Indole-3-Carbinol Enhances Alternative Activation of Macrophages via AHR Pathway and Glucose Transporter Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Studies and Treatments
2.2. RNA Extraction and Real-Time RT-PCR
2.3. Flow Cytometric Evaluation of Surface Markers
2.4. Cytokine Assays
2.5. Statistical Analysis
3. Results
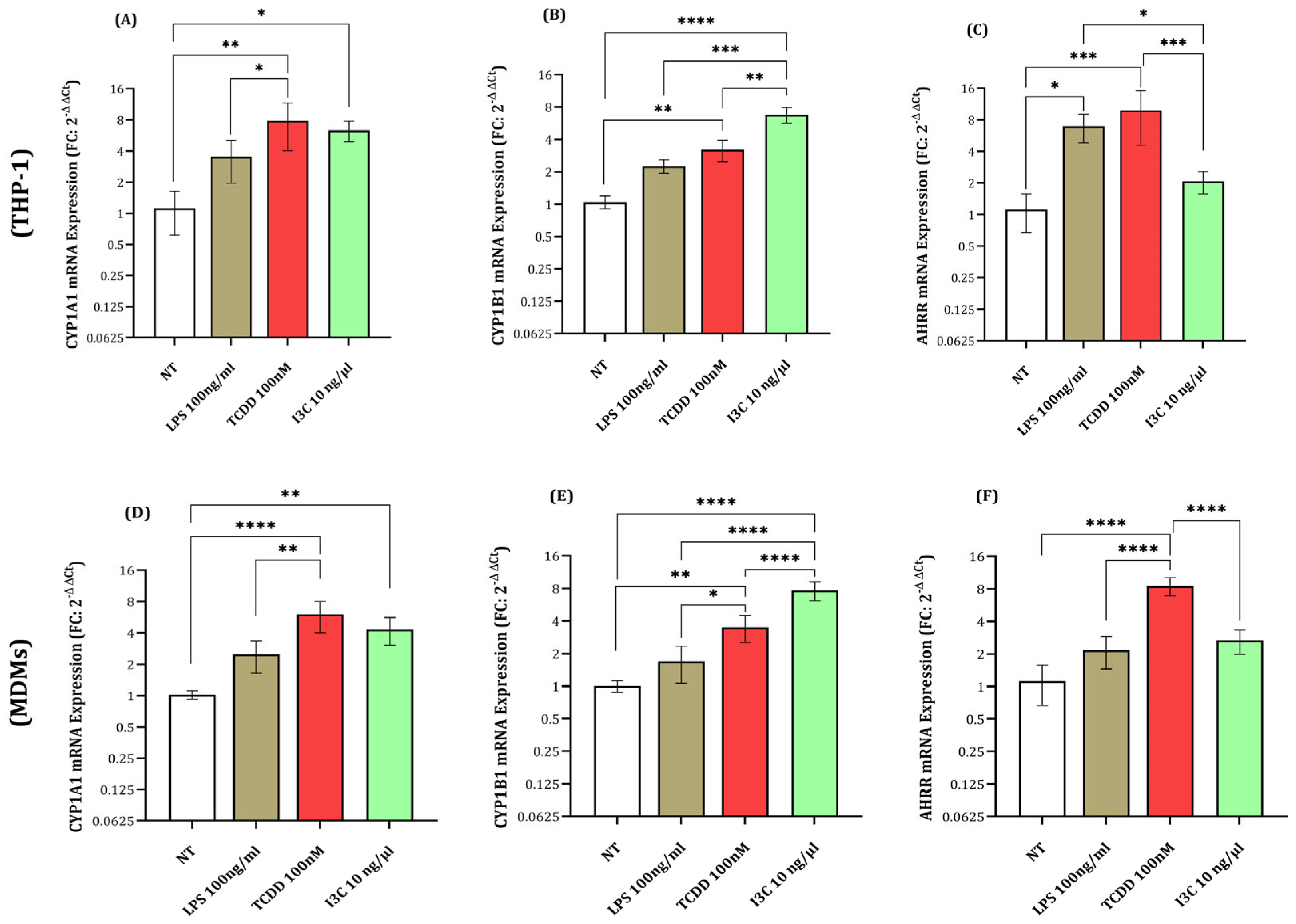
3.1. Expression of AHR Activation Genes (CYP1A1, CYP1B1, and AHRR) in Response to I3C, TCDD, and LPS
3.2. I3C Alters Macrophage Polarization: Increased CD163 Expression and Reduced CD16 and CD86 Levels
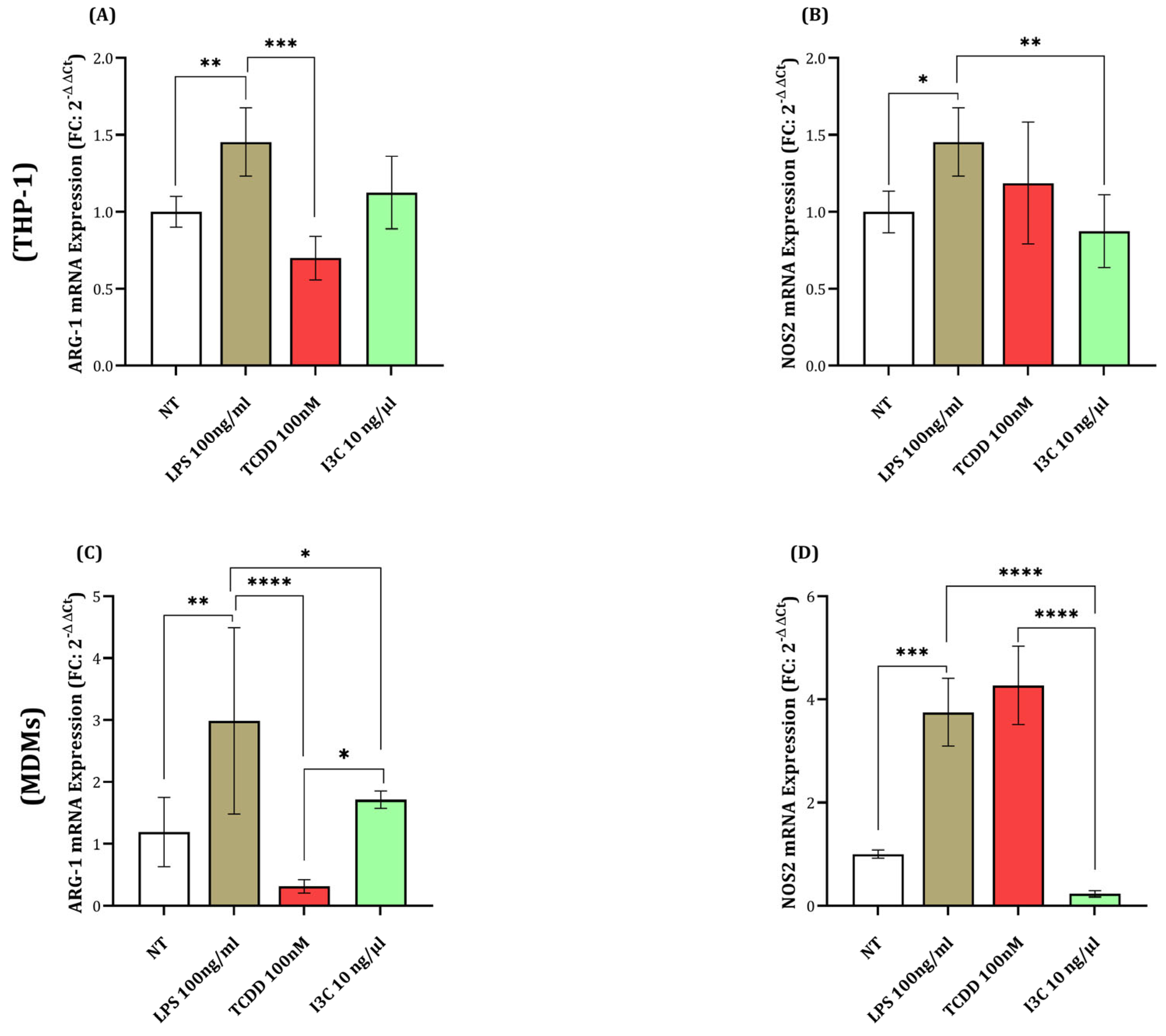
3.3. I3C Promotes ARG1 Expression While Reducing NOS2 Levels in Macrophages
3.4. I3C Treatment Results in Elevated IL-10 and TGF-β and Modulated IL-12 and TNF-α Levels in Macrophages
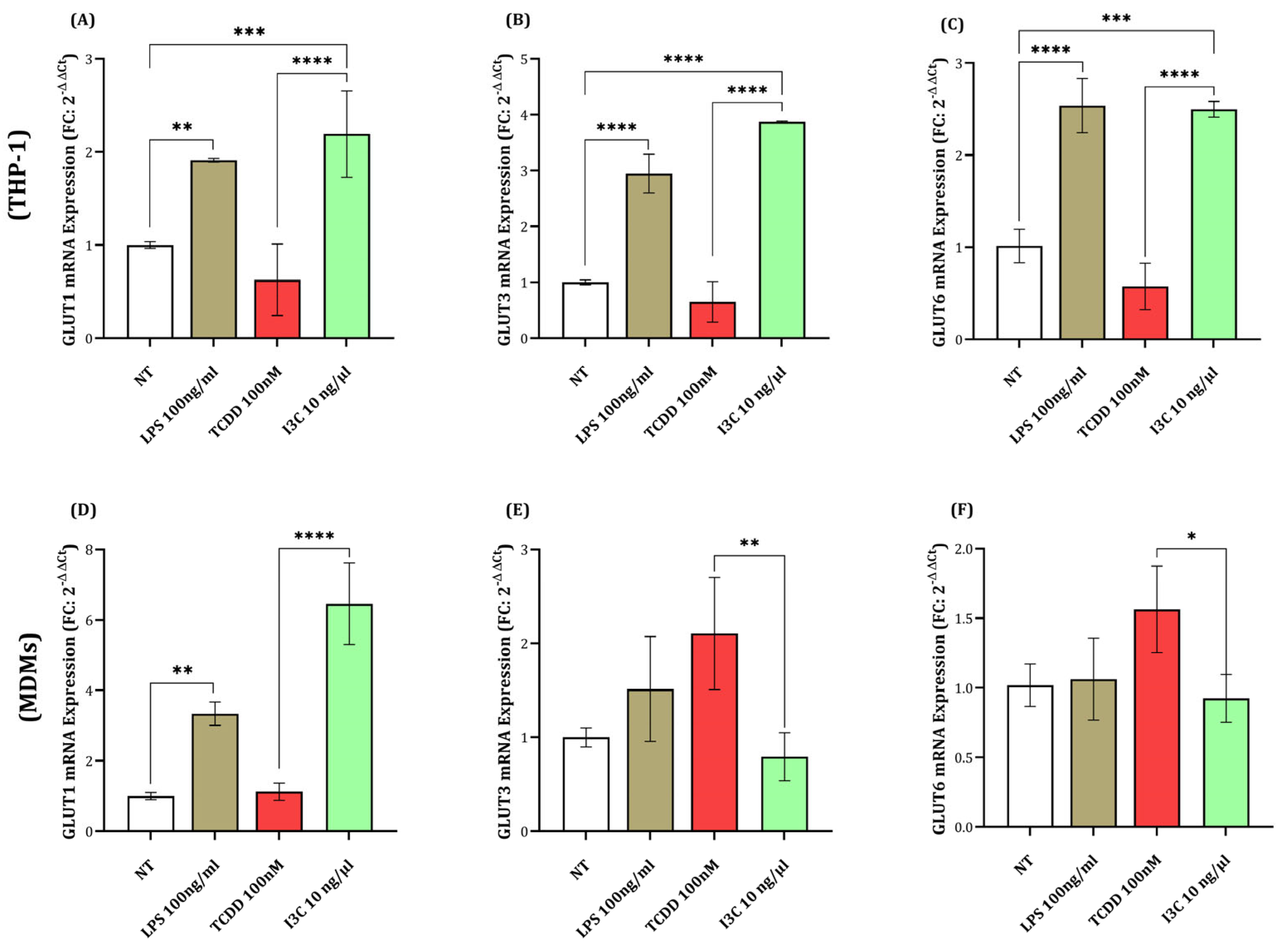
3.5. I3C-Induced Upregulation of GLUT1, GLUT3, and GLUT6 in Macrophages
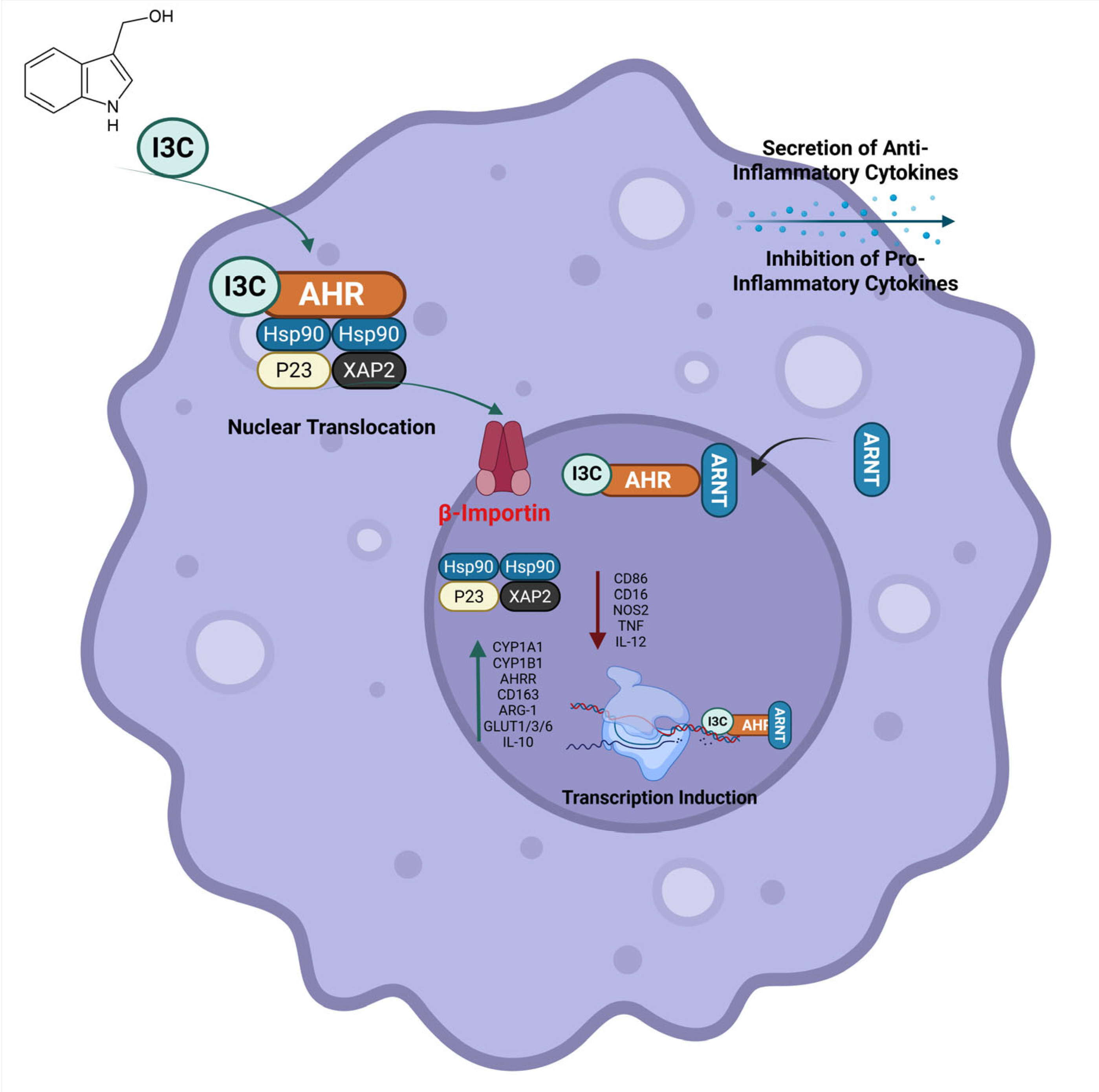
3.6. Indole-3-Carbinol Promotes Anti-Inflammatory Macrophage Polarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ta, W.; Chawla, A.; Pollard, J. Origins and hallmarks of macrophages: Development, homeostasis, and disease. Nature 2013, 496, 445–455. [Google Scholar]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Lichtnekert, J.; Kawakami, T.; Parks, W.C.; Duffield, J.S. Changes in macrophage phenotype as the immune response evolves. Curr. Opin. Pharmacol. 2013, 13, 555–564. [Google Scholar] [CrossRef]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Horhold, F.; Eisel, D.; Oswald, M.; Kolte, A.; Roll, D.; Osen, W.; Eichmuller, S.B.; Konig, R. Reprogramming of macrophages employing gene regulatory and metabolic network models. PLoS Comput. Biol. 2020, 16, e1007657. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Dahm, K.; Kammoun, H.L. Macrophage polarization in obesity and type 2 diabetes: Weighing down our understanding of macrophage function? Front. Immunol. 2014, 5, 470. [Google Scholar] [CrossRef]
- Stunault, M.I.; Bories, G.; Guinamard, R.R.; Ivanov, S. Metabolism plays a key role during macrophage activation. Mediat. Inflamm. 2018, 2018, 2426138. [Google Scholar] [CrossRef]
- Gauthier, T.; Chen, W. Modulation of macrophage immunometabolism: A new approach to fight infections. Front. Immunol. 2022, 13, 780839. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhang, T.; Ma, Y.; Tong, X.; Fan, H. The role of immunometabolism in macrophage polarization and its impact on acute lung injury/acute respiratory distress syndrome. Front. Immunol. 2023, 14, 1117548. [Google Scholar] [CrossRef]
- Caruana, B.T.; Byrne, F.L.; Knights, A.J.; Quinlan, K.G.R.; Hoehn, K.L. Characterization of Glucose Transporter 6 in Lipopolysaccharide-Induced Bone Marrow-Derived Macrophage Function. J. Immunol. 2019, 202, 1826–1832. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Newsholme, P.; Curi, R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma and burns. In Proceedings of the Biochemical Society Symposium, London, UK; 1987; pp. 145–162. [Google Scholar]
- Newsholme, P.; Gordon, S.; Newsholme, E.A. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem. J. 1987, 242, 631–636. [Google Scholar] [CrossRef]
- Jeon, E.H.; Park, T.S.; Jang, Y.; Hwang, E.; Kim, S.-J.; Song, K.-D.; Weinstein, D.A.; Lee, Y.M.; Park, B.-C.; Jun, H.S. Glucose-6-phosphate transporter mediates macrophage proliferation and functions by regulating glycolysis and mitochondrial respiration. Biochem. Biophys. Res. Commun. 2020, 524, 89–95. [Google Scholar] [CrossRef]
- Curi, R.; de Siqueira Mendes, R.; de Campos Crispin, L.A.; Norata, G.D.; Sampaio, S.C.; Newsholme, P. A past and present overview of macrophage metabolism and functional outcomes. Clin. Sci. 2017, 131, 1329–1342. [Google Scholar] [CrossRef]
- Quintana, F.J. The aryl hydrocarbon receptor: A molecular pathway for the environmental control of the immune response. Immunology 2013, 138, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Korecka, A.; Dona, A.; Lahiri, S.; Tett, A.J.; Al-Asmakh, M.; Braniste, V.; D’Arienzo, R.; Abbaspour, A.; Reichardt, N.; Fujii-Kuriyama, Y.; et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef]
- Mohammadi, S.; Memarian, A.; Sedighi, S.; Behnampour, N.; Yazdani, Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity 2018, 51, 199–209. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research 2015, 4, 1465. [Google Scholar] [CrossRef]
- Mohammadi, S.; Seyedhosseini, F.S.; Behnampour, N.; Yazdani, Y. Indole-3-carbinol induces G1 cell cycle arrest and apoptosis through aryl hydrocarbon receptor in THP-1 monocytic cell line. J. Recept. Signal Transduct. 2017, 37, 506–514. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sedighi, S.; Memarian, A.; Yazdani, Y.J.L. Overexpression of interferon-γ and indoleamine 2, 3-dioxygenase in systemic lupus erythematosus: Relationship with the disease activity. LaboratoriumsMedizin 2017, 41, 41–47. [Google Scholar] [CrossRef]
- Ociepa-Zawal, M.; Rubiś, B.; Laciński, M.; Trzeciak, W.H. The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim. Pol. 2007, 54, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Gargaro, M.; Scalisi, G.; Manni, G.; Mondanelli, G.; Grohmann, U.; Fallarino, F. The landscape of AhR regulators and coregulators to fine-tune AhR functions. Int. J. Mol. Sci. 2021, 22, 757. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.; Pimpão, A.; Correia, M.; Rodrigues, T.; Monteiro, E.; Morello, J.; Pereira, S. Pharmacological blockage of the AHR-CYP1A1 axis: A call for in vivo evidence. J. Mol. Med. 2022, 100, 215–243. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M.J. The central role of cytochrome P450 in xenobiotic metabolism—A brief review on a fascinating enzyme family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Guzman-Navarro, G.; León, M.B.d.; Martín-Estal, I.; Durán, R.C.-D.; Villarreal-Alvarado, L.; Vaquera-Vazquez, A.; Cuevas-Cerda, T.; Garza-García, K.; Cuervo-Pérez, L.E.; Barbosa-Quintana, Á.; et al. Prenatal indole-3-carbinol administration activates aryl hydrocarbon receptor-responsive genes and attenuates lung injury in a bronchopulmonary dysplasia model. Exp. Biol. Med. 2021, 246, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Uchida, K.; Fukushima, K.; Satoh, M.; Koyama, T.; Tsuchiya, M.; Saito, H.; Uchiyama, K.; Takahira, N.; Inoue, G.; et al. Correlation between CD163 expression and resting pain in patients with hip osteoarthritis: Possible contribution of CD163+ monocytes/macrophages to pain pathogenesis. J. Orthop. Res. 2022, 40, 1365–1374. [Google Scholar] [CrossRef]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, N.; Zhang, X.; Liu, Y.; Gao, X.; Wang, L. Study on the imbalance of M1/M2 macrophage polarization in severe chronic periodontitis. Technol. Health Care 2023, 31, 117–124. [Google Scholar] [CrossRef]
- Dixon, K.J.; Wu, J.; Walcheck, B. Engineering anti-tumor monoclonal antibodies and Fc receptors to enhance ADCC by human NK cells. Cancers 2021, 13, 312. [Google Scholar] [CrossRef]
- Hullsiek, R.; Li, Y.; Snyder, K.M.; Wang, S.; Di, D.; Borgatti, A.; Lee, C.; Moore, P.F.; Zhu, C.; Fattori, C.; et al. Examination of IgG Fc receptor CD16A and CD64 expression by canine leukocytes and their ADCC activity in engineered NK cells. Front. Immunol. 2022, 13, 841859. [Google Scholar] [CrossRef]
- Mir, M.; Albaradeh, R.; Agrewala, J. Innate–effector immune response elicitation against tuberculosis through anti-B7-1 (CD80) and anti-B7-2 (CD86) signaling in macrophages. Int. J. Phar. Biol Allied Sci. 2013, 2, 1024–1043. [Google Scholar]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ macrophages in inflammatory and malignant diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef]
- Gantzel, R.H.; Kjær, M.B.; Laursen, T.L.; Kazankov, K.; George, J.; Møller, H.J.; Grønbæk, H. Macrophage activation markers, soluble CD163 and mannose receptor, in liver fibrosis. Front. Med. 2021, 7, 615599. [Google Scholar] [CrossRef] [PubMed]
- Martí i Líndez, A.-A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Iacono, A.; Carvalho, A.; Orabona, C.; Volpi, C.; Pallotta, M.T.; Matino, D.; Esposito, S.; Grohmann, U. Amino acid metabolism as drug target in autoimmune diseases. Autoimmun. Rev. 2019, 18, 334–348. [Google Scholar] [CrossRef]
- Haydar, D.; Cory, T.J.; Birket, S.E.; Murphy, B.S.; Pennypacker, K.R.; Sinai, A.P.; Feola, D.J. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J. Immunol. 2019, 203, 1021–1030. [Google Scholar] [CrossRef]
- Hezaveh, K.; Shinde, R.S.; Klötgen, A.; Halaby, M.J.; Lamorte, S.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; Grünwald, B.T. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 2022, 55, 324–340.e328. [Google Scholar] [CrossRef]
- Shreshtha, S.; Sharma, P.; Kumar, P.; Sharma, R.; Singh, S. Nitric Oxide: It’s Role Immunity. J. Clin. Diagn. Res. 2018, 12. [Google Scholar] [CrossRef]
- Rostoka, E.; Isajevs, S.; Baumane, L.; Line, A.; Silina, K.; Dzintare, M.; Sharipova, J.; Svirina, D.; Kalvinsh, I.; Sjakste, N. Effects of lycopene, indole-3-carbinol, and luteolin on nitric oxide production and iNOS expression are organ-specific in rats. Arh. Za Hig. Rada I Toksikol. 2010, 61, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, M.; Hsu, C.; Liu, M.; Chan, M.; Chen, Y.-H. Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes. Int. J. Obes. 2011, 35, 1530–1538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emampanahi, M.; Masoudi Rad, S.; Saghaeian Jazi, M.; Mansour Samaei, N.; Behnampour, N.; Mohammadi, S.; Fakhari, E. Association between interleukin-10 gene polymorphisms and severe chronic periodontitis. Oral Dis. 2019, 25, 1619–1626. [Google Scholar] [CrossRef]
- Mohammadi, S.; Saghaeian Jazi, M.; Zare Ebrahimabad, M.; Eghbalpour, F.; Abdolahi, N.; Tabarraei, A.; Yazdani, Y. Interleukin 10 gene promoter polymorphisms (rs1800896, rs1800871 and rs1800872) and haplotypes are associated with the activity of systemic lupus erythematosus and IL10 levels in an Iranian population. Int. J. Immunogenet. 2019, 46, 20–30. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Zhao, X.; Di, Q.; Liu, H.; Quan, J.; Ling, J.; Zhao, Z.; Xiao, Y.; Wu, H.; Wu, Z.; Song, W.; et al. MEF2C promotes M1 macrophage polarization and Th1 responses. Cell. Mol. Immunol. 2022, 19, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. M1 and M2 polarization of macrophages: A mini-review. Med Biol. Sci. Eng. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage immunometabolism: Where are we (going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Shapiro, H.; Lutaty, A.; Ariel, A. Macrophages, meta-inflammation, and immuno-metabolism. Sci. World J. 2011, 11, 2509–2529. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef]
- Cheng, J.; Cai, W.; Zong, S.; Yu, Y.; Wei, F. Metabolite transporters as regulators of macrophage polarization. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 395, 13–25. [Google Scholar] [CrossRef]
- Bai, G.; Matsuba, T.; Niki, T.; Hattori, T. Stimulation of THP-1 macrophages with LPS increased the production of osteopontin-encapsulating exosome. Int. J. Mol. Sci. 2020, 21, 8490. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Jung, H.; Yang, H.; Yoo, M.; Kim, C.; Kim, K.J. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 2007, 56, 45–50. [Google Scholar] [CrossRef]
- Schliefsteiner, C.; Ibesich, S.; Wadsack, C. Placental Hofbauer cell polarization resists inflammatory cues in vitro. Int. J. Mol. Sci. 2020, 21, 736. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E. Indoles derived from glucobrassicin: Cancer chemoprevention by indole-3-carbinol and 3, 3’-diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef]
- Banerjee, S.; Kong, D.; Wang, Z.; Bao, B.; Hillman, G.G.; Sarkar, F.H. Attenuation of multi-targeted proliferation-linked signaling by 3, 3′-diindolylmethane (DIM): From bench to clinic. Mutat. Res. Mol. Mech. Mutagen. 2011, 728, 47–66. [Google Scholar] [CrossRef]
- Rasheed, A.; Rayner, K.J. Macrophage responses to environmental stimuli during homeostasis and disease. Endocr. Rev. 2021, 42, 407–435. [Google Scholar] [CrossRef]



| Gene ID | Gene Name | Forward Primer Sequence | Reverse Primer Sequence | Tm (°C) | Product Size (bp) |
|---|---|---|---|---|---|
| 1543 | CYP1A1 | CTCCTCAACCTCCTGCTACC | GTCACTGATACCACCACATACC | 59 | 129 |
| 1545 | CYP1B1 | GCTCACCAGTGCGATTTCA | GCTTGCCTCTTGCTTCTTATTG | 59 | 187 |
| 383 | ARG1 | GGTGGCAGAAGTCAAGAAGAAC | GTGGTTGTCAGTGGAGTGTTG | 60 | 159 |
| 3593 | IL-12B | CAGGAGGTCAAGGCTATGGT | ACTTACGGTGTTTCTGTGTCAT | 59 | 181 |
| 57491 | AHRR | CGGGATGTTGCCCAAAAGTG | AAGCGTGTGTCTTAGGACGG | 61 | 182 |
| 4843 | NOS2 | GCAGAGAACTCAGCCTCATTC | GGAAGAGACCTGTGCCTTGA | 60.0 | 107 |
| 3586 | IL-10 | ACATCAGGGTGGCGACTC | CGTTCACAGAGAAGCTCAGTAA | 59.0 | 185 |
| 6513 | GLUT1 | CACCACCTCACTCCTGTTACT | TTCTGTCTCACTCCCATCCAAA | 59 | 115 |
| 6515 | GLUT3 | TCCCCTCCGCTGCTCACTATTT | ATCTCCATGACGCCGTCCTTTC | 61.0 | 190 |
| 11182 | GLUT6 | TCACCAAGTCCTTCCTGCCAGT | CACAGCAGCCTGTGAACACCAG | 61 | 105 |
| 2597 | GAPDH | GAAATCCCATCACCATCTTC | TCTGAGTGGCAGTGATGTC | 60.0 | 345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omrani, D.; Mohammadi, S.; Malekzadeh, M.; Saeidi, M.; Seyedhosseini, F.S.; Al-Harrasi, A.; Yazdani, Y. Indole-3-Carbinol Enhances Alternative Activation of Macrophages via AHR Pathway and Glucose Transporter Regulation. Immuno 2025, 5, 15. https://doi.org/10.3390/immuno5020015
Omrani D, Mohammadi S, Malekzadeh M, Saeidi M, Seyedhosseini FS, Al-Harrasi A, Yazdani Y. Indole-3-Carbinol Enhances Alternative Activation of Macrophages via AHR Pathway and Glucose Transporter Regulation. Immuno. 2025; 5(2):15. https://doi.org/10.3390/immuno5020015
Chicago/Turabian StyleOmrani, Delara, Saeed Mohammadi, Moein Malekzadeh, Mohsen Saeidi, Fakhri Sadat Seyedhosseini, Ahmed Al-Harrasi, and Yaghoub Yazdani. 2025. "Indole-3-Carbinol Enhances Alternative Activation of Macrophages via AHR Pathway and Glucose Transporter Regulation" Immuno 5, no. 2: 15. https://doi.org/10.3390/immuno5020015
APA StyleOmrani, D., Mohammadi, S., Malekzadeh, M., Saeidi, M., Seyedhosseini, F. S., Al-Harrasi, A., & Yazdani, Y. (2025). Indole-3-Carbinol Enhances Alternative Activation of Macrophages via AHR Pathway and Glucose Transporter Regulation. Immuno, 5(2), 15. https://doi.org/10.3390/immuno5020015








