Abstract
Mastic oil (MO) is extracted from the resin of the bark of Pistacia lentiscus var. chia, a tree abundantly grown in the Greek island of Chios. Various biological activities, such as antimicrobial, anticancer and antioxidant, have been associated with the dietary intake of MO. However, little is known about MO’s potential anti-inflammatory effects, while some of its main chemical constituents were reported to exert significant anti-inflammatory activity. This study aims to assay the bioactivity of MO on in vitro and in vivo experimental inflammation models, in particular on LPS-stimulated RAW264.7 macrophages, murine primary peritoneal macrophages and a model of zymosan-induced peritonitis in BALB/c mice. The per os administration of MO inhibited the recruitment of macrophages into the peritoneal cavity of zymosan-treated mice, but did not affect neutrophil mobilisation or the levels of IL-6 or TNF-α in the peritoneal fluid. Similarly, IL-6 and TNF-α secretion in primary LPS-stimulated macrophages was not affected by MO, but the levels of phosphoproteins that activate inflammation in macrophages were differentially regulated. Finally, MO and some of its individual constituents reduced nitric oxide (NO), prostaglandin E2 and TNF-α levels in supernatants of LPS-stimulated RAW264.7 cells and inhibited their phagocytosis rate. Our data imply that MO may promote an anti-inflammatory transition in macrophages due to the combined bioactivities of its individual constituents. Thus, as a mixture of various compounds, MO seems to affect multiple molecular mechanisms that are involved in the development of inflammation. Therefore, more research, focusing on MO’s individual constituents and employing various pre-clinical inflammation models that activate different mechanisms, is required for a detailed investigation of the oil’s potential anti-inflammatory activity.
1. Introduction
Inflammation is a biological response that aims at eliminating a cell injury source, which could either be physical or chemically-induced tissue injury or invading pathogens [1]. Overall, inflammation is a protective mechanism that helps the organism by eliminating harmful agents and initiating the healing process. However, repetitive exposure to triggering stimuli or unresolved inflammation may lead to chronic conditions associated with a broad range of diseases [2]. The most obvious group of such conditions is autoimmune diseases such as rheumatoid arthritis, diabetes, inflammatory bowel disease, gout and systemic lupus erythematosus [3]. Other inflammation-related diseases are atherosclerosis [4], neurodegenerative diseases including Alzheimer’s disease [5], Parkinson’s disease [6] and amyotrophic lateral sclerosis (ALS) [7], liver [8], lung [9], kidney [10] and skin diseases [11] and, of course, cancer [1,12].
Many of the anti-inflammatory medications currently used are nonsteroidal anti-inflammatory drugs (NSAIDs). Hippocrates used an extract from parts of the willow tree almost 3500 years ago to treat inflammation, but it was not until the late 1700s when the active compound in this extract was identified as salicylic acid (aspirin) [13]. Aspirin and ibuprofen are among the most-used NSAIDs whose mechanism of action, as we now know, is the inhibition of the COX enzyme that catalyses the conversion of arachidonic acid to prostaglandins E2, prostacyclins and thromboxanes [14]. Despite their use for over a century, it is well known that COX inhibitors are associated with a number of side effects, most of which are accompanied by gastrointestinal symptoms, while kidney and liver problems have also been reported [13]. Thus, the discovery of new anti-inflammatory compounds is of great interest and, once again, the plant kingdom poses as the most valuable source of novel substances in drug development.
Phytochemicals have long been studied for their biological properties [15,16,17,18,19,20,21]. Some of the most prominent plant-derived agents with anti-inflammatory activity are resveratrol, capsaicin, quercetin, curcumin, epigallocatechin-3-gallate (EGCG) and colchicine [22]. In fact, there is a considerable amount of literature on the anti-inflammatory activity of plant-derived compounds [23,24,25,26,27,28,29]. The bioactivities of herbal and plant extracts are related to the presence of molecules of various chemical classes. In certain essential oils, volatile terpenes and terpenoid constituents have been shown to exert a wide range of bioactivities [30,31]. Essential oils are concentrated hydrophobic liquids, extracted from various plants parts that are rich in volatile compounds.
Mastic oil (MO), the essential oil extracted from the resin of the plant Pistacia lentiscus var. chia, is rich in terpenes and terpenoids [20,32]. Pistacia lentiscus var. chia is a plant that has been cultivated for its aromatic resin mostly in the southern part of the island of Chios in Greece. MO is extracted from the resin that is released from the incised bark of the aromatic plant. This resin, known as mastic gum, apart from being traditionally used as a flavouring agent, has also been incorporated into folk medicine, mainly for the treatment of gastrointestinal disorders. Besides Greece, different Pistacia lentiscus L. species have long been cultivated in the Mediterranean and Middle Eastern areas. Extracts of the plant have been traditionally used by various ethnic groups against respiratory, cardiovascular, renal, oral cavity and tooth diseases and as antimicrobial, antipyretic and analgesic remedies [30].
Mastic gum (MG) preparations have been shown to exert significant anti-inflammatory activities that are connected to MG’s strong antioxidant properties. By blocking the PKC-dependent activation of NADPH oxidases, MG led to the inhibition of TNF-α or angiotensin II-induced hydrogen peroxide and superoxide production in endothelial and smooth muscle cells [33]. MG’s anti-inflammatory activity seems to be partly mediated by the inhibition of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) expression, as observed in macrophages, and the suppression of the adhesion molecules VCAM-1 and ICAM-1, as shown in TNF-α-stimulated endothelial cells, resulting in the inhibition of pro-inflammatory interleukins and TNF-α production [34,35]. Further in vitro studies have demonstrated that MG inhibits the production of pro-inflammatory molecules such as nitric oxide (NO) and prostaglandin E2 (PGE2) by activated macrophages, mainly via the inhibition of iNOS and COX-2 protein expression rather than by NO scavenging [36]. Besides macrophages, MG has been shown to exert anti-inflammatory properties in human aortic endothelial cells as well [37].
The potential anti-inflammatory activity of MG has also been investigated in in vivo experimental models. The local application of MG ointment attenuated inflammatory responses in murine experimental models of allergic dermatitis. Reduced ear swelling and itching behaviour as well as the downregulation of IL-1β, IL-33, TARC and TSLP levels in skin tissue were observed. Moreover, the migration of dendritic cells, helper T cells and IgE-positive B cells was inhibited [38].
In a gastric ulcer model in rats, MG reduced gastric and colonic mucosal hyperaemia and haemorrhagic infiltration as well as mucosal oedema to the same extent as the control standard drug, omeprazole. Moreover, MG significantly lowered the serum TNF-α and IL-1β levels [39]. Similarly in rats, in an acetic acid-induced colitis model, MG inhibited TNF-α levels in colon tissue and significantly limited the overall colitis index [40]. In another study employing a different rat colitis model (trinito-benzene sulfonic acid-induced), all TNF-α, IL-6, IL-8, ICAM-1 and malonaldehyde (MDA) tissue levels were inhibited. Accordingly, in MG-treated mice, the significant amelioration of colitis extracts was observed histologically [41].
Human studies further support these findings [34,35]. Orally administered MG, in patients with mild or moderately active Crohn’s disease, inhibited IL-6 and CRP (C-reactive protein) plasma levels, while the activity index of the disease was significantly limited [42]. Also in patients with inflammatory bowel disease (IBD), MG supplementation seems to induce favourable changes in oxidative stress biomarkers such as a decrease in oxidised LDL, plasma cysteine and faecal lysozyme [43,44] and elevated IL-17A serum levels [45].
It becomes evident that there is now substantial evidence for the anti-inflammatory properties of mastic gum. However, most studies tend to focus on the resin, the crystallised natural form of mastic gum. Notably, its essential oil represents a superior, more concentrated source of the bioactive compounds present in mastic resin. Nevertheless, in the literature, there seem to be no studies assessing the anti-inflammatory properties of MO. Maxia et al. described the anti-inflammatory properties exerted by the essential oil extracted from the leaves of P. lentiscus in carrageenan-induced paw oedema and cotton-pellet-induced granuloma rat models. In both models, the essential oil inhibited serum TNF-α and IL-6 levels [46]. The oil extracted from the fruit of the plant was also examined in LPS-activated macrophages where NO inhibition was observed [47].
Based on the significant amount of data available on the anti-inflammatory properties of mastic gum resin and considering the importance of the health-promoting properties of MO, we decided to investigate the bioactivity of MO and its constituents on in vitro and in vivo experimental inflammation models. It is worth noting that our group has previously reported the significant antitumor properties exerted by MO in colon cancer in in vitro as well as in vivo models [20]. Herein, we employed LPS-stimulated RAW264.7 mouse macrophages, murine primary peritoneal macrophages and a model of zymosan-induced peritonitis in BALB/c mice. Our data suggest that MO could promote an anti-inflammatory transition in macrophages due to the combined bioactivities of its individual constituents that seem to exert different anti-inflammatory effects. Thus, multiple molecular mechanisms that are involved in the development of inflammation seem to be affected by MO. To the best of our knowledge, this is the first study assaying the anti-inflammatory properties of the essential oil extracted from mastic resin.
2. Materials and Methods
2.1. Plant Material, Essential Oil and Monoterpenes
The mastic gum was kindly provided by Chios Mastic Gum Growers Association L.L.C. (Chios, Greece) and the MO was extracted with small experimental distillation equipment under vacuum in VIORYL’s research laboratories (VIORYL S.A., 28th km National Road Athens-Lamia, Afidnes, 19014, Greece) according to the previously described methodology [20]. The composition of the extracted oil was analysed by gas chromatography–mass spectrometry (GC-MS) (GC: 6890 A, Agilent Technologies, Santa Clara, CA, USA; MSD: 5973, Agilent Technologies, Santa Clara, CA, USA) using an HP-1 MS column (25 m, 0.2 mm i.d., 0.33 µm film thickness). The detailed analysis process and chemical composition of the oil are described in Spyridopoulou et al. [20]. The monoterpene compounds α-pinene 90–93% (TREATT, Suffolk, UK), β-pinene 97% (Lluch Essence, Barcelona, Spain), myrcene 91–93% (Takasago International Corporation, Tokyo, Japan), limonene 99% (VIORYL, Athens, Greece) and linalool 98% (BASF, Ludwigshafen, Germany) were kindly provided by VIORYL.
2.2. Chemicals and Reagents
Acetic acid, dimethyl sulfoxide (DMSO), trichloroacetic acid (TCA), sulforhodamine B (SRB), Trizma base, zymosan (Z4250), lipopolysaccharides (LPS) (L4391), thioglycolate (T9032) and Griess (G4410) were purchased from Sigma-Aldrich (St. Louis, MO, USA); antibodies, Fc block, Fix/Perm kit and GolgiPlug inhibitor for flow cytometry were purchased from BD Biosciences (Franklin Lakes, NJ, USA) as stated; Dulbecco’s Modified Eagle’s Medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 and phosphate-buffered saline (PBS) were purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA); trypsin, foetal bovine serum (FBS), gentamicin and penicillin/streptomycin were purchased from Biosera (Boussens, France). A PathScan multi-target ELISA kit was purchased from Cell Signaling (Danvers, MA, USA); cytokine ELISA kits were purchased from eBioscience (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Cell and Bacterial Cultures
RAW264.7 murine macrophages were grown in DMEM medium supplemented with 10% heat inactivated foetal bovine serum, 2 mM glutamine, 100 μL/mL penicillin and 100 μg/mL streptomycin, as described in Spyridopoulou et al. [48]. Isolated peritoneal cells were maintained in RPMI with the same supplementation as DMEM and additionally 50 μg/mL gentamicin. All cells were cultured in a humidified incubator at 37 °C, 5% CO2.
Lactobacillus casei ATCC 393 (DSMZ, Braunschweig, Germany) bacteria were grown as described in Aindelis et al. [49]. For the phagocytosis assay, the bacteria were harvested in late-log/early stationary phase and labelled with CFSE (CellTrace CFSE Cell Proliferation kit, Invitrogen, Waltham, MA, USA) according to the methodology described in Tiptiri-Kourpeti et al. [50].
2.4. Determination of Tolerable Concentrations in RAW264.7 Macrophages
In order for the tolerable concentrations of MO and its constituents to be determined, 104 RAW264.7 cells per well were seeded in 96-well plates and left overnight to adhere. The following day, the adherent cells were treated with various concentrations of MO for 24 h. The viability of the treated cells was estimated with the SRB assay, as previously mentioned [51]. Briefly, the treated cells were fixed with 10% TCA and then stained with 0.057% w/v SRB. After extensive washes with 1% acetic acid, bound stain was dissolved with 10 mM Tris base and the absorbance at 510 nm was measured. Viability was determined as the percentage growth of the treated cells compared to the control cells cultured in DMEM.
2.5. Zymosan-induced Peritonitis
In order to evaluate the potential anti-inflammatory activity of MO, a model of zymosan-induced peritonitis was used. Twenty-eight female BALB/c mice (6–8 weeks old, weight 20–25 g) were separated into two independent groups (14 mice per group). Briefly, a 10% v/v mixture of MO in corn oil (0.58 g of MO/kg body weight) was administered orally to each mouse in the MO group for three days. The MO dose used in our experiments can be regarded as safe as it was previously determined by our group [20]. The control mice only received an equal volume of corn oil (100 μL). Oral administration was performed using a gavage needle. On the third day, one hour after the last dose of either MO or corn oil, 0.5 mL of a zymosan solution in PBS (2 mg/mL) was injected i.p. in each mouse and half of the animals of each group (seven animals per group) were euthanised by cervical dislocation 6 h later. The remaining 14 mice (7 animals from each group) were sacrificed 24 h after zymosan injection. Peritoneal cavities were flushed with 4 mL of cold PBS in order to collect migrated cells. Peritoneal lavage was centrifuged at 4 °C, 500× g for 5 min and supernatants were stored at −80 °C while cells were collected and processed further. The experiment was repeated twice to confirm the results.
2.6. Analysis of Neutrophil and Macrophage Migration
Immune cell migration in the peritoneum was investigated using flow cytometry. Peritoneal cells were collected as mentioned above and washed once with FACS buffer (PBS containing 2.5% FBS and 2.5 mM EDTA). Non-specific binding was blocked with Mouse BD Fc Block (553142, BD Pharmigen) and then the cells were stained with fluorescent antibodies against CD11b (APC-Cy7, 561039, BD Pharmigen), F4/80 (PE, 565410, BD Pharmigen) and Ly6G (PE, 551461, BD Pharmigen) for 40 min at 4 °C. The stained cells were washed twice with FACS buffer, resuspended in PBS, and analysed with an Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, MA, USA). Data analysis was performed with FlowJo v.10 software (TreeStar, Inc., Ashland, OR, USA).
2.7. Ex-Vivo Evaluation of Mastic Oil’s Anti-Inflammatory Activity
The migrated cells collected from mice that received zymosan injections were collected as previously mentioned. The cells were washed twice with PBS and cultured in RPMI supplemented with 10% heat-inactivated foetal bovine serum, 2 mM glutamine, 100 μL/mL penicillin, 100 μg/mL streptomycin and 50 μg/mL gentamicin. The cells were then treated with 0.2 mg/mL zymosan for 24 h and supernatants were collected, centrifuged at 4 °C, 1000× g for 5 min to remove cells and debris, and cytokine concentration was determined by ELISA.
2.8. Isolation of Peritoneal Macrophages
The intra-peritoneal injection of thioglycolate was employed for the isolation of peritoneal macrophages from mice. Seven female BALB/c mice (6–8 weeks old, weight 20–25 g) were used in total. Briefly, 3 mL of 3% w/v thioglycolate was injected in the peritoneum of the mice. Four days later, the migrated cells were isolated by the injection of 5 mL cold PBS in the peritoneal cavity, and after application of mild pressure, the PBS was retrieved. This peritoneal lavage was centrifuged for 5 min at 4 °C, 500× g. The cells were collected, diluted in RPMI supplemented with 10% heat-inactivated foetal bovine serum, 2 mM glutamine, 100 μg/mL penicillin, 100 μg/mL streptomycin and 50 μg/mL gentamicin, counted and cultured in RPMI medium for four hours at 37 °C. Following this incubation period, the plates were extensively washed with PBS for non-adherent cells to be removed and the remaining cells were incubated at 37 °C overnight. The experiment was repeated two times.
2.9. Evaluation of Inflammatory Regulators in LPS-Stimulated Macrophages
The peritoneal macrophages were isolated as previously stated and following the overnight incubation period. MO at a concentration of 8.9 μg/mL was added to the cultured macrophages, and 30 min later, the cells were stimulated with 100 ng/mL of LPS and cultured for another 24 h. RAW264.7 cells were seeded on 100 mm plates at a density of 3 × 106 cells per plate and left overnight to adhere. The following day, MO was added (8.9 μg/mL) for 30 min and then the cells were stimulated with 100 ng/mL LPS. The control cells only received LPS and the negative control was cultured in plain medium (unstimulated). After treatment, the cells were observed under a bright field microscope for the identification of morphological changes, indicative of their differentiation. Supernatants were collected, centrifuged for 5 min at 4 °C, 1000× g to remove cellular debris and stored at −80 °C for subsequent ELISA analysis. The remaining cells were washed with cold PBS and protein extracts were prepared by the addition of lysis buffer directly onto the culture dish and incubating at 4 °C for 5 min. The cells were then scraped from the dish, sonicated on ice, centrifuged for 10 min at 4 °C, 12,000× g and the supernatant was collected. Sample protein concentration was determined with the BCA assay utilising a standard curve (BCA kit, Invitrogen, Waltham, MA, USA) and the levels of key regulatory proteins of the inflammatory response were determined with a commercially available kit (PathScan® Inflammation Multi-Target Sandwich ELISA Kit #7276), following the manufacturer’s instructions, ensuring that equal amounts of protein were loaded into each well.
2.10. Cytokine Quantification
The concentrations of IL-6 (eBioscience, 88-7064) and TNF-α (eBioscience, 88-7324) were determined in peritoneal lavage and cell culture supernatants utilising sandwich ELISA according to the manufacturer’s instructions. The production of IL-10 in the peritoneal macrophages was evaluated using flow cytometry. The peritoneal macrophages were treated with MO and LPS and cultured for 24 h. The secretion of interleukins was blocked with a protein transport inhibitor (BD GolgiPlug, 555029, BD Pharmigen) 4 h prior to cell collection. The macrophages were then collected, washed once with FACS buffer, permeabilised with a BD Cytofix/Cytoperm Fixation/Permeabilization kit (554714, BD Pharmigen) and stained with an antibody against IL-10 (PE, 554467, BD Pharmigen) for 40 min at 4 °C. The stained cells were washed twice, suspended in PBS and analysed with an Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, MA, USA). Data analysis was performed with FlowJo v.10 software (TreeStar, Inc., Ashland, OR, USA).
2.11. Evaluation of NO and PGE2 Production
The production of NO following LPS treatment was estimated with the Griess reaction [52]. Equal amounts of culture supernatant and Griess reagent were mixed and incubated for 15 min. Following this, absorbance at 540 nm was measured with a plate reader (Enspire, Perkin Elmer, Waltham, MA, USA) and the nitrite concentration was quantified using a sodium nitrite standard curve. The production of PGE2 was analysed employing the Prostaglandin E2 Parameter Assay Kit (KGE004B, R&D Systems, Minneapolis, MN, USA).
2.12. Phagocytosis Analysis
In order to investigate the effect of MO and its major components on the macrophage phagocytosis rate, Lactobacillus casei ATCC 393 (L. casei) was used as an in vitro pathogen model. Briefly, L. casei was grown in MRS broth at 37 °C without agitation. The bacteria were harvested in the late-log/early stationary phase (109 CFUs/mL). In order to collect the cells, the bacterial cultures were centrifuged at 1700× g for 15 min at 4 °C. Next, the cells were washed in PBS, stained with CFSE and resuspended in DMEM for the phagocytosis assay. RAW264.7 cells’ nuclei were stained with Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA, USA) and their cytoplasm with CellBrite® Red (Biotium, Hayward, CA, USA) according to the manufacturer’s instructions. Labelled cells were treated with MO or its constituents in non-toxic concentrations (Table 1) for 1 h. Next, the cells were co-cultured with CFSE-stained L. casei (2 × 107 CFU/mL) for 4 h in either MO/constituents containing or plain (control) medium. The medium used for the phagocytosis assay was DMEM supplemented with 10% heat-inactivated foetal bovine serum, 2 mM glutamine, 100 μL/mL penicillin and 100 μg/mL streptomycin. After treatment, the cells were washed, fixed and observed under a Zeiss Axio Scope A1 fluorescence microscope and image acquisition was performed using the ZEN Blue imaging software by Carl Zeiss Microscopy (Zeiss, Göttingen, Germany). Multiple photos per treatment were taken and analysed with ImageJ software (NIH, Bethesda, MD, USA). RAW264.7 cells engulfing at least one bacterium were counted as ‘phagocytosing cells’ compared to cells that did not appear to be associated with bacteria (non-phagocytosing cells) for each experimental condition. The %Phagocytosis rate was calculated as the percentage of phagocytosing/non-phagocytosing cells.

Table 1.
Maximum non-toxic concentrations of MO or its major constituents in RAW264.7 cells as determined by the SRB assay.
2.13. Statistical Analysis
All data shown are representative of at least three independent experiments. Sigma Plot v.11 software (Systat Software Inc., San José, CA, USA) was used for statistical analysis and graphing. For statistical comparisons between groups, a Student’s t-test, one-way ANOVA or Mann–Whitney test for non-parametric variables was performed. Differences between groups were considered significant when p < 0.05.
2.14. Ethics Statement
The animals were housed in polycarbonate cages at room temperature and were provided with commercial food and tap water ad libitum. The animal experiments were approved by the Animal Care and Use Committee of the Veterinary Department of Ioannina Prefecture (licence number EL20BIO02) since it complied with the requirements set by Directive 86/609/EEC and PD 160/91, which was the legislation in force at the time of experimentation. All animal experiments were conducted in light of the “3 Rs” (replacement, refinement, reduction) and none of the mice used for the experiments were subjected to pain or discomfort.
3. Results
Herein, we investigated the potential anti-inflammatory activity of MO and its major constituents. The composition of MO has been previously described by our team, analysed by gas chromatography–mass spectrometry (GC-MS). Briefly, volatile monoterpenes and a sesquiterpene (caryophyllene) were identified, present at different percentages and covering 94.12% of the total chromatographic area. The five major constituents of MO were found to be the monoterpenes α-pinene (67.71%), myrcene (18.81%), β-pinene (3.05%), limonene (0.89%) and linalool (0.73%) [20].
MO’s anti-inflammatory potency was initially assayed employing a preclinical acute peritonitis model. BALB/c mice received the essential oil per os for 3 days, acute peritonitis was induced with the intra-peritoneal injection of zymosan, and the migration of immune cells to the site of inflammation was evaluated using flow cytometry. Neutrophil recruitment was not affected at any examined time point (Figure 1a); however, macrophage migration was reduced by approximately 20% at the initial stages of acute inflammation in the MO-treated mice (Figure 1b). The overall intensity of the inflammatory response was also not significantly influenced by the consumption of MO, as evidenced by the similar levels of IL-6 and TNF-α that were detected with ELISA in the peritoneal fluid (Figure 1c) as well as in the supernatants of ex vivo restimulated peritoneal-infiltrated cells with zymosan (Figure 1d).
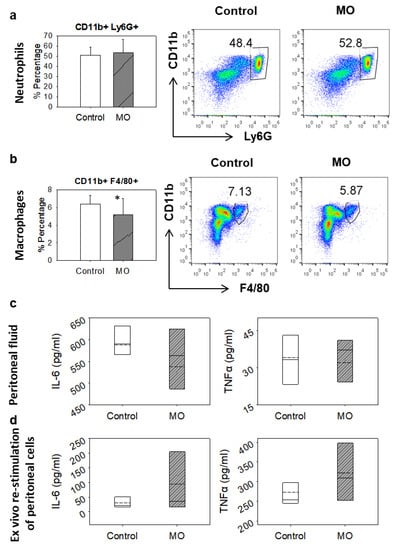
Figure 1.
Effect of orally administered MO in the zymosan-induced peritonitis BALB/c mouse model. (a) Neutrophil and (b) macrophage recruitment in the peritoneal cavity 6 h after zymosan challenge, analysed by flow cytometry. Treated mice (n = 7) received 0.58 g/kg body weight of MO orally in a 10% v/v mixture with corn oil for three days prior to zymosan administration. Control animals received an equal volume of plain corn oil. Bars in (a,b) represent the percentage of CD11b+ Ly6G+ (neutrophils) or CD11b+ F4/80+ (macrophages) cells, respectively. (c) Peritoneal IL-6 and TNF-α levels 6 h after zymosan challenge, determined by ELISA. (d) IL-6 and TNF-α levels analysed by ELISA, in the supernatants of cells migrated to peritoneum, collected from either control or MO-treated animals and rechallenged with zymosan ex vivo for 24 h. Box plots of the cytokine levels in (c,d) represent the first to the third quartiles; solid lines indicate median and dashed lines indicate mean values. Data are representative of at least three independent experiments. Asterisk indicates statistically significant difference compared to control (Student’s t-test, p ≤ 0.05).
Considering that the initiation of the inflammatory response is dominated by neutrophils, we decided to focus our efforts on a more targeted analysis of the effect of MO on the macrophage-driven inflammation. Taking into account the composition of the essential oil, we also decided to include in our analyses the major constituents of the oil. For this purpose, we utilised in vitro and ex vivo cultures of RAW264.7 cells and thioglycolate-elicited peritoneal macrophages, respectively.
Macrophage migration to the peritoneum was induced with the intra-peritoneal injection of thioglycolate and isolated cells were then cultured and treated with LPS and MO. The treatment of isolated macrophages with MO for 24 h did not appear to notably affect the stimulation of macrophages by LPS, as the levels of secreted IL-6 and TNF-α were not noticeably reduced in any group (Figure 2a,b), despite the observed downregulation of p38 phosphorylation (Figure 2c). In addition, the production of IL-10 was severely decreased after the introduction of the essential oil (Figure 2d).
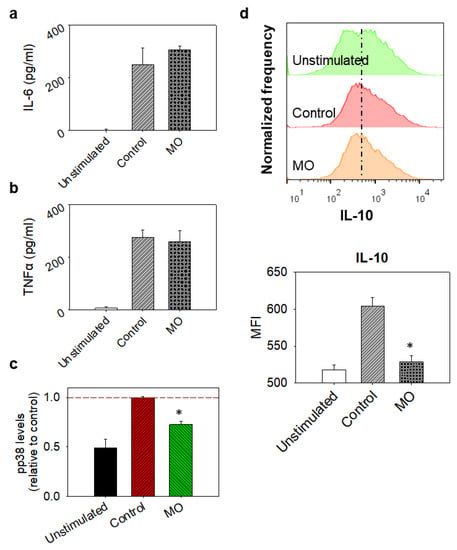
Figure 2.
Ex vivo effects of MO in thioglycolate-elicited murine peritoneal macrophages. Cells were collected from the peritoneum of BALB/c mice, 4 days after i.p injection of thioglycolate and left to adhere on culture dishes. MO-treated macrophages were incubated with 8.9 μg/mL MO for 30 min before the addition of 100 ng/mL LPS; control cells were incubated in LPS only, while unstimulated cells were grown in plain medium. Culture supernatants were collected 24 h later. (a) IL-6 and (b) TNF-α levels in macrophage culture supernatants analysed by ELISA. Bars represent cytokine levels. Data are presented as mean ± SD of at least three independent experiments. (c) Phospho-p38 (pp38) MAPK levels in cells, determined with the PathScan® Inflammation Multi-Target Sandwich ELISA Kit. Bars represent pp38 levels expressed as a relative to control ratio. Dashed line indicates normalised control levels as reference. (d) Intracellular IL-10 detection in cells by flow cytometry. Bars represent intracellular IL-10 levels and data are presented as mean ± SD of at least seven (n = 7) animals. MFI stands for median fluorescence intensity. Results are representative of at least three independent experiments. Asterisks indicate statistically significant difference compared to control (Student’s t-test, p ≤ 0.05).
As mentioned above, the anti-inflammatory potential of the essential oil and its constituents was also evaluated in RAW264.7 macrophages. Tolerable concentrations were determined using the SRB assay (Figure 3 and Table 1) and then the effect of each component on LPS-stimulated RAW264.7 cells was examined.
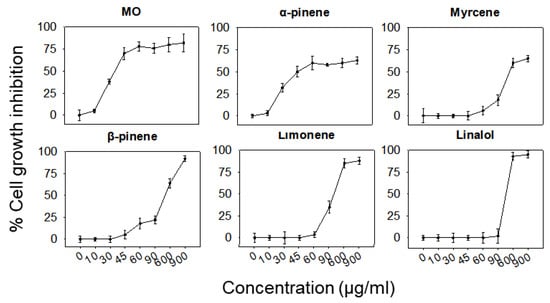
Figure 3.
Effect of MO or its major constituents on the growth rate of RAW264.7 cells after 24 h of treatment. Cell growth inhibition was analysed using the SRB assay. Values represent means ± SD of at least four replicates. Data shown are representative of three independent experiments.
The observation of stimulated cells under a microscope revealed a significant reduction in the number of differentiated cells after treatment with the complete essential oil or each of its constituents, except limonene (Figure 4a). In a similar manner, all treatments reduced the amount of NO produced following the induction of inflammation with LPS, detected with the Griess assay, as shown in Figure 4b. In order to better understand the activity of the various ingredients, we then examined the production of key inflammatory mediators such as prostaglandins and cytokines. All of the compounds except α-pinene were found to reduce the amount of prostaglandins (Figure 4c) and α-pinene was also unable to decrease the secretion of TNF-α along with myrcene (Figure 4d), while in all other treatments the production of both prostaglandins and TNF-α was significantly diminished (Figure 4c,d). Surprisingly, no reduction in IL-6 was observed for any of the compounds (Figure 4e). Furthermore, no changes in the levels and/or phosphorylation status of key inflammation regulatory proteins (NFkB p65, pSAPK, pSTAT3, PIkB-a) was observed in LPS-stimulated RAW264.7 cells pre-treated with a range of MO concentrations (Figure 4f).
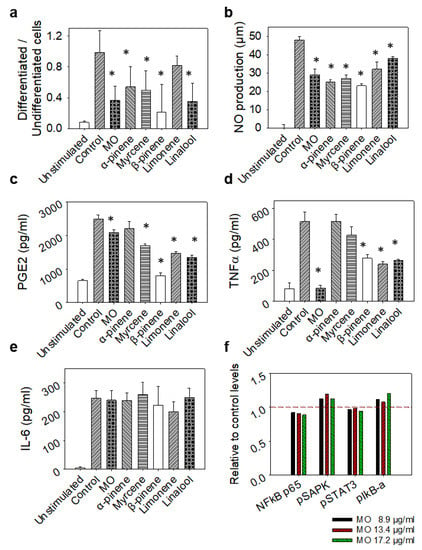
Figure 4.
Effect of non-toxic concentrations of MO or its major constituents on LPS-stimulated RAW264.7 murine macrophages after 24 h of treatment. Control cells were only treated with LPS, while unstimulated cells were grown in plain medium (DMEM). (a) Ratio of differentiated to undifferentiated cells analysed by bright field microscopy. (b) NO levels in cells´ supernatant determined by the Griess assay. (c) PGE2, (d) TNF-α and (e) IL-6 levels in supernatants determined by ELISA. Results are presented as mean ± SD values of three replicates. (f) NFkB p65, pSAPK, pSTAT3 and pIkB-a levels determined by ELISA, in LPS-stimulated RAW264.7 macrophages treated with increasing concentrations of MO. Results are expressed as a relative to control ratio. Dashed line indicates normalised control levels as reference. Data shown are representative of three independent experiments. Asterisks indicate statistically significant difference compared to control (one-way ANOVA, p ≤ 0.05).
Finally, as a means of assessing the stimulation of macrophages, we examined their phagocytic potential. Unstimulated RAW264.7 cells were treated with MO or its constituents and then incubated with stained L. casei as a target. As shown in Figure 5, all treatments, excluding myrcene, noticeably limited the phagocytic activity of RAW264.7 cells to the point that in most cases, the phagocytosis of the bacterium was halved.
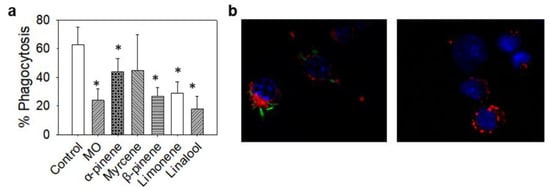
Figure 5.
Effect of non-toxic concentrations of MO or its major constituents on the phagocytosis rate of RAW264.7 cells. CFSE-stained L. casei (green) was used as an in vitro pathogen model. RAW264.7 nuclei were stained with Hoechst 33342 (blue) and their cytoplasm with CellBrite (red). Cells were co-cultured with L. casei in the presence of MO or its major constituents for 4 h. Control cells were exposed to L. casei in plain medium. (a) %Phagocytosis rate of L. casei bacteria by RAW264.7 cells calculated as percentage of phagocytosing to non-phagocytosing cells. Data are representative of three independent experiments and are presented as mean ±SD. (b) Fluorescence microscopy images of control RAW264.7 cells phagocytosing L. casei bacteria (left) and MO-treated RAW264.7 cells exhibiting limited phagocytic activity. Asterisks indicate statistically significant difference compared to control (one way ANOVA, p ≤ 0.05).
4. Discussion
Mastic oil (MO) is the oil extracted from the resin (mastic gum) of the plant Pistacia lentiscus var. chia. The benefits of mastic gum consumption have long been known to humans and it has traditionally been used for the treatment of various gastrointestinal disorders. Mastic gum is currently used as a supplement in liquors, drinks, foods, toothpaste, lotions and other cosmetics. Despite its broad use in food and cosmetics, it was not until the last few years that considerable attention was drawn to MO from the scientific community due to the anticancer, antimicrobial, antioxidant and other health-promoting biological properties attributed to it [20,53,54,55]. Our group has previously shown that the oral administration of MO attenuates tumour growth in a murine colon cancer model [20]. It is noteworthy that different preparations from various parts of the mastic plant have been shown to exert significant anti-inflammatory activities in in vitro [47,56] and in vivo preclinical [38,39,40,57,58,59] and clinical [34,35] studies. Most studies, though, investigate the mastic resin and not its essential oil, which is a significantly more concentrated source of bioactive compounds. Herein, we examined the anti-inflammatory properties of mastic oil (MO) in in vitro and in vivo experimental inflammation models.
A significant reduction (−20%) in macrophage migration was observed in the early stages of inflammation in the zymosan-induced peritonitis model in BALB/c mice that had orally received MO (Figure 1). Interestingly, neutrophil migration was not affected. In a different acute inflammation model in rats (carrageenan-induced pleurisy), Bouriche et al. described that orally administered extracts from the leaves of the plant significantly inhibited neutrophil migration, although macrophages were not assayed [57]. Similarly, in a different study also employing inflammation models in rats, the authors conclude that the topical application of the oil extracted from the leaves of the mastic tree attenuated leukocyte migration [46].
Subsequently, MO’s effect on the production and/or secretion of various inflammation mediators was examined. Since MO is a mixture of volatile compounds, having analysed its chemical composition, we also assayed its five major constituents, i.e., α-pinene, myrcene, limonene, β-pinene and linalool. Crucially, sub-toxic concentrations of the various compounds were used for all of the experiments (Figure 3 and Table 1). The production of TNF-α seems to be significantly reduced by MO in LPS-stimulated RAW264.7-derived macrophages. Linalool, limonene and β-pinene also inhibit TNF-α levels, although to a lesser extent (Figure 4d). Furthermore, ΝO production is reduced both by MO and its constituents with α-pinene and β-pinene exhibiting the highest inhibition rates (Figure 4b). Similarly, MO and all of its constituents, with the exception of α-pinene, inhibit PGE2 production. Surprisingly, β-pinene exhibited the highest inhibition rate (Figure 4c). β-pinene is also the compound that limits the LPS-induced differentiation in RAW264.7 cells (Figure 4a).
These results are in agreement with various studies describing the anti-inflammatory effects of these monoterpenes such as the study by Kim et al. where α-pinene was shown to reduce NO production in macrophages isolated from rats [60]. Accordingly, Yoon et. al. [61] showed that limonene suppresses LPS-induced NO, PGE2 and TNF-α in LPS induced-RAW264.7 macrophages. Myrcene was also reported to suppress pro-inflammatory mediators such as TNF-α [62] and NO [63]. Likewise, linalool was shown to inhibit LPS-induced inflammation in RAW264.7 cells mediating TNF-α and IL-6 inhibition, among other effects [64]. The significant anti-inflammatory effects exerted by the individual constituents of MO are a strong indication for the oil’s potential against inflammation. However, there are no studies assaying the oil extracted from mastic resin. Oil extracted from the fruit of the mastic plant was also shown to suppress NO production in LPS-stimulated RAW264.7 cells [47], while the resin, as a solid extrudate, was shown to inhibit both NO and PGE2 [36].
Moreover, we observed that MO, linalool, β-pinene, limonene and α-pinene inhibit the phagocytosis rate of L. casei bacteria by RAW264.7 cells compared to the untreated control cells (Figure 5). Data presented in the review by da Silveira e Sa et al., describing the inhibition of phagocytic activity of peritoneal macrophages by many monoterpenes, are in agreement with our results [65]. Finally, both MO and all of its constituents significantly reduced the amount of RAW264.7 cells that underwent LPS-induced morphological changes, with β-pinene and MO exerting the most pronounced effects (Figure 4a). This reduction in differentiated macrophages also reflects a reduction in the number of the activated macrophages that are recruited during inflammation.
Besides RAW264.7-derived macrophages, cells isolated from the peritoneum of thioglycolate-challenged mice were also employed. The ex vivo stimulation of primary macrophages with LPS in the presence of MO led to an inhibition in IL-10 production and the downregulation of p38 phosphorylation, while neither TNF-α nor IL-6 levels were affected. p38 MAPKs (mitogen-activated protein kinases) are key MAPKs involved in the production of inflammatory mediators, including TNF-α and IL-6 [66]. Environmental signals (cellular stress or cytokines) lead to the phosphorylation of p38, activating its signalling pathway. The p38 MAPK pathway was originally identified as a master regulator of pro-inflammatory cytokine production [67]. Hence, the identification of compounds/inhibitors targeting the p38 MAPK pathway has been considered a promising strategy for the treatment of inflammatory diseases. Despite the promising preclinical results, several of the molecules identified did not perform well in clinical trials and only achieved transient regulation of the inflammation process. It has been recently proposed that this could be explained by the dependency of p38′s anti-inflammatory activity to IL-10 levels [68]. IL-10 is a cytokine broadly expressed by many immune cells that also exhibits anti-inflammatory properties [69]. It was described that, in the absence of IL-10, p38 phosphorylation in macrophages promotes protective effects against inflammation [68]. In our experiments, MO inhibited both IL-10 and phospho-p38 levels, but did not affect TNF-α and IL-6 production (Figure 2). Furthermore, we observed that the levels of phosphoproteins that activate inflammation in macrophages are differentially regulated by MO in the LPS-based inflammation model in the RAW264.7-derived macrophages employed, even though small differences were detected compared to the control. These results indicate that MO may act on specific branches of the cross-talk pathways between cytokines and signalling processes during inflammation.
Considering all of the above results, mastic oil (MO), as a mixture of various compounds, seems to affect multiple inflammation-related cellular mechanisms. As expected, the modulation of the inflammatory response in murine macrophages by MO relies on the combined activity of all of its constituents that seem to act on various pathways involved in the development of inflammation. β-pinene could play a central role in the anti-inflammatory activity of MO, as it exerts the most potent effects in the regulation of most of the parameters examined. Therefore, further research is proposed that focuses on individual constituents and, most importantly, β-pinene, and employing various preclinical inflammation models that activate different mechanisms in order to characterise mastic oil’s anti-inflammatory activity. Finally, taking into consideration its multiple effects on many pathways of inflammation, MO could be used for the development of nutritional and pharmaceutical supplements with anti-inflammatory activity.
Author Contributions
Conceptualisation, K.C.; methodology, G.A., K.S. and A.T.-K.; validation, G.A., A.T.-K. and K.S.; formal analysis, G.K., K.S., A.T.-K. and G.A.; investigation, G.K., G.A. and K.S.; resources, K.C.; writing—original draft preparation, K.S., G.A. and G.K.; writing—review and editing, K.C., A.T.-K. and G.K.; visualisation, K.S.; supervision, K.C.; project administration, K.C.; funding acquisition, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
The research project was supported by (a) the Hellenic Foundation for Research and Innovation (HFRI) under the “First Call for H.F.R.I. Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment” (Project Number: H.F.R.I.-FM17C3-2007) and (b) the Operational Program “National Action Cooperation 2011—Partnerships of Production and Research Institutions in Focused Research and Technology Sectors” (Project Nr. 11SYN_2_566). Part of the work was implemented utilising the facilities of “InTechThrace: Integrated Technologies in biomedical research: Multilevel biomarker analysis in Thrace” (MIS Code 5047285), under the Operational Program “Competitiveness, Entrepreneurship & Innovation” (EPAnEK), co-funded by the European Regional Development Fund (ERDF) and national resources (Partnership Agreement 2014–2020).
Institutional Review Board Statement
The in vivo experimental protocol followed in this study was approved by the Animal Care and Use Committee of the Veterinary Department of Ioannina Prefecture (licence number EL20BIO02). All animal procedures were carried out in accordance with the principle of the “3 Rs” (replacement, refinement, reduction) and none of the mice used were subjected to any pain or discomfort.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data are available from the authors upon request.
Acknowledgments
The authors would like to thank VIORYL (VIORYL S.A., 28th km National Road Athens-Lamia, Afidnes, 19014, Greece) for kindly providing the mastic oil and monoterpenes used in this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J. Immunol. Res. 2019, 2019, 403796. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- McCombe, P.A.; Lee, J.D.; Woodruff, T.M.; Henderson, R.D. The Peripheral Immune System and Amyotrophic Lateral Sclerosis. Front. Neurol. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Michalick, L.; Kuebler, W.M. TRPV4—A Missing Link Between Mechanosensation and Immunity. Front. Immunol. 2020, 11, 413. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Câmara, N.O.S. Inflammation in renal diseases: New and old players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review—Current Concepts in Inflammatory Skin Diseases Evolved by Transcriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2020, 21, 699. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27. [Google Scholar] [CrossRef]
- Mahesh, G.; Kumar, K.A.; Reddanna, P. Overview on the Discovery and Development of Anti-Inflammatory Drugs: Should the Focus Be on Synthesis or Degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef]
- Fitzpatrick, F. Cyclooxygenase Enzymes: Regulation and Function. Curr. Pharm. Des. 2005, 10, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT Food Sci. Technol. 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Bardouki, H.; Panayiotidis, M.; Galanis, A.; Kourkoutas, Y.; Chlichlia, K.; et al. Phytochemical Profile and Evaluation of the Biological Activities of Essential Oils Derived from the Greek Aromatic Plant Species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Aravidou, T.; Lampri, E.; Effraimidou, E.; Pappa, A.; Chlichlia, K. Antitumor Potential of Lippia citriodora Essential Oil in Breast Tumor-Bearing Mice. Antioxidants 2021, 10, 875. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Vamvakias, M.; Bardouki, H.; Galanis, A.; Chlichlia, K.; Kourkoutas, Y.; Panayiotidis, M.; Pappa, A. Chemical Composition and Evaluation of the Biological Properties of the Essential Oil of the Dietary Phytochemical Lippia citriodora. Molecules 2018, 23, 123. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 3782. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Fitsiou, E.; Spyridopoulou, K.; Vasileiadis, S.; Iliopoulos, C.; Galanis, A.; Vekiari, S.; Pappa, A.; Chlichlia, K. Evaluation of Antioxidant and Antiproliferative Properties of Cornus mas L. Fruit Juice. Antioxidants 2019, 8, 377. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-Derived Anti-Inflammatory Compounds: Hopes and Disappointments regarding the Translation of Preclinical Knowledge into Clinical Progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.D.R.; Arantes, M.B.; de Faria Pereira, S.M.; da Cruz, L.L.; de Souza Passos, M.; de Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Li, R.W.; David Lin, G.; Myers, S.P.; Leach, D.N. Anti-inflammatory activity of Chinese medicinal vine plants. J. Ethnopharmacol. 2003, 85, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Myers, S.P.; Leach, D.N.; Lin, G.D.; Leach, G. A cross-cultural study: Anti-inflammatory activity of Australian and Chinese plants. J. Ethnopharmacol. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2015, 30, 1343–1352. [Google Scholar] [CrossRef]
- Cuéllar, M.J.; Giner, R.M.; Recio, M.C.; Máez, S.; Ríos, J.L. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia 2001, 72, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; Mordujovich De Buschiazzo, P.; Ríos, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033. [Google Scholar] [CrossRef]
- Milia, E.; Bullitta, S.M.; Mastandrea, G.; Szotáková, B.; Schoubben, A.; Langhansová, L.; Quartu, M.; Bortone, A.; Eick, S. Leaves and fruits preparations of Pistacia lentiscus L.: A review on the ethnopharmacological uses and implications in inflammation and infection. Antibiotics 2021, 10, 425. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Dimou, S.; Dionysopoulou, M.; Argyropoulou, A.; Diallinas, G.; Halabalaki, M. Chemical Profiling of Pistacia lentiscus var. chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity. Processes 2021, 9, 418. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Bikineyeva, A.; Dikalova, A.; Nazarewicz, R.; Lerakis, S.; Dikalov, S. Anti-inflammatory activity of Chios mastic gum is associated with inhibition of TNF-alpha induced oxidative stress. Nutr. J. 2011, 10, 64. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, I.; Karatzas, T.; Korou, L.M.; Katsilambros, N.; Perrea, D. Beneficial health effects of Chios Gum Mastic and Peroxisome proliferator-Activated receptors: Indications of common mechanisms. J. Med. Food 2015, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Satoh, K.; Takahashi, K.; Watanabe, S.; Nakamura, W.; Maki, J.; Hatano, H.; Takekawa, F.; Shimada, C.; Sakagami, H. Re-evaluation of anti-inflammatory activity of mastic using activated macrophages. In Vivo 2009, 23, 583–589. [Google Scholar]
- Loizou, S.; Paraschos, S.; Mitakou, S.; Chrousos, G.P.; Lekakis, I.; Moutsatsou, P. Chios Mastic Gum Extract and Isolated Phytosterol Tirucallol Exhibit Anti-Inflammatory Activity in Human Aortic Endothelial Cells. Exp. Biol. Med. 2009, 234, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, R.; Kato, N.; Koike, M.; Iwashita, N.; Takagi, Y.; Fukuyama, T. Topical treatment with mastic (resin from Pistacia lentiscus) elicits anti-inflammatory and anti-pruritic responses by modulating keratinocyte activation in a mouse model of allergic dermatitis. Phytomedicine 2021, 91, 153679. [Google Scholar] [CrossRef]
- Kakagia, D.; Papalois, A.; Lambropoulou, M.; Papachristou, F.; Trypsiannis, G.; Anagnostopoulos, C.; Pitiakoudis, M.; Tsaroucha, A. The Use of Pistacia lentiscus chia Resin versus Omeprazole in Protecting Male Rats Peptic Mucosa against Cold Restraint Stress. J. Crit. Care Med. 2020, 6, 100–110. [Google Scholar] [CrossRef]
- Ostovan, M.; Fazljou, S.M.B.; Khazraei, H.; Araj Khodaei, M.; Torbati, M. The Anti-Inflammatory Effect of Pistacia lentiscus in a Rat Model of Colitis. J. Inflamm. Res. 2020, 13, 369–376. [Google Scholar] [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Papalois, A.; Agrogiannis, G.; Triantafillidis, J.K.; Andrikopoulos, N.K. Pistacia lentiscus resin regulates intestinal damage and inflammation in trinitrobenzene sulfonic acid-induced colitis. J. Med. Food 2011, 14, 1403–1411. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.Z.; Andrikopoulous, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Forbes, A.; Tzavara, C.; Smyrnioudis, I.; Kaliora, A.C. Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: A double-blind and placebo-controlled randomised trial. Phytother. Res. 2019, 33, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Amerikanou, C.; Dimitropoulou, E.; Gioxari, A.; Papada, E.; Tanaini, A.; Fotakis, C.; Zoumpoulakis, P.; Kaliora, A.C. Linking the IL-17A immune response with NMR-based faecal metabolic profile in IBD patients treated with Mastiha. Biomed. Pharmacother. 2021, 138, 111535. [Google Scholar] [CrossRef] [PubMed]
- Maxia, A.; Sanna, C.; Frau, M.A.; Piras, A.; Karchuli, M.S.; Kasture, V. Anti-inflammatory activity of Pistacia lentiscus essential oil: Involvement of IL-6 and TNF-α. Nat. Prod. Commun. 2011, 6, 1543–1544. [Google Scholar] [CrossRef]
- Chaabani, E.; Abert Vian, M.; Dakhlaoui, S.; Bourgou, S.; Chemat, F.; Ksouri, R. Pistacia lentiscus L. edible oil: Green extraction with bio-based solvents, metabolite profiling and in vitro anti-inflammatory activity. OCL 2019, 26, 25. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Aindelis, G.; Pappa, A.; Chlichlia, K. Anticancer Activity of Biogenic Selenium Nanoparticles: Apoptotic and Immunogenic Cell Death Markers in Colon Cancer Cells. Cancers 2021, 13, 5335. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune responses raised in an experimental colon carcinoma model following oral administration of lactobacillus casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Bardouki, H.; Vamvakias, M.; Panas, P.; Paraskevas, P.; Kourkoutas, Y. Assessment of Antimicrobial Efficiency of Pistacia lentiscus and Fortunella margarita Essential Oils against Spoilage and Pathogenic Microbes in Ice Cream and Fruit Juices. Microbiol. Res. 2022, 13, 667–680. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Kalousi, F.D.; Pollastro, F.; Christodoulou, E.C.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.G. Apoptotic, Anti-Inflammatory Activities and Interference with the Glucocorticoid Receptor Signaling of Fractions from Pistacia lentiscus L. var. chia Leaves. Plants 2022, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, H.; Saidi, A.; Ferradji, A.; Belambri, S.A.; Senator, A. Anti-inflammatory and immunomodulatory properties of Pistacia lentiscus extracts. J. Appl. Pharm. Sci. 2016, 6, 140–146. [Google Scholar] [CrossRef]
- Dellai, A.; Souissi, H.; Borgi, W.; Bouraoui, A.; Chouchane, N. Antiinflammatory and antiulcerogenic activities of Pistacia lentiscus L. leaves extracts. Ind. Crops Prod. 2013, 49, 879–882. [Google Scholar] [CrossRef]
- Boutemine, I.M.; Amri, M.; Amir, Z.C.; Fitting, C.; Mecherara-Idjeri, S.; Layaida, K.; Sennoun, N.; Berkane, S.; Cavaillon, J.M.; Touil-Boukoffa, C. Gastro-protective, therapeutic and anti-inflammatory activities of Pistacia lentiscus L. fatty oil against ethanol-induced gastric ulcers in rats. J. Ethnopharmacol. 2018, 224, 273–282. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene Suppresses Lipopolysaccharide-Induced Production of Nitric Oxide, Prostaglandin E2, and Pro-inflammatory Cytokines in RAW 264.7 Macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef]
- Islam, A.U.S.; Hellman, B.; Nyberg, F.; Amir, N.; Jayaraj, R.L.; Petroainu, G.; Adem, A. Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model. Molecules 2020, 25, 4492. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Da Silveira E Sá, R.; Andrade, L.N.; De Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-Kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Raza, A.; Crothers, J.W.; McGill, M.M.; Mawe, G.M.; Teuscher, C.; Krementsov, D.N. Anti-inflammatory roles of p38α MAPK in macrophages are context dependent and require IL-10. J. Leukoc. Biol. 2017, 102, 1219–1227. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).