Pre- and Post-Transplant Anti-BKV IgG Responses and HLA Associations in BK Virus Reactivation Among Renal Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Recruitment
2.2. Sample Collection and Storage
- Pre-transplant: Blood samples were obtained before transplantation to assess baseline anti-BKV IgG levels and HLA typing.
- During viremia onset: In patients who developed BK viremia, additional blood samples were collected at the time of confirmed viremia detection.
- Post-transplant: A third sample was obtained at least one year post-transplant to evaluate long-term changes in anti-BKV IgG levels.
2.3. Laboratory Procedures
Detection of BK Virus DNA in Blood
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Anti-BKV IgG
2.5. HLA Typing
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Patient Demographics and Clinical Characteristics
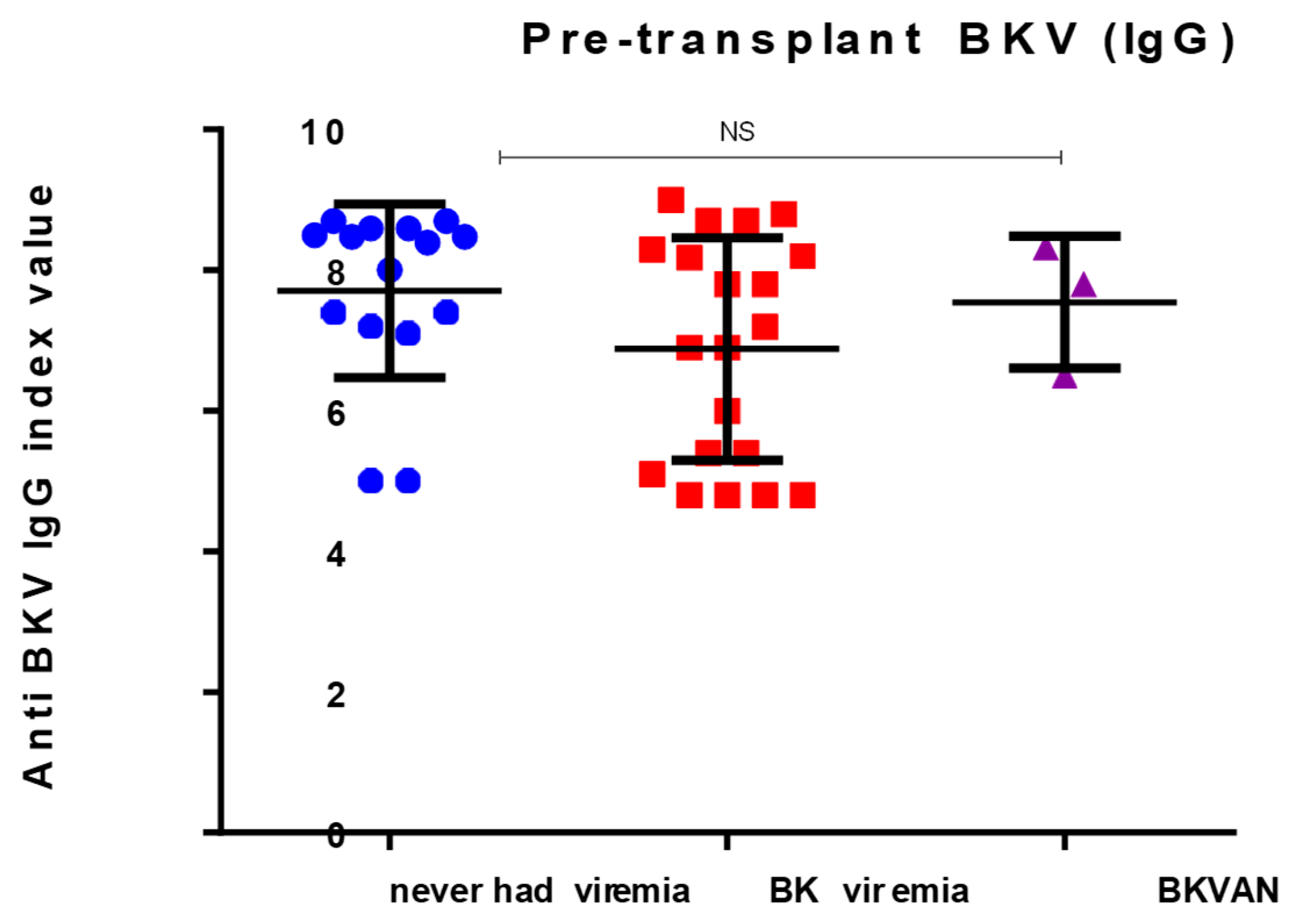
3.2. Pre- and Post-Transplant Anti-BKV IgG Levels
3.3. Longitudinal Changes in Anti-BKV IgG Levels
3.4. Association Between HLA Alleles and BK Viremia
3.5. Multivariate Analysis of Risk Factors for BK Viremia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuwirt, H.; Rudnicki, M.; Schratzberger, P.; Pirklbauer, M.; Kronbichler, A.; Mayer, G. Immunosuppression after renal transplantation. memo—Mag. Eur. Med. Oncol. 2019, 12, 216–221. [Google Scholar] [CrossRef]
- Asif, R.U.; Ghani, E.; Rathore, M.A.; Mushtaq, S.; Ahmed, F.; Hussain, H. Evaluation of new-onset BK viruria in post-renal transplant recipients by quantitative PCR. Transpl. Immunol. 2024, 87, 102136. [Google Scholar] [CrossRef]
- Cohen-Bucay, A.; Ramirez-Andrade, S.E.; Gordon, C.E.; Francis, J.M.; Chitalia, V.C. Advances in BK Virus Complications in Organ Transplantation and Beyond. Kidney Med. 2020, 2, 771–786. [Google Scholar] [CrossRef]
- Kant, S.; Dasgupta, A.; Bagnasco, S.; Brennan, D.C. BK Virus Nephropathy in Kidney Transplantation: A State-of-the-Art Review. Viruses 2022, 14, 1616. [Google Scholar] [CrossRef]
- Wong, G.; Marsh, J.; Howell, M.; Lim, W.H.; Chadban, S.; Coates, T.; Hawley, C.; Campbell, S.; Larkins, N.; Snelling, T.; et al. Screening and Management Practices for Polyoma (BK) Viremia and Nephropathy in Kidney Transplant Recipients From the Lands Down Under: Addressing the Unknowns and Rationale for a Multicenter Clinical Trial. Kidney Int. Rep. 2020, 5, 1777–1780. [Google Scholar] [CrossRef]
- Dalianis, T.; Eriksson, B.-M.; Felldin, M.; Friman, V.; Hammarin, A.-L.; Herthelius, M.; Ljungman, P.; Mölne, J.; Wennberg, L.; Swartling, L. Management of BK-virus infection—Swedish recommendations. Infect. Dis. 2019, 51, 479–484. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, C.; Wang, Y.; Yu, Z.; Wu, Z.; Zhou, Y.; Yan, Z.; Luo, J.; Xia, R.; Zeng, W.; et al. Dynamic risk prediction of BK polyomavirus reactivation after renal transplantation. Front. Immunol. 2022, 13, 971531. [Google Scholar] [CrossRef]
- Blazquez-Navarro, A.; Roch, T.; Wehler, P.; Stervbo, U.; Bauer, C.; Wolk, K.; Sabat, R.; Dang-Heine, C.; Thomusch, O.; Reinke, P.; et al. Lack of predictive capacity of pre-transplant anti-BK virus antibodies for post-transplant reactivation. J. Nephrol. 2023, 36, 1071–1073. [Google Scholar] [CrossRef]
- Nourie, N.; Boueri, C.; Tran Minh, H.; Divard, G.; Lefaucheur, C.; Salmona, M.; Gressens, S.B.; Louis, K. BK Polyomavirus Infection in Kidney Transplantation: A Comprehensive Review of Current Challenges and Future Directions. Int. J. Mol. Sci. 2024, 25, 12801. [Google Scholar] [CrossRef]
- Demey, B.; Bentz, M.; Descamps, V.; Morel, V.; Francois, C.; Castelain, S.; Helle, F.; Brochot, E. BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation. Int. J. Mol. Sci. 2022, 23, 7240. [Google Scholar] [CrossRef]
- Hisadome, Y.; Noguchi, H.; Nakafusa, Y.; Sakihama, K.; Mei, T.; Kaku, K.; Okabe, Y.; Masutani, K.; Ohara, Y.; Ikeda, K.; et al. Association of Pretransplant BK Polyomavirus Antibody Status with BK Polyomavirus Infection After Kidney Transplantation: A Prospective Cohort Pilot Study of 47 Transplant Recipients. Transplant. Proc. 2020, 52, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Jung, S.; Chung, B.H.; Yang, C.W.; Oh, E.J. Pretransplant BKV-IgG serostatus and BKV-specific ELISPOT assays to predict BKV infection after kidney transplantation. Front. Immunol. 2023, 14, 1243912. [Google Scholar] [CrossRef] [PubMed]
- Dakroub, F.; Touzé, A.; Akl, H.; Brochot, E. Pre-transplantation assessment of BK virus serostatus: Significance, current methods, and obstacles. Viruses 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Saláková, M.; Ludvíková, V.; Hamšíková, E.; Kolářová, M.; Šroller, V.; Viklický, O.; Wohlfahrtová, M. Pretransplantation seroreactivity in kidney donors and recipients as a predictive factor for posttransplant BKPyV-DNAemia. Front. Immunol. 2022, 13, 929946. [Google Scholar] [CrossRef]
- Chong, S.M.Y.; Hung, R.K.Y.; Yuen Chang, F.; Atkinson, C.; Fernando, R.; Harber, M.; Magee, C.N.; Salama, A.D.; Reeves, M. Composition of the neutralising antibody response predicts risk of BK virus DNAaemia in recipients of kidney transplants. eBioMedicine 2024, 110, 105430. [Google Scholar] [CrossRef]
- Mahdil, B.M. A glow of HLA typing in organ transplantation. Clin. Transl. Sci. 2013, 2, 6. [Google Scholar] [CrossRef]
- Kamenaric, M.B.; Ivkovic, V.; Vojtusek, I.K.; Zunec, R.; Burek Kamenaric, M.; Ivkovic, V.; Kovacevic Vojtusek, I.; Zunec, R. The role of hla and kir immunogenetics in bk virus infection after kidney transplantation. Viruses 2020, 12, 1417. [Google Scholar] [CrossRef]
- Bohl, D.L.; Storch, G.A.; Ryschkewitsch, C.; Gaudreault-Keener, M.; Mark, A.S.; Major, E.O.; Brennan, D.C. Donor Origin of BK Virus in Renal Transplantation and Role of HLA C7 in Susceptibility to Sustained BK Viremia. Am. J. Transplant. 2005, 5, 2213–2221. [Google Scholar] [CrossRef]
- Masutani, K.; Ninomiya, T.; Randhawa, P. HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol. Dial. Transplant. 2013, 28, 3119–3126. [Google Scholar] [CrossRef]
- Solis, M.; Velay, A.; Porcher, R.; Domingo-Calap, P.; Soulier, E.; Joly, M.; Meddeb, M.; Kack-Kack, W.; Moulin, B.; Bahram, S.; et al. Neutralizing Antibody–Mediated Response and Risk of BK Virus–Associated Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 326–334. [Google Scholar] [CrossRef]
- Šťastná-Marková, M.; Hamšíková, E.; Hainz, P.; Hubáček, P.; Kroutilová, M.; Kryštofová, J.; Ludvíková, V.; Musil, J.; Pecherková, P.; Saláková, M.; et al. Pretransplant BK Virus-Specific T-Cell-Mediated Immunity and Serotype Specific Antibodies May Have Utility in Identifying Patients at Risk of BK Virus-Associated Haemorrhagic Cystitis after Allogeneic HSCT. Vaccines 2021, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Bohl, D.L.; Brennan, D.C.; Ryschkewitsch, C.; Gaudreault-Keener, M.; Major, E.O.; Storch, G.A. BK virus antibody titers and intensity of infections after renal transplantation. J. Clin. Virol. 2008, 43, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.; Bohl, D.; Brennan, D.; Ruppert, K.; Ramaswami, B.; Storch, G.; March, J.; Shapiro, R.; Viscidi, R. Longitudinal Analysis of Levels of Immunoglobulins against BK Virus Capsid Proteins in Kidney Transplant Recipients. Clin. Vaccine Immunol. 2008, 15, 1564–1571. [Google Scholar] [CrossRef]
- Binggeli, S.; Egli, A.; Schaub, S.; Binet, I.; Mayr, M.; Steiger, J.; Hirsch, H.H. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am. J. Transplant. 2007, 7, 1131–1139. [Google Scholar] [CrossRef]
- Schachtner, T.; Müller, K.; Stein, M.; Diezemann, C.; Sefrin, A.; Babel, N.; Reinke, P. BK virus-specific immunity kinetics: A predictor of recovery from polyomavirus BK-associated nephropathy. Am. J. Transplant. 2011, 11, 2443–2452. [Google Scholar] [CrossRef]
- Teutsch, K.; Schweitzer, F.; Knops, E.; Kaiser, R.; Pfister, H.; Verheyen, J.; Göbel, H.; Cingöz, T.; Di Cristanziano, V. Early identification of renal transplant recipients with high risk of polyomavirus-associated nephropathy. Med. Microbiol. Immunol. 2015, 204, 657–664. [Google Scholar] [CrossRef]
- Couture, A.; Garnier, A.; Docagne, F.; Boyer, O.; Vivien, D.; Le-Mauff, B.; Latouche, J.B.; Toutirais, O. HLA-class II artificial antigen presenting cells in CD4+ T cell-based immunotherapy. Front. Immunol. 2019, 10, 1081. [Google Scholar] [CrossRef]




| Parameters | Recipients = 38 | Viremia = 18 | |||||
|---|---|---|---|---|---|---|---|
| Non-Viremia | Viremia | Low Viral Loads | p-Value | No BKVAN | BKVAN | p-Value (p < 0.05) | |
| No. patients (%) | 15 (39.5) | 18 (47.4) | 5 (13) | 15 (83.3) | 3 (16.6) | ||
| Patient’s sex (male) | 8 (53.3) | 12 (66.6) | 2 (40) | 0.2 | 10 (66.6) | 2 (66.6) | NS |
| Age (mean ±/− SD) | 58.17 ± 14.7 | 56 ± 13.7 | 59.6 ± 12.6 | 0.8 | 57 ± 11 | 46.6 ± 5 | NS |
| First → graft (%) | 11 (73.3) | 17 (100) | 4 (80) | NS | 15 (100) | 3 (100) | NS |
| Underlying condition (%) | |||||||
| Obstructive urology (%) | 3 (20) | 0 | 1 (20) | NS | NS | ||
| Hypertension (%) | 2 (13) | 6 (35) | 2 (40) | NS | 6 (40) | 0 | NS |
| Polycystic kidney (%) | 4 (26.6) | 1 (5.8) | 0 | NS | 1 (6.6) | 0 | NS |
| Other (%) | 6 (40.0) | 11 (58.8) | 2 | NS | 8 (60) | 3 | NS |
| Induction of Immune Suppression (%) | |||||||
| Campath | 70.9 | 71.5 | 40 | NS | 12 (80) | 3 (100) | |
| Simulect | 29.1 | 28.5 | 60 | NS | 3 (20) | 0 | |
| Maintenance suppression (%) | |||||||
| MMF + Advagraf | 4 (16) | 2 (28) | 1 (20) | NS | 1 (6.6) | 1 (30) | |
| Azathioprine | 4 (16) | 4 (22) | 0 | NS | 3 (20) | 1 (30) | |
| MMF + prograf | 3 (12.5) | 2 (11) | 1 (20) | NS | 2 (13) | 0 | |
| Advagraf (%) | 2 (8.3) | 2 (28) | 1 (20) | NS | 5 (30) | 1 (30) | |
| MMF | 6 (25) | 3 (42) | 2 (40) | NS | 3 (20) | 0 | |
| MMF + TAC (%) | 5 (20) | 1 (5) | 0 | 1 (6.6) | 0 | ||
| HLA mismatch mean ± SD | |||||||
| A | 0.88 ± 0.58 | 0.88 ± 0.78 | 1 ± 0.7 | NS | 0.92 ± 0.37 | 1 | |
| B | 0.77 ± 0.54 | 0.77 ± 0.83 | 0.4 ± 0.54 | NS | 0.84 ± 0.3 | 0.66 ± 0.033 | |
| DR | 0.66 ± 0.68 | 0.44 ± 0.72 | 0.6 ± 0.54 | NS | 0.92 ± 0.57 | 0.66 ± 33 | 0.4 |
| A + B + DR | 2.2 ± 1.4 | 2.1 ± 1.9 | 2.4 ± 1.5 | NS | 2.7 ± 1.9 | 2.3 ± 1.33 | |
| Acute rejection episode (%) | 3 (12.5) | 3 (16.6) | 1 (33) | ||||
| Post-operation period (months) | 3.5 | 10 | |||||
| BKV Status | Pre-Transplant Seroprevalence (%) | Pre- Transplant Ab Titre | Post Operation > 1 Year | p- Value |
|---|---|---|---|---|
| Never BKV | 66.6 | 7.704 ± 0.31 | 4.627 ± 2.9 | 0.7 |
| BKV viremia | 94.4 | 6.87± 0.32 | 10.21 ± 0.5 | <0.05 |
| BVPyAN | 100 | 7.5 ± 0.94 | 10.50 ± 0.23 | <0.04 |
| p = 0.14 |
| BKV Viremia N = 23 (%) | No Viremia N = 15 (%) | p Value (p < 0.05) | |
|---|---|---|---|
| HLA-A1 (%) | 6 (26) | 6 (40) | 0.7 |
| A2 | 6 (26) | 5 (33) | 0.8 |
| HLA-B8 | 2 (8.6) | 5 (33) | 0.2 |
| B44 | 14 (60) | 3 (20) | 0.02 * |
| B51 | 1 (4.3) | 1 (6.6) | 1 |
| HLA-DR1 | 6 (26) | 2 (13) | 0.6 |
| DR3 | 2 (8.6) | 2 (13) | 1 |
| DR4 | 7 (30) | 2 (13) | 0.3 |
| DR7 | 2 (8.6) | 4 (26) | 0.37 |
| DR11 | 1 (4.3) | 2 (13) | 0.5 |
| DR13 | 1 (4.3) | 4 (26) | 0.15 |
| DR15 | 11 (47) | 1 (6.6) | 0.01 * |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 0.46 | 0.009–0.15 | 0.6 |
| Gender | 0.42 | 0.63–1.12 | 0.08 |
| HLA-B44 | 0.296 | 0.11–0.73 | 0.009 |
| HLA-DR15 | 0.44 | 0.18–1.08 | 0.07 |
| HLA-A2 | 0.51–3.20 | 0.59 | |
| HLA-mismatch | 0.28 | ||
| A | 2.9 | 0.6–13.7 | 0.177 |
| B | 2.3 | 0.06–27 | 0.489 |
| DR | 0.18 | 0.22–1.5 | 0.115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallatah, D.I.; Christmas, S. Pre- and Post-Transplant Anti-BKV IgG Responses and HLA Associations in BK Virus Reactivation Among Renal Transplant Recipients. Immuno 2025, 5, 16. https://doi.org/10.3390/immuno5020016
Fallatah DI, Christmas S. Pre- and Post-Transplant Anti-BKV IgG Responses and HLA Associations in BK Virus Reactivation Among Renal Transplant Recipients. Immuno. 2025; 5(2):16. https://doi.org/10.3390/immuno5020016
Chicago/Turabian StyleFallatah, Deema Ibrahim, and Steve Christmas. 2025. "Pre- and Post-Transplant Anti-BKV IgG Responses and HLA Associations in BK Virus Reactivation Among Renal Transplant Recipients" Immuno 5, no. 2: 16. https://doi.org/10.3390/immuno5020016
APA StyleFallatah, D. I., & Christmas, S. (2025). Pre- and Post-Transplant Anti-BKV IgG Responses and HLA Associations in BK Virus Reactivation Among Renal Transplant Recipients. Immuno, 5(2), 16. https://doi.org/10.3390/immuno5020016





