2019, 14, e0225375. [Google Scholar] [CrossRef]
- Smith, V.H.; Crews, T. Applying Ecological Principles of Crop Cultivation in Large-Scale Algal Biomass Production. Algal Res. 2014, 4, 23–34. [Google Scholar] [CrossRef]
- Mooij, P.R.; Stouten, G.R.; van Loosdrecht, M.C.; Kleerebezem, R. Ecology-Based Selective Environments as Solution to Contamination in Microalgal Cultivation. Curr. Opin. Biotechnol. 2015, 33, 46–51. [Google Scholar] [CrossRef]
- Hassanpour, M.; Abbasabadi, M.; Ebrahimi, S.; Hosseini, M.; Sheikhbaglou, A. Gravimetric Enrichment of High Lipid and Starch Accumulating Microalgae. Bioresour. Technol. 2015, 196, 17–21. [Google Scholar] [CrossRef]
- Kleerebezem, R.; van Loosdrecht, M.C. Mixed Culture Biotechnology for Bioenergy Production. Curr. Opin. Biotechnol. 2007, 18, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from Algae: Challenges and Prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; Van Loosdrecht, M.C.M. Production of Polyhydroxyalkanoates by Mixed Microbial Cultures. Bioprocess. Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.G.; Fick, J. Algal Cultivation in Urban Wastewater: An Efficient Way to Reduce Pharmaceutical Pollutants. J. Appl. Phycol. 2017, 29, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, W.; Fu, Z.; Cheng, Y.; Min, M.; Liu, Y.; Zhang, Y.; Chen, P.; Ruan, R. Effect of Wastewater-Borne Bacteria on Algal Growth and Nutrients Removal in Wastewater-Based Algae Cultivation System. Bioresour. Technol. 2014, 167, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, P.; Peter, J.; Koter, S.; Mateos, R.; Kumar, G.; Koók, L.; Rózsenberszki, T.; Pientka, Z.; Kujawski, W.; Kim, S.-H.; et al. Possibilities for the Biologically-Assisted Utilization of CO2-Rich Gaseous Waste Streams Generated during Membrane Technological Separation of Biohydrogen. J. CO2 Util. 2020, 36, 231–243. [Google Scholar] [CrossRef]
- Comley, J.G.; Scott, J.A.; Laamanen, C.A. Utilizing CO2 in Industrial off-Gas for Microalgae Cultivation: Considerations and Solutions. Crit. Rev. Biotechnol. 2024, 44, 910–923. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of Algae Consortium in a Dairy Farm Wastewater for Biodiesel Production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Passero, M.; Cragin, B.; Coats, E.R.; McDonald, A.G.; Feris, K. Dairy Wastewaters for Algae Cultivation, Polyhydroxyalkanote Reactor Effluent Versus Anaerobic Digester Effluent. Bioenergy Res. 2015, 8, 1647–1660. [Google Scholar] [CrossRef]
- Vieira Costa, J.A.; Cruz, C.G.; da Rosa, A.P.C. Insights into the Technology Utilized to Cultivate Microalgae in Dairy Effluents. Biocatal. Agric. Biotechnol. 2021, 35, 102106. [Google Scholar] [CrossRef]
- Zewdie, D.T.; Ali, A.Y. Cultivation of Microalgae for Biofuel Production: Coupling with Sugarcane-Processing Factories. Energy Sustain. Soc. 2020, 10, 27. [Google Scholar] [CrossRef]
- Suriya narayanan, G.; kumar, G.; seepana, S.; Elankovan, R.; Premalatha, M. Utilization of Unfiltered LPG-Burner Exhaust-Gas Emission Using Microalga Coelastrella sp. J. CO2 Util. 2019, 29, 283–295. [Google Scholar] [CrossRef]
- Acedo, M.; Gonzalez Cena, J.R.; Kiehlbaugh, K.M.; Ogden, K.L. Coupling Carbon Capture from a Power Plant with Semi-Automated Open Raceway Ponds for Microalgae Cultivation. J. Vis. Exp. 2020, 162, e61498. [Google Scholar] [CrossRef]
- Sirikulrat, K.; Pekkoh, J.; Pumas, C. Illumination System for Growth and Net Energy Ratio Enhancement of Arthrospira (Spirulina) Platensis Outdoor Cultivation in Deep Raceway Pond. Bioresour. Technol. Rep. 2021, 14, 100661. [Google Scholar] [CrossRef]
- Li, S.; Luo, S.; Guo, R. Efficiency of CO2 Fixation by Microalgae in a Closed Raceway Pond. Bioresour. Technol. 2013, 136, 267–272. [Google Scholar] [CrossRef]
- Dey, S.; Bhattacharya, A.; Kumar, P.; Malik, A. High-Rate CO2 Sequestration Using a Novel Venturi Integrated Photobioreactor and Subsequent Valorization to Microalgal Lipids. Green. Chem. 2020, 22, 7962–7973. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Liao, Q.; Zhu, X.; Xia, A.; Zhu, X.; Chang, J.-S. Boosting Photo-Biochemical Conversion and Carbon Dioxide Bio-Fixation of Chlorella Vulgaris in an Optimized Photobioreactor with Airfoil-Shaped Deflectors. Bioresour. Technol. 2021, 337, 125355. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, J.; Lai, X.; Guo, W.; Yang, W. Developing a Three-Dimensional Tangential Swirl Plate Photobioreactor to Enhance Mass Transfer and Flashlight Effect for Microalgal CO2 Fixation. Chem. Eng. Sci. 2021, 244, 116837. [Google Scholar] [CrossRef]
- Yaqoubnejad, P.; Rad, H.A.; Taghavijeloudar, M. Development a Novel Hexagonal Airlift Flat Plate Photobioreactor for the Improvement of Microalgae Growth That Simultaneously Enhance CO2 Bio-Fixation and Wastewater Treatment. J. Environ. Manag. 2021, 298, 113482. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.; Lai, X.; Zhang, X.; Yang, W.; Park, J.-Y.; Kim, H.; Xu, L. Enhancing Microalgal Biomass Productivity with an Optimized Flow Field Generated by Double Paddlewheels in a Flat Plate Photoreactor with CO2 Aeration Based on Numerical Simulation. Bioresour. Technol. 2020, 314, 123762. [Google Scholar] [CrossRef]
- Barbera, E.; Sforza, E.; Guidobaldi, A.; Di Carlo, A.; Bertucco, A. Integration of Dye-Sensitized Solar Cells (DSC) on Photobioreactors for Improved Photoconversion Efficiency in Microalgal Cultivation. Renew. Energy 2017, 109, 13–21. [Google Scholar] [CrossRef]
- Hermadi, I.; Setiadianto, I.R.; Al Zahran, D.F.; Simbolon, M.N.; Saefurahman, G.; Wibawa, D.S.; Arkeman, Y. Development of Smart Algae Pond System for Microalgae Biomass Production. IOP Conf. Ser. Earth Environ. Sci. 2021, 749, 012068. [Google Scholar] [CrossRef]
- Carrasquilla-Batista, A.; Chacon-Rodriguez, A.; Murillo-Vega, F.; Niinez-Montero, K.; Goomez-Espinoza, O.; Guerrero-Barrantes, M. Characterization of Biomass Pellets from Chlorella Vulgaris Microalgal Production Using Industrial Wastewater. In Proceedings of the 2017 International Conference in Energy and Sustainability in Small Developing Economies (ES2DE), Funchal, Portugal, 10–12 July 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, S.; Chen, J.P. Carbon Capture and Utilization by Algae with High Concentration CO2 or Bicarbonate as Carbon Source. Sci. Total Environ. 2024, 918, 170325. [Google Scholar] [CrossRef] [PubMed]
- Do, C.V.T.; Nguyen, N.T.T.; Tran, T.D.; Pham, M.H.T.; Pham, T.Y.T. Capability of Carbon Fixation in Bicarbonate-Based and Carbon Dioxide-Based Systems by Scenedesmus Acuminatus TH04. Biochem. Eng. J. 2021, 166, 107858. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Roh, K.; Han, J.-I. The Use of Bicarbonate for Microalgae Cultivation and Its Carbon Footprint Analysis. Green. Chem. 2019, 21, 5053–5062. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, S.; Ji, Y.; Schwaneberg, U.; Chi, Z. Progress toward a Bicarbonate-Based Microalgae Production System. Trends Biotechnol. 2022, 40, 180–193. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Heo, J.; Kim, H.-S.; Han, J.-I. Bicarbonate-Based Cultivation of Dunaliella salina for Enhancing Carbon Utilization Efficiency. Bioresour. Technol. 2017, 237, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Sarangi, P.K.; Dalai, A.K.; Kozinski, J.A. A Broad Introduction to First-, Second-, and Third-Generation Biofuels. In Recent Advancements in Biofuels and Bioenergy Utilization; Springer: Singapore, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Mohd Azhar, S.H.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A Review on Third Generation Bioethanol Feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A Review on Feedstocks, Production Processes, and Yield for Different Generations of Biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Sokhansanj, S.; Lim, C.J. Co-Pelletization of Microalgae Chlorella Vulgaris and Pine Sawdust to Produce Solid Fuels. Fuel Process. Technol. 2018, 177, 129–139. [Google Scholar] [CrossRef]
- Miranda, M.T.; Sepúlveda, F.J.; Arranz, J.I.; Montero, I.; Rojas, C.V. Physical-Energy Characterization of Microalgae Scenedesmus and Experimental Pellets. Fuel 2018, 226, 121–126. [Google Scholar] [CrossRef]
- Cui, X.; Yang, J.; Shi, X.; Lei, W.; Huang, T.; Bai, C. Experimental Investigation on the Energy Consumption, Physical, and Thermal Properties of a Novel Pellet Fuel Made from Wood Residues with Microalgae as a Binder. Energies 2019, 12, 3425. [Google Scholar] [CrossRef]
- Choi, H.I.; Lee, J.S.; Choi, J.W.; Shin, Y.S.; Sung, Y.J.; Hong, M.E.; Kwak, H.S.; Kim, C.Y.; Sim, S.J. Performance and Potential Appraisal of Various Microalgae as Direct Combustion Fuel. Bioresour. Technol. 2019, 273, 341–349. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Musiał, T.; Urbaniak, D.; Otwinowski, H. Analysis of Microalgae Pellets Combustion in a Circulating Fluidized-Bed. E3S Web Conf. 2017, 14, 02035. [Google Scholar] [CrossRef]
- Cui, X.; Yang, J.; Wang, Z.; Shi, X. Better Use of Bioenergy: A Critical Review of Co-Pelletizing for Biofuel Manufacturing. Carbon Capture Sci. Technol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, G.; Zhang, H.; Xu, J.; Ge, H.; Shen, L.; Song, T. Coupling Effects of Heating Pelleting and Torrefaction on Black Pellets Production from Microalga Nannochloropsis Oceanica Residues. Fuel 2023, 353, 129007. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, J.; Yuan, X.; Li, H.; Li, C.; Xiao, Z.; Huang, H.; Wang, H.; Zeng, G. Co-Pelletization of Sewage Sludge and Biomass: The Density and Hardness of Pellet. Bioresour. Technol. 2014, 166, 435–443. [Google Scholar] [CrossRef]
- Mostafa, M.E.; Hu, S.; Wang, Y.; Su, S.; Hu, X.; Elsayed, S.A.; Xiang, J. The Significance of Pelletization Operating Conditions: An Analysis of Physical and Mechanical Characteristics as Well as Energy Consumption of Biomass Pellets. Renew. Sustain. Energy Rev. 2019, 105, 332–348. [Google Scholar] [CrossRef]
- Kastanaki, E.; Vamvuka, D. A Comparative Reactivity and Kinetic Study on the Combustion of Coal–Biomass Char Blends. Fuel 2006, 85, 1186–1193. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, L.; Wang, Z.; Wang, Z.; Xu, Y.; Sun, F.; Sun, Z.; Liu, Z.; Zhu, L. Experimental Study on Combustion and Pollutants Emissions of Oil Sludge Blended with Microalgae Residue. J. Energy Inst. 2018, 91, 877–886. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Wei, Z.; Chen, Z.; Evrendilek, F.; Huang, W. Oxy-Fuel Co-Combustion Dynamics of Phytoremediation Biomass and Textile Dyeing Sludge: Gas-to-Ash Pollution Abatement. Sci. Total Environ. 2022, 825, 153656. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, F.; Cheng, Z.; Chan, Q.N.; Kook, S.; Yeoh, G.H. Emissions Characteristics of NOx and SO2 in the Combustion of Microalgae Biomass Using a Tube Furnace. J. Energy Inst. 2017, 90, 806–812. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. Production of Bio-Coal, Bio-Methane and Fertilizer from Seaweed via Hydrothermal Carbonisation. Algal Res. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Jeeru, L.R.; Abdul, F.K.; Anireddy, J.S.; Ch, V.P.; Dhanavath, K.N. Optimization of Process Parameters for Conventional Pyrolysis of Algal Biomass into Bio–Oil and Bio–Char Production. Chem. Eng. Process.-Process Intensif. 2023, 185, 109311. [Google Scholar] [CrossRef]
- Yadav, V.; Baruah, B.P.; Khare, P. Comparative Study of Thermal Properties of Bio-Coal from Aromatic Spent with Low Rank Sub-Bituminous Coals. Bioresour. Technol. 2013, 137, 376–385. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Rizzo, A.M.; Pari, L. Review and Experimental Study on Pyrolysis and Hydrothermal Liquefaction of Microalgae for Biofuel Production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Suyono, E.A.; Haryadi, W.; Zusron, M.; Nuhamunada, M.; Rahayu, S.; Nugroho, A.P. The Effect of Salinity on Growth, Dry Weight and Lipid Content of the Mixed Microalgae Culture Isolated from Glagah as Biodiesel Substrate. J. Life Sci. 2015, 10, 229–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Sun, M.; Peng, Q.; Li, A. Photosynthetic Physiological Performance and Proteomic Profiling of the Oleaginous Algae Scenedesmus Acuminatus Reveal the Mechanism of Lipid Accumulation under Low and High Nitrogen Supplies. Photosynth. Res. 2018, 138, 73–102. [Google Scholar] [CrossRef]
- Mao, B.; Li, G.; Wang, M.; Deng, X.; Gao, K.; Zhang, B. Using Nitrogen Starvation and Excess Phosphorus for Two-Stage Algae Cultivation to Improve Butanol Production of Lipid-Extracted Algae. Renew. Energy 2024, 220, 119652. [Google Scholar] [CrossRef]
- Nayak, M.; Suh, W.I.; Chang, Y.K.; Lee, B. Exploration of Two-Stage Cultivation Strategies Using Nitrogen Starvation to Maximize the Lipid Productivity in Chlorella sp. HS2. Bioresour. Technol. 2019, 276, 110–118. [Google Scholar] [CrossRef]
- Xiong, W.; Gao, C.; Yan, D.; Wu, C.; Wu, Q. Double CO2 Fixation in Photosynthesis–Fermentation Model Enhances Algal Lipid Synthesis for Biodiesel Production. Bioresour. Technol. 2010, 101, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.T.; Ullrich, N.; Joo, S.; Waffenschmidt, S.; Goodenough, U. Algal Lipid Bodies: Stress Induction, Purification, and Biochemical Characterization in Wild-Type and Starchless Chlamydomonas reinhardtii. Eukaryot. Cell 2009, 8, 1856–1868. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Possible Methods for Biodiesel Production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic Biodiesel: Challenges and Opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Świderek, K.; Tuñón, I.; Moliner, V. Predicting Enzymatic Reactivity: From Theory to Design. WIREs Comput. Mol. Sci. 2014, 4, 407–421. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Tailoring Multipurpose Biocatalysts via Protein Engineering Approaches: A Review. Catal. Lett. 2019, 149, 2204–2217. [Google Scholar] [CrossRef]
- Bose, A.; Keharia, H. Production, Characterization and Applications of Organic Solvent Tolerant Lipase by Pseudomonas aeruginosa AAU2. Biocatal. Agric. Biotechnol. 2013, 2, 255–266. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Enzymatic Reactors for Biodiesel Synthesis: Present Status and Future Prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, J.; Akram, S.; Mumtaz, M.W.; Danish, M.; Irfan, A.; Touqeer, T.; Rashid, U.; Ghani, W.A.W.A.K.; Choong, T.S.Y. Nanobiocatalysts for Biodiesel Synthesis through Transesterification—A Review. Catalysts 2021, 11, 171. [Google Scholar] [CrossRef]
- Ameen, M.; Ahmad, M.; Zafar, M.; Munir, M.; Mujtaba, M.M.; Sultana, S.; Rozina; El-Khatib, S.E.; Soudagar, M.E.M.; Kalam, M.A. Prospects of Catalysis for Process Sustainability of Eco-Green Biodiesel Synthesis via Transesterification: A State-Of-The-Art Review. Sustainability 2022, 14, 7032. [Google Scholar] [CrossRef]
- Rai, M.; dos Santos, J.C.; Soler, M.F.; Franco Marcelino, P.R.; Brumano, L.P.; Ingle, A.P.; Gaikwad, S.; Gade, A.; da Silva, S.S. Strategic Role of Nanotechnology for Production of Bioethanol and Biodiesel. Nanotechnol. Rev. 2016, 5, 231–250. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Liu, X. An Overview of Algae Bioethanol Production. Int. J. Energy Res. 2014, 38, 965–977. [Google Scholar] [CrossRef]
- Karishma, S.; Saravanan, A.; Senthil Kumar, P.; Rangasamy, G. Sustainable Production of Biohydrogen from Algae Biomass: Critical Review on Pretreatment Methods, Mechanism and Challenges. Bioresour. Technol. 2022, 366, 128187. [Google Scholar] [CrossRef] [PubMed]
- Kendir, E.; Ugurlu, A. A Comprehensive Review on Pretreatment of Microalgae for Biogas Production. Int. J. Energy Res. 2018, 42, 3711–3731. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of Microalgae to Improve Biogas Production: A Review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Phwan, C.K.; Chew, K.W.; Sebayang, A.H.; Ong, H.C.; Ling, T.C.; Malek, M.A.; Ho, Y.-C.; Show, P.L. Effects of Acids Pre-Treatment on the Microbial Fermentation Process for Bioethanol Production from Microalgae. Biotechnol. Biofuels 2019, 12, 191. [Google Scholar] [CrossRef]
- Onumaegbu, C.; Mooney, J.; Alaswad, A.; Olabi, A.G. Pre-Treatment Methods for Production of Biofuel from Microalgae Biomass. Renew. Sustain. Energy Rev. 2018, 93, 16–26. [Google Scholar] [CrossRef]
- Yoon, M.; Choi, J.; Lee, J.-W.; Park, D.-H. Improvement of Saccharification Process for Bioethanol Production from Undaria sp. by Gamma Irradiation. Radiat. Phys. Chem. 2012, 81, 999–1002. [Google Scholar] [CrossRef]
- Budarin, V.L.; Zhao, Y.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.W.; Macquarrie, D.J.; Clark, J.H. Microwave-Mediated Pyrolysis of Macro-Algae. Green. Chem. 2011, 13, 2330. [Google Scholar] [CrossRef]
- Choi, J.-A.; Hwang, J.-H.; Dempsey, B.A.; Abou-Shanab, R.A.I.; Min, B.; Song, H.; Lee, D.S.; Kim, J.R.; Cho, Y.; Hong, S.; et al. Enhancement of Fermentative Bioenergy (Ethanol/Hydrogen) Production Using Ultrasonication of Scenedesmus Obliquus YSW15 Cultivated in Swine Wastewater Effluent. Energy Environ. Sci. 2011, 4, 3513. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of Hydrothermal Pretreatment on the Anaerobic Digestion of Pelagic Sargassum for Biogas and Fertiliser Recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
- Kim, J.K.; Um, B.-H.; Kim, T.H. Bioethanol Production from Micro-Algae, Schizocytrium sp., Using Hydrothermal Treatment and Biological Conversion. Korean J. Chem. Eng. 2012, 29, 209–214. [Google Scholar] [CrossRef]
- Okuda, K.; Oka, K.; Onda, A.; Kajiyoshi, K.; Hiraoka, M.; Yanagisawa, K. Hydrothermal Fractional Pretreatment of Sea Algae and Its Enhanced Enzymatic Hydrolysis. J. Chem. Technol. Biotechnol. 2008, 83, 836–841. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; García, J.C.; Lopez, F.; Villar, J.C.; Diaz, M.J. Steam Explosion and Enzymatic Pre-Treatments as an Approach to Improve the Enzymatic Hydrolysis of Eucalyptus Globulus. Biomass Bioenergy 2012, 42, 97–106. [Google Scholar] [CrossRef]
- Lorente, E.; Hapońska, M.; Clavero, E.; Torras, C.; Salvadó, J. Microalgae Fractionation Using Steam Explosion, Dynamic and Tangential Cross-Flow Membrane Filtration. Bioresour. Technol. 2017, 237, 3–10. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Bassin, I.D.; Cammarota, M.C. Microalgae and Cyanobacteria Biomass Pretreatment Methods: A Comparative Analysis of Chemical and Thermochemical Pretreatment Methods Aimed at Methane Production. Fermentation 2022, 8, 497. [Google Scholar] [CrossRef]
- Greetham, D.; Adams, J.M.; Du, C. The Utilization of Seawater for the Hydrolysis of Macroalgae and Subsequent Bioethanol Fermentation. Sci. Rep. 2020, 10, 9728. [Google Scholar] [CrossRef]
- Hebbale, D.; Ramachandra, T.V. Optimal Sugar Release from Macroalgal Feedstock with Dilute Acid Pretreatment and Enzymatic Hydrolysis. Biomass Convers. Biorefin. 2023, 13, 8287–8300. [Google Scholar] [CrossRef]
- AlMomani, F.; Shawaqfah, M.; Alsarayreh, M.; Khraisheh, M.; Hameed, B.H.; Naqvi, S.R.; Berkani, M.; Varjani, S. Developing Pretreatment Methods to Promote the Production of Biopolymer and Bioethanol from Residual Algal Biomass (RAB). Algal Res. 2022, 68, 102895. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Bhushan, S.; Rana, M.S.; Bhandari, M.; Sharma, A.K.; Simsek, H.; Prajapati, S.K. Enzymatic Pretreatment of Algal Biomass Has Different Optimal Conditions for Biogas and Bioethanol Routes. Chemosphere 2021, 284, 131264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, X.; Wang, Z.; Sun, Y.; Zhu, S.; Li, L.; Lv, P. Optimization of Enzymatic Hydrolysis for Effective Lipid Extraction from Microalgae Scenedesmus sp. Renew. Energy 2018, 125, 1049–1057. [Google Scholar] [CrossRef]
- Miranda, J.R.; Passarinho, P.C.; Gouveia, L. Bioethanol Production from Scenedesmus Obliquus Sugars: The Influence of Photobioreactors and Culture Conditions on Biomass Production. Appl. Microbiol. Biotechnol. 2012, 96, 555–564. [Google Scholar] [CrossRef]
- Sohail, M.; Siddiqi, R.; Ahmad, A.; Khan, S.A. Cellulase Production from Aspergillus Niger MS82: Effect of Temperature and PH. New Biotechnol. 2009, 25, 437–441. [Google Scholar] [CrossRef]
- Dura, A.; Błaszczak, W.; Rosell, C.M. Functionality of Porous Starch Obtained by Amylase or Amyloglucosidase Treatments. Carbohydr. Polym. 2014, 101, 837–845. [Google Scholar] [CrossRef]
- Timilsina, P.M.; Pandey, G.R.; Shrestha, A.; Ojha, M.; Karki, T.B. Purification and Characterization of a Noble Thermostable Algal Starch Liquefying Alpha-Amylase from Aeribacillus Pallidus BTPS-2 Isolated from Geothermal Spring of Nepal. Biotechnol. Rep. 2020, 28, e00551. [Google Scholar] [CrossRef]
- Ratuchne, A.; Knob, A. A New and Unusual β-Glucosidase from Aspergillus Fumigatus: Catalytic Activity at High Temperatures and Glucose Tolerance. Biocatal. Agric. Biotechnol. 2021, 35, 102064. [Google Scholar] [CrossRef]
- Percival Zhang, Y.-H.; Himmel, M.E.; Mielenz, J.R. Outlook for Cellulase Improvement: Screening and Selection Strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Kubicek, C.P. Genetic Engineering of Trichoderma reesei Cellulases and Their Production. Microb. Biotechnol. 2017, 10, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Solid State Fermentation for Production of Microbial Cellulases: Recent Advances and Improvement Strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P.W. Applications of Fungal Cellulases in Biofuel Production: Advances and Limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386. [Google Scholar] [CrossRef]
- Imran, M.; Anwar, Z.; Irshad, M.; Asad, M.J.; Ashfaq, H. Cellulase Production from Species of Fungi and Bacteria from Agricultural Wastes and Its Utilization in Industry: A Review. Adv. Enzyme Res. 2016, 04, 44–55. [Google Scholar] [CrossRef]
- Ajeje, S.B.; Hu, Y.; Song, G.; Peter, S.B.; Afful, R.G.; Sun, F.; Asadollahi, M.A.; Amiri, H.; Abdulkhani, A.; Sun, H. Thermostable Cellulases/Xylanases From Thermophilic and Hyperthermophilic Microorganisms: Current Perspective. Front. Bioeng. Biotechnol. 2021, 9, 794304. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Kumar, S. Bioprospecting Thermophilic/Thermotolerant Microbes for Production of Lignocellulosic Ethanol: A Future Perspective. Renew. Sustain. Energy Rev. 2015, 51, 699–717. [Google Scholar] [CrossRef]
- Bhalla, A.; Bansal, N.; Kumar, S.; Bischoff, K.M.; Sani, R.K. Improved Lignocellulose Conversion to Biofuels with Thermophilic Bacteria and Thermostable Enzymes. Bioresour. Technol. 2013, 128, 751–759. [Google Scholar] [CrossRef]
- Kasana, R.C.; Gulati, A. Cellulases from Psychrophilic Microorganisms: A Review. J. Basic Microbiol. 2011, 51, 572–579. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; dos Santos Vasconcelos, M.R.; Passarini, M.R.Z.; Vieira, G.A.L.; Lopes, V.C.P.; Mainardi, P.H.; dos Santos, J.A.; de Azevedo Duarte, L.; Otero, I.V.R.; da Silva Yoshida, A.M.; et al. Marine-Derived Fungi: Diversity of Enzymes and Biotechnological Applications. Front. Microbiol. 2015, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.A.; Oliveira, M.M.; Curvelo, A.A.S.; Fonseca, L.P.; Porto, A.L.M. Hydrolysis of Cellulose from Sugarcane Bagasse by Cellulases from Marine-Derived Fungi Strains. Int. Biodeterior. Biodegrad. 2017, 121, 66–78. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Hilário, S.; Van de Peer, Y.; Esteves, A.C.; Alves, A. Genomic and Metabolomic Analyses of the Marine Fungus Emericellopsis Cladophorae: Insights into Saltwater Adaptability Mechanisms and Its Biosynthetic Potential. J. Fungi 2021, 8, 31. [Google Scholar] [CrossRef]
- Loulergue, P.; Balannec, B.; Fouchard-Le Graët, L.; Cabrol, A.; Sayed, W.; Djelal, H.; Amrane, A.; Szymczyk, A. Air-Gap Membrane Distillation for the Separation of Bioethanol from Algal-Based Fermentation Broth. Sep. Purif. Technol. 2019, 213, 255–263. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen Production from Microalgae for Environmental Sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef]
- Anwar, M.; Lou, S.; Chen, L.; Li, H.; Hu, Z. Recent Advancement and Strategy on Bio-Hydrogen Production from Photosynthetic Microalgae. Bioresour. Technol. 2019, 292, 121972. [Google Scholar] [CrossRef]
- Mandotra, S.K.; Sharma, C.; Srivastava, N.; Ahluwalia, A.S.; Ramteke, P.W. Current Prospects and Future Developments in Algal Bio-Hydrogen Production: A Review. Biomass Convers. Biorefin. 2023, 13, 8575–8592. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Kondo, A.; Chang, J.-S. Recent Insights into Biohydrogen Production by Microalgae—From Biophotolysis to Dark Fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]
- Ghirardi, M.L. Implementation of Photobiological H2 Production: The O2 Sensitivity of Hydrogenases. Photosynth. Res. 2015, 125, 383–393. [Google Scholar] [CrossRef]
- Hallenbeck, P. Biological Hydrogen Production; Fundamentals and Limiting Processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of Bioenergy and Biochemicals from Industrial and Agricultural Wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Yoon, J.-J.; Park, H.-D.; Kim, Y.J.; Lim, D.J.; Kim, S.-H. Feasibility of Biohydrogen Production from Gelidium amansii. Int. J. Hydrog. Energy 2011, 36, 13997–14003. [Google Scholar] [CrossRef]
- Lee, D.-J.; Show, K.-Y.; Su, A. Dark Fermentation on Biohydrogen Production: Pure Culture. Bioresour. Technol. 2011, 102, 8393–8402. [Google Scholar] [CrossRef]
- Turon, V.; Baroukh, C.; Trably, E.; Latrille, E.; Fouilland, E.; Steyer, J.-P. Use of Fermentative Metabolites for Heterotrophic Microalgae Growth: Yields and Kinetics. Bioresour. Technol. 2015, 175, 342–349. [Google Scholar] [CrossRef]
- Abusweireh, R.S.; Rajamohan, N.; Sonne, C.; Vasseghian, Y. Algae Biogas Production Focusing on Operating Conditions and Conversion Mechanisms—A Review. Heliyon 2023, 9, e17757. [Google Scholar] [CrossRef]
- Kamusoko, R.; Jingura, R.M.; Chikwambi, Z.; Parawira, W. Biogas: Microbiological Research to Enhance Efficiency and Regulation. In Handbook of Biofuels; Academic Press: Cambridge, MA, USA, 2022; pp. 485–497. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A Comprehensive Review on Operating Parameters and Different Pretreatment Methodologies for Anaerobic Digestion of Municipal Solid Waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Einarsson, R.; Persson, U.M. Analyzing Key Constraints to Biogas Production from Crop Residues and Manure in the EU—A Spatially Explicit Model. PLoS ONE 2017, 12, e0171001. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Hussain, A.; Verma, C. Design Considerations and Operational Performance of Anaerobic Digester: A Review. Cogent Eng. 2016, 3, 1181696. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic Digestion from the Viewpoint of Microbiological, Chemical, and Operational Aspects—A Review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Manassa, P.; Dawson, M.; Fitzgerald, S.K. Oxidation Reduction Potential as a Parameter to Regulate Micro-Oxygen Injection into Anaerobic Digester for Reducing Hydrogen Sulphide Concentration in Biogas. Bioresour. Technol. 2014, 173, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Vongvichiankul, C.; Deebao, J.; Khongnakorn, W. Relationship between PH, Oxidation Reduction Potential (ORP) and Biogas Production in Mesophilic Screw Anaerobic Digester. Energy Procedia 2017, 138, 877–882. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and Perspectives of Anaerobic Co-Digestion: A Review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Faraji, M.; Saidi, M. Hydrogen-Rich Syngas Production via Integrated Configuration of Pyrolysis and Air Gasification Processes of Various Algal Biomass: Process Simulation and Evaluation Using Aspen Plus Software. Int. J. Hydrog. Energy 2021, 46, 18844–18856. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Li, M. Syngas Production from Algae Biomass Gasification: The Case of China. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1474–1482. [Google Scholar] [CrossRef]
- Tavares, R.; Monteiro, E.; Tabet, F.; Rouboa, A. Numerical Investigation of Optimum Operating Conditions for Syngas and Hydrogen Production from Biomass Gasification Using Aspen Plus. Renew. Energy 2020, 146, 1309–1314. [Google Scholar] [CrossRef]
- Gu, H.; Tang, Y.; Yao, J.; Chen, F. Study on Biomass Gasification under Various Operating Conditions. J. Energy Inst. 2019, 92, 1329–1336. [Google Scholar] [CrossRef]
- Azizi, K.; Keshavarz Moraveji, M.; Najafabadi, H.A. A Review on Bio-Fuel Production from Microalgal Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2018, 82, 3046–3059. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-Pyrolysis of Biomass and Waste Plastics as a Thermochemical Conversion Technology for High-Grade Biofuel Production: Recent Progress and Future Directions Elsewhere Worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A Review on Co-Pyrolysis of Biomass: An Optional Technique to Obtain a High-Grade Pyrolysis Oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Duan, P.; Jin, B.; Xu, Y.; Wang, F. Co-Pyrolysis of Microalgae and Waste Rubber Tire in Supercritical Ethanol. Chem. Eng. J. 2015, 269, 262–271. [Google Scholar] [CrossRef]
- Yan, W.-H.; Wang, K.; Duan, P.-G.; Wang, B.; Wang, F.; Shi, X.-L.; Xu, Y.-P. Catalytic Hydropyrolysis and Co-Hydropyrolysis of Algae and Used Engine Oil for the Production of Hydrocarbon-Rich Fuel. Energy 2017, 133, 1153–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Rajagopalan, K.; Lei, H.; Ruan, R.; Sharma, B.K. An Overview of a Novel Concept in Biomass Pyrolysis: Microwave Irradiation. Sustain. Energy Fuels 2017, 1, 1664–1699. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Tong, D.; Hu, C. Microwave-Enhanced Pyrolysis of Natural Algae from Water Blooms. Bioresour. Technol. 2016, 212, 311–317. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical Conversion of Microalgal Biomass for Biofuel Production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Microbially Induced Calcium Carbonate Precipitation: A Widespread Phenomenon in the Biological World. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Sharma, M.; Satyam, N.; Reddy, K.R. Strength Enhancement and Lead Immobilization of Sand Using Consortia of Bacteria and Blue-Green Algae. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020049. [Google Scholar] [CrossRef]
- Lin, W.; Lin, W.; Cheng, X.; Chen, G.; Ersan, Y.C. Microbially Induced Desaturation and Carbonate Precipitation through Denitrification: A Review. Appl. Sci. 2021, 11, 7842. [Google Scholar] [CrossRef]
- Dranseike, D.; Cui, Y.; Ling, A.S.; Donat, F.; Bernhard, S.; Bernero, M.; Areeckal, A.; Qin, X.-H.; Oakey, J.S.; Dillenburger, B.; et al. Dual Carbon Sequestration with Photosynthetic Living Materials. bioRxiv 2023, 12, 22.572991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muto, K.; Ito, S.; Ishii, D. Liquid Transport in Bio-Inspired Capillary-Driven Open-Air Channels. MRS Adv. 2017, 2, 1111–1116. [Google Scholar] [CrossRef]
- Li, G.; Yao, J. Snap-Off during Imbibition in Porous Media: Mechanisms, Influencing Factors, and Impacts. Eng 2023, 4, 2896–2925. [Google Scholar] [CrossRef]
- Yao, J.; Oakey, J. Geometrically-Mediated Snap-off of Water-in-Oil Emulsion Droplets in Microfluidic Flow Focusing Devices. J. Oil Gas. Petrochem. Sci. 2018, 1, 42–46. [Google Scholar] [CrossRef]
- Brinckmann, S.A.; Patra, N.; Yao, J.; Ware, T.H.; Frick, C.P.; Fertig, R.S. Stereolithography of SiOC Polymer-Derived Ceramics Filled with SiC Micronwhiskers. Adv. Eng. Mater. 2018, 20, 1800593. [Google Scholar] [CrossRef]
- Brinckmann, S.A.; Yao, J.; Young, J.C.; Jones, M.H.; Fertig, R.S., III; Frick, C.P. Additive Manufacturing of SiCNO Polymer-Derived Ceramics via Step-Growth Polymerization. Open Ceram. 2023, 15, 100414. [Google Scholar] [CrossRef]
- Kana, T.M.; Geider, R.J.; Critchley, C. Regulation of Photosynthetic Pigments in Micro-algae by Multiple Environmental Factors: A Dynamic Balance Hypothesis. New Phytol. 1997, 137, 629–638. [Google Scholar] [CrossRef]
- Vener, A.V. Environmentally Modulated Phosphorylation and Dynamics of Proteins in Photosynthetic Membranes. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 449–457. [Google Scholar] [CrossRef]
- Durall, C.; Lindblad, P. Mechanisms of Carbon Fixation and Engineering for Increased Carbon Fixation in Cyanobacteria. Algal Res. 2015, 11, 263–270. [Google Scholar] [CrossRef]
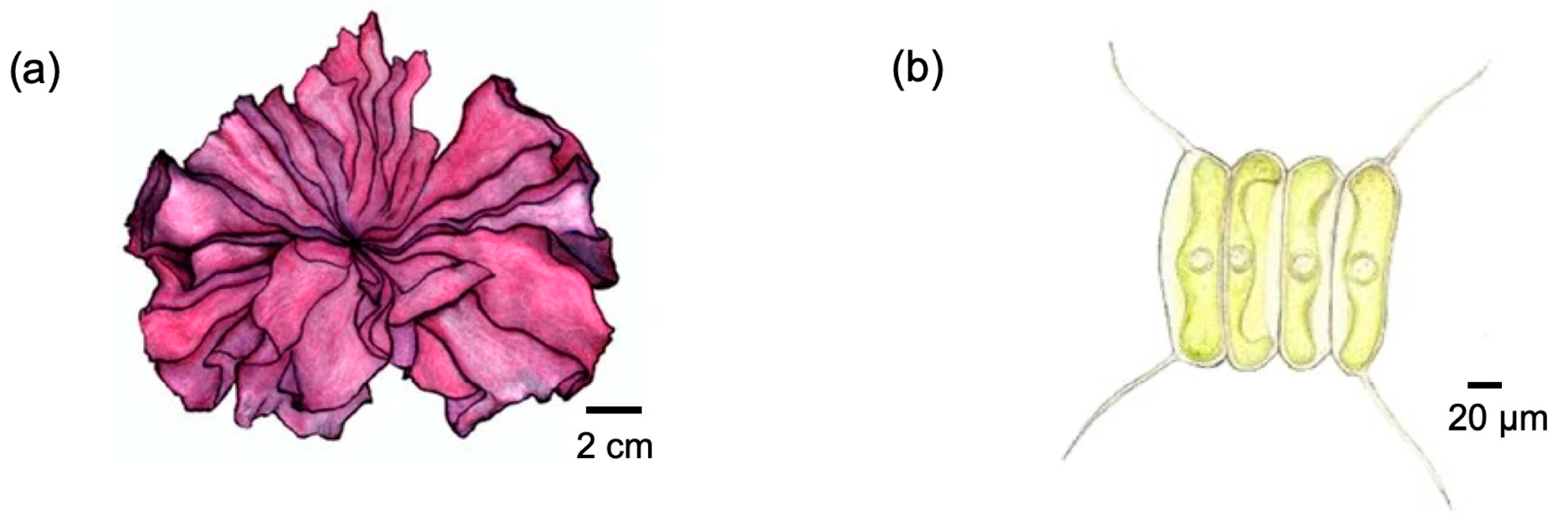
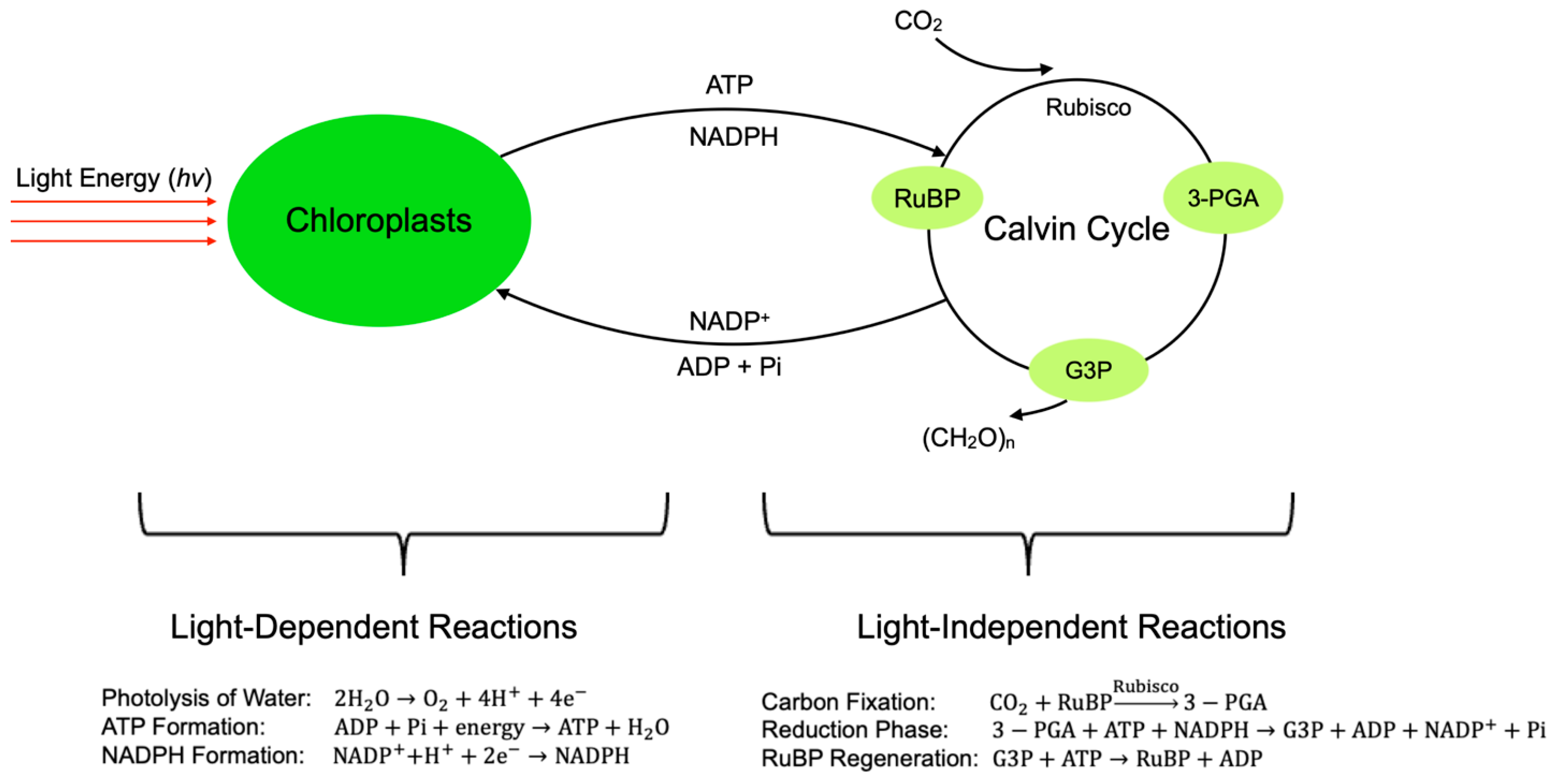
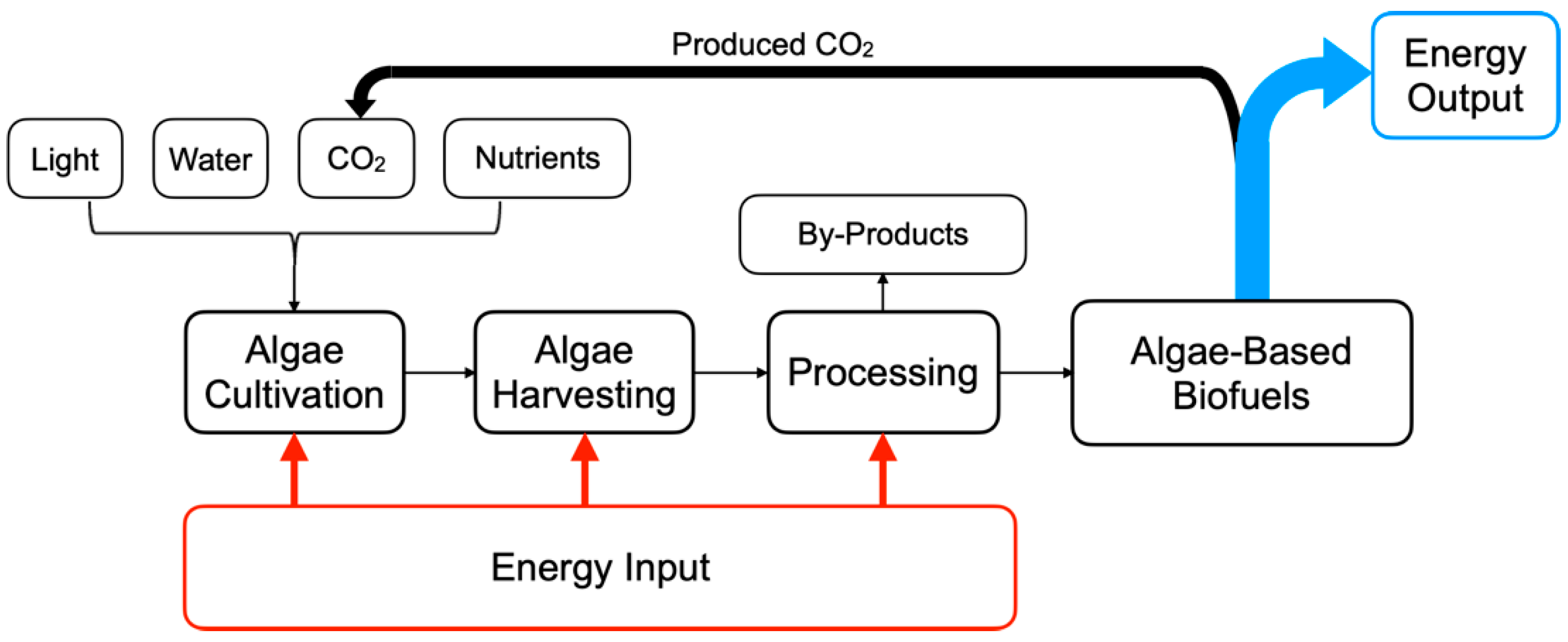
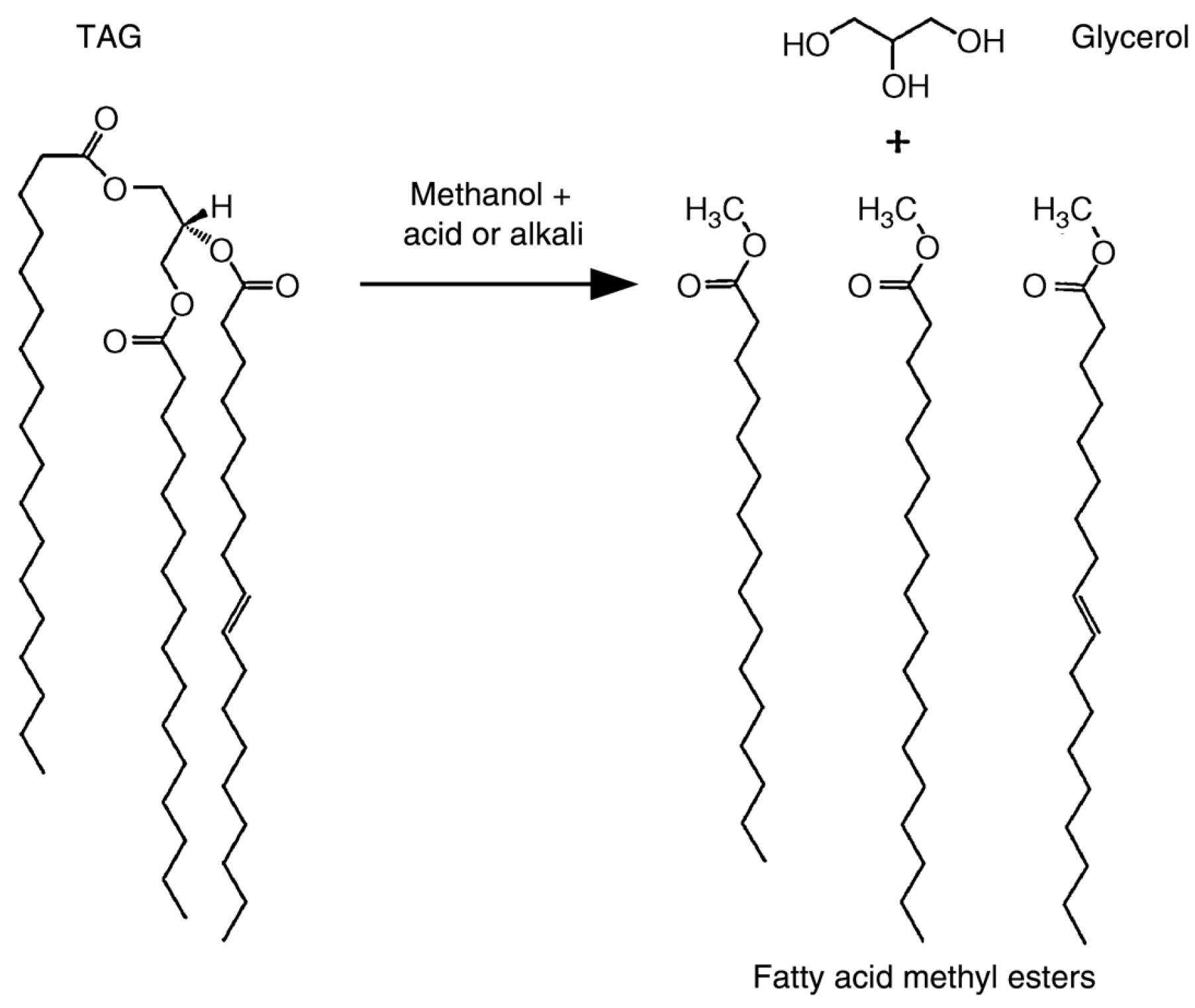


| Algae Type | Characteristics | Growth Environment | Subcategories | Suitability for CCUS | References |
|---|---|---|---|---|---|
| Macroalgae | Complex structures composed of multicellular organisms; Low protein and lipid content but high carbohydrates and moisture | Coastal or shallow water areas, usually requiring substrate attachment | Classified into four groups based on pigmentation: Cyanophyta, Chlorophyta, Heterokontophyta, and Rhodophyta | High productivity; Bioremediation of contaminants; High conversion capability from inorganic carbon to biomass | [53,59,60] |
| Microalgae | Small-sized structures with single-celled or few-celled composition; Rich in proteins and lipids | Widely distributed in various aquatic environments | Classified into three groups based on pigmentation: Bacillariophyceae, Chlorophyceae, and Chrysophyceae | Rapid growth rate; High photosynthetic rate; High conversion efficiency | [53,60] |
| Pretreatment Methods | Technical Details | Applicable Conditions | Characteristics | References | |
|---|---|---|---|---|---|
| Physical Pretreatment | Mechanical comminution | Grind, slice, and pulverize algal biomass to reduce particle sizes and cellulose crystallinity. | Effective for macroalgae with rigid cell walls and high cellulose crystallinity | Increasing the surface area-to-volume ratio of algal particles improves processing efficiency; Controlling the particle size within 3–6 mm optimizes energy consumption. | [193,198] |
| Irradiation | Gamma rays disrupt algal cell walls, increase surface area, and reduce cellulose crystallinity. Microwave irradiation generates internal heat, creating an explosion effect to break down resistant cell walls. | Gamma irradiation is suitable for macroalgae with recalcitrant cellulose structures; Microwave irradiation is suitable for microalgae | Combining this method with other pretreatments significantly accelerates saccharification; High energy requirement | [199,200] | |
| Ultrasonication | Ultrasound generates shear forces to disrupt cell walls and reduce particle size. | Suitable for microalgae with tough cell walls and larger biomass particles | Processing efficiency is influenced by ultrasound power, temperature, and pH, requiring adjustment of treatment conditions; While it consumes high energy, its efficiency can shorten pretreatment time, ultimately lowering overall energy demand. | [201,202] | |
| Physicochemical Pretreatment | Hydrothermal pretreatment | Hot water under high pressure hydrates cellulose and removes hemicellulose. | The most effective method for macroalgae with high cellulose and hemicellulose content | Water as the sole solvent; Short reaction time; Reduce sugar degradation loss; Minimize fermentation inhibitors | [203,204] |
| Steam explosion | High-pressure steam rapidly heats and depressurizes size-reduced biomass, causing explosive expansion that ruptures algal cell walls and increases surface area. | Suitable for microalgae with high cellulose content | High energy consumption; Higher cost; The addition of chemicals can improve the conversion rate of polysaccharides to monosaccharides. | [205,206] | |
| Supercritical carbon dioxide | By permeating biomass with supercritical carbon dioxide, and sudden depressurization causes the rupture of cell walls and other structures. | Suitable for both of macroalgae and microalgae with high cellulose content. | Cost-effective; Easy to extract and recover; Environmentally friendly | [193] | |
| Chemical Pretreatment | Alkaline pretreatment | Saponification is utilized by alkaline substances to break the ester bonds between hemicellulose and other components. | Suitable for both macroalgae and microalgae with high hemicellulose content | Reduce fermentation inhibition; Lower production costs. | [207,208] |
| Acid pretreatment | Acidic solutions effectively disrupt algal cell walls. | Widely applicable to both microalgae and macroalgae, and the most effective method for microalgae | High sugar yield; Simple operation; By-products like furfural and hydroxymethylfurfural (HMF) may impact fermentation and require additional treatment. | [197,209] | |
| Sodium chlorite treatment | Sodium chlorate generates chlorine dioxide (ClO2) in an acid, converting lignin into soluble compounds. | Suitable for lignin-containing macroalgae | Remove lignin from biomass while maximizing the retention of carbohydrates; Significantly enhance the efficiency of sugar extraction. | [210,211] | |
| Biological Pretreatment | Microorganisms are used to partially decompose biomass for saccharification | Different microorganisms are effective under specific conditions for macroalgae and microalgae saccharification | Low energy consumption; Safe and environmentally friendly | [193,212] | |
| Enzymatic Pretreatment | Enzymes are employed to target and degrade specific biomass compounds | Commonly applied to microalgae | Specific compound breakdown; Mild reaction conditions; Long processing time; High enzyme costs | [213] | |
| Enzymes | Optimal Temperature | Optimal pH | Applicable Algae | References |
|---|---|---|---|---|
| Cellulase | 30–45 °C | 4.5–5 | Macroalgae | [216] |
| Amyloglucosidase | 20–70 °C | 3.50–5.50 | Microalgae | [217] |
| α-amylase | 95–115 °C | 5.0–7.5 | Microalgae | [218] |
| β-glucosidase | less than 80 °C | 4.0–6.5 | Macroalgae | [219] |
| Sugar Types | Fermenting Microorganisms | Characteristics of Fermenting Microorganisms | References |
|---|---|---|---|
| Pentoses (Five-Carbon Sugars) | Xylose-fermenting microorganisms, including Candida shehatae and Pichia stipitis | Specific fermentation procedures and conditions are required; Repeated batch operations can enhance bioethanol production capacity. | [193] |
| Hexoses (Six-Carbon Sugars) | Yeasts, with Saccharomyces cerevisiae as a typical example | High bioethanol tolerance; Resistance to inhibitory substances; High osmotic resistance; Efficient bioethanol production | [57] |
| Bacteria, with Zymomonas mobilis as a typical example | Anaerobic fermentation; Rapid fermentation rate; Efficient bioethanol production; Limited tolerance to phenolic compounds | [57] |
| Algae-Based Biofuels | Production Technology | Characteristics | |
|---|---|---|---|
| Solid Algae-Based Biofuels | Solid biomass pellet | Pelletization | Efficient utilization of agal biomass; A straightforward manufacturing process; Convenient use for direct combustion |
| Biocoal | Pyrolysis and carbonization | Utilization of carbon-based components of algal biomass; Similar characteristics to coal; Applicable to carbon-intensive industries | |
| Liquid Algae-Based Biofuels | Biodiesel | Transesterification | Utilization of algal lipid components; High energy density; Glycerol as a valuable by-product with extensive application |
| Bioethanol | Fermentation | Utilization of algal carbohydrates; High energy density; Promising transportation fuel alternatives to gasoline | |
| Gaseous Algae-Based Biofuels | Biohydrogen | Biological photolysis and dark fermentation | Utilization of algal carbohydrates, lipids, and proteins; Water as the only by-product when used as fuel; Integration with biorefinery for enhanced energy recovery and by-product utilization |
| Biogas | Anaerobic digestion | Utilization of algal carbohydrates, lipids, and proteins; Digestate as fertilizer by-product | |
| Syngas | Gasification | Utilization of algal carbohydrates, lipids, and proteins; Direct use as fuel, or further converting into other fuels | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yao, J. A Review of Algae-Based Carbon Capture, Utilization, and Storage (Algae-Based CCUS). Gases 2024, 4, 468-503. https://doi.org/10.3390/gases4040024
Li G, Yao J. A Review of Algae-Based Carbon Capture, Utilization, and Storage (Algae-Based CCUS). Gases. 2024; 4(4):468-503. https://doi.org/10.3390/gases4040024
Chicago/Turabian StyleLi, Guihe, and Jia Yao. 2024. "A Review of Algae-Based Carbon Capture, Utilization, and Storage (Algae-Based CCUS)" Gases 4, no. 4: 468-503. https://doi.org/10.3390/gases4040024
APA StyleLi, G., & Yao, J. (2024). A Review of Algae-Based Carbon Capture, Utilization, and Storage (Algae-Based CCUS). Gases, 4(4), 468-503. https://doi.org/10.3390/gases4040024









