Combining CAR T Cell Therapy and Oncolytic Virotherapy for Pediatric Solid Tumors: A Promising Option
Abstract
1. Introduction
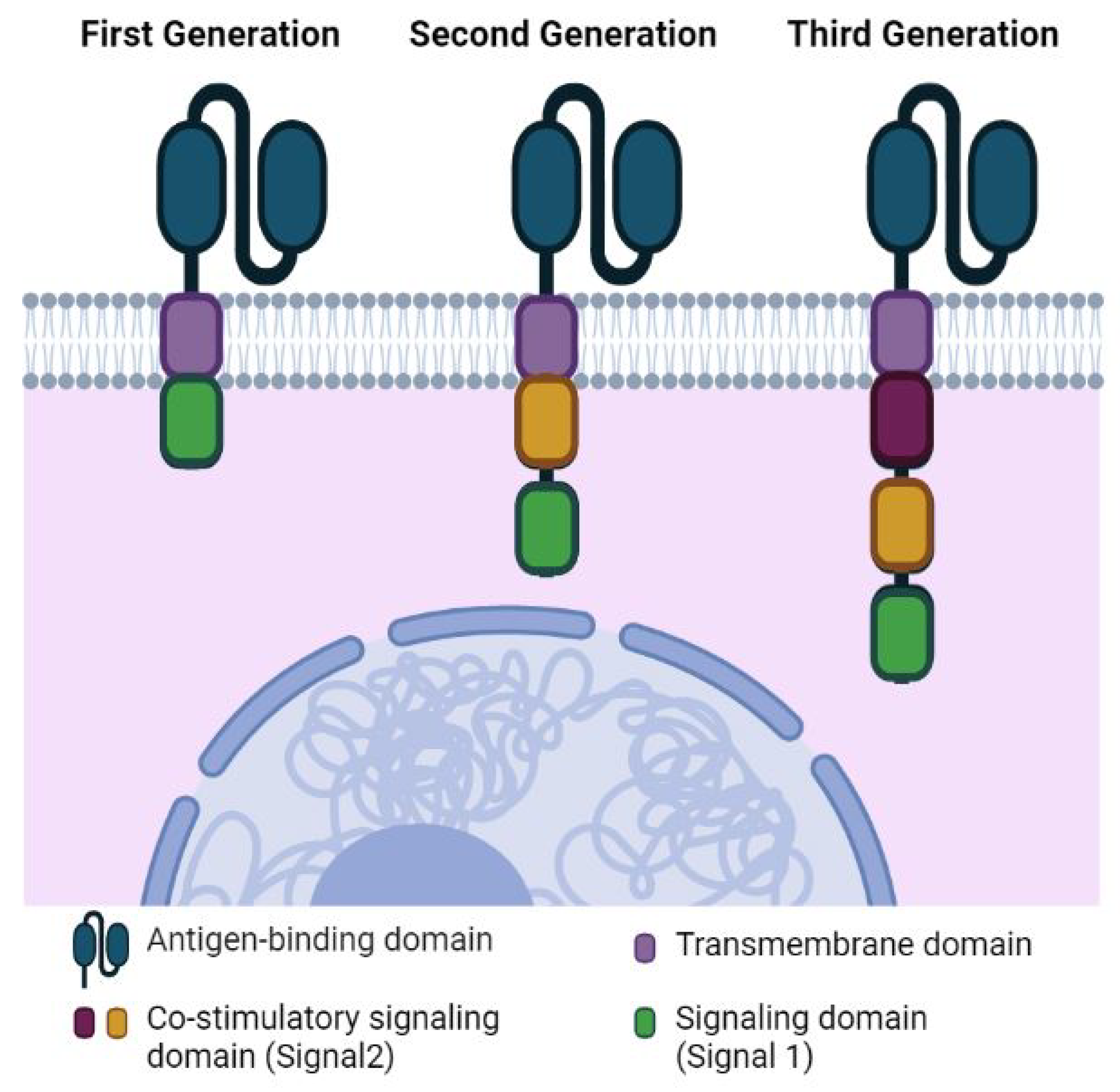
2. CAR T Cell Therapy for Pediatric Solid Tumors
3. Oncolytic Virotherapy in Pediatric Solid Tumors
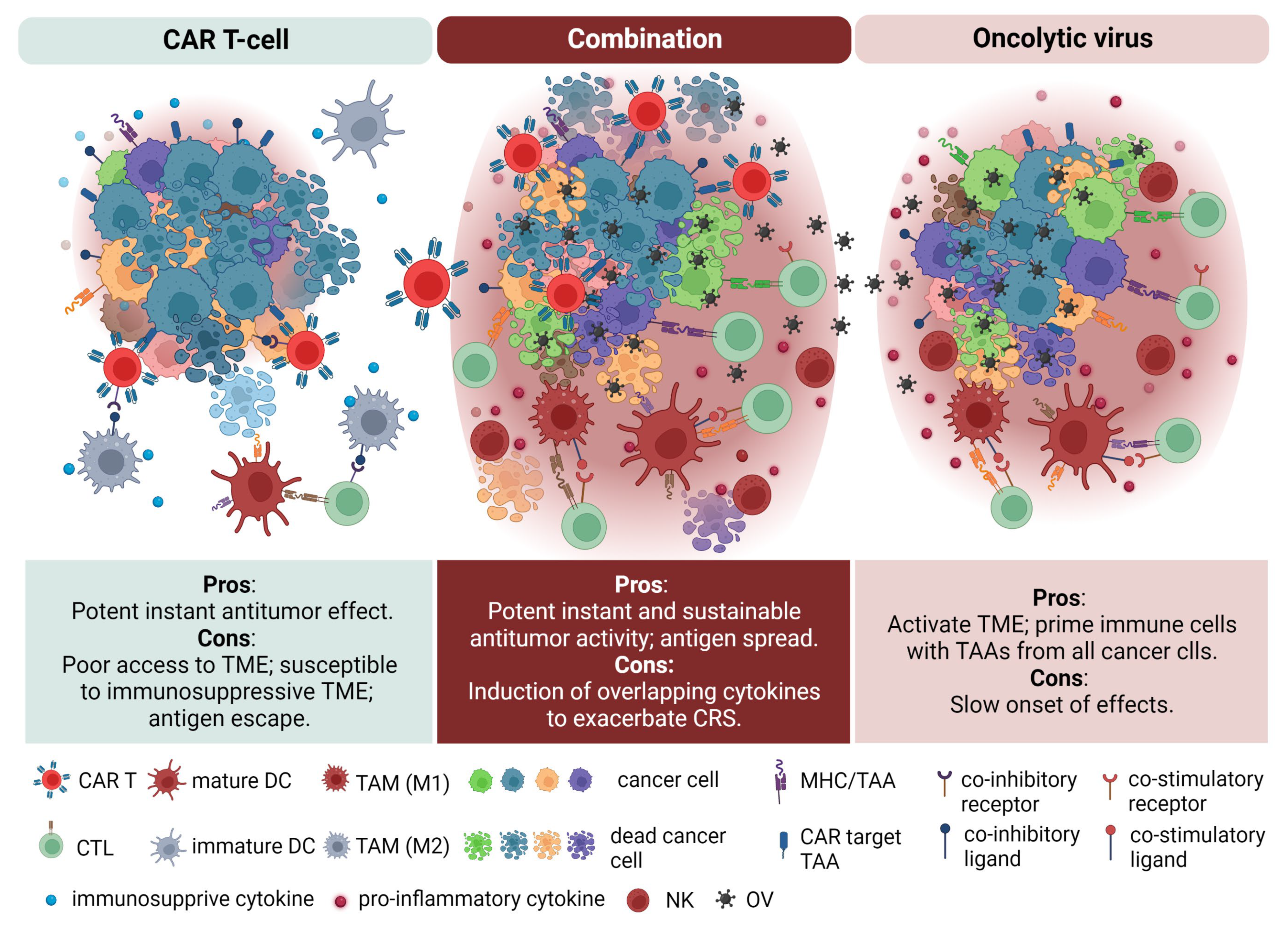
4. Combination of CAR T Cell Therapy and Oncolytic Virotherapy in Pediatric Solid Tumors
5. Future Perspectives and Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Pappo, A.; Dyer, M.A. Pediatric solid tumor genomics and developmental pliancy. Oncogene 2015, 34, 5207–5215. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.R.; Wilson, R.K.; Zhang, J.; Mardis, E.R.; Pui, C.-H.; Ding, L.; Ley, T.J.; Evans, W.E. The Pediatric Cancer Genome Project. Nat. Genet. 2012, 44, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Rahal, Z.; Abdulhai, F.; Kadara, H.; Saab, R. Genomics of adult and pediatric solid tumors. Am. J. Cancer Res. 2018, 8, 1356–1386. [Google Scholar] [PubMed]
- ICCC Recode Third Edition ICD-O-3/IARC 2017. Available online: https://seer.cancer.gov/iccc/iccc-iarc-2017.html#fn (accessed on 27 February 2022).
- Trubicka, J.; Grajkowska, W.; Dembowska-Bagińska, B. Molecular Markers of Pediatric Solid Tumors—Diagnosis, Optimizing Treatments, and Determining Susceptibility: Current State and Future Directions. Cells 2022, 11, 1238. [Google Scholar] [CrossRef]
- Butler, E.; Ludwig, K.; Pacenta, H.L.; Klesse, L.J.; Watt, T.C.; Laetsch, T.W. Recent progress in the treatment of cancer in children. CA Cancer J. Clin. 2021, 71, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Nigro, O.; Ferrari, A.; Casanova, M.; Orbach, D.; Leruste, A.; Gatz, S.A.; Frappaz, D.; Massimino, M. Controversies on the possible role of immune checkpoint inhibitors in pediatric cancers: Balancing irAEs and efficacy. Tumori J. 2021, 107, 276–281. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.; Wainberg, Z.; Cho, J.; Schellens, J.; Soria, J.; Wen, P.; Zielinski, C.; Cabanillas, M.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Choucair, K.; Morand, S.; Stanbery, L.; Edelman, G.; Dworkin, L.; Nemunaitis, J. TMB: A promising immune-response biomarker, and potential spearhead in advancing targeted therapy trials. Cancer Gene Ther. 2020, 27, 841–853. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Martinez, M.; Moon, E.K. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front. Immunol. 2019, 10, 128. [Google Scholar] [CrossRef]
- Hou, A.J.; Chen, L.C.; Chen, Y.Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 2021, 20, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Boccalatte, F.; Mina, R.; Aroldi, A.; Leone, S.; Suryadevara, C.M.; Placantonakis, D.G.; Bruno, B. Advances and Hurdles in CAR T Cell Immune Therapy for Solid Tumors. Cancers 2022, 14, 5108. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of Oncolytic Viruses: Genesis to Genetic Engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Pérez-Larraya, J.G.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef]
- June, C.H.; Riddell, S.R.; Schumacher, T.N. Adoptive cellular therapy: A race to the finish line. Sci. Transl. Med. 2015, 7, 280ps7. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, R.; Racila, E. CD19 Antigen in Leukemia and Lymphoma Diagnosis and Immunotherapy. Leuk. Lymphoma 1995, 18, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.J.; Rivière, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [PubMed]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves fourth CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Baruch, E.N.; Berg, A.L.; Besser, M.J.; Schachter, J.; Markel, G. Adoptive T cell therapy: An overview of obstacles and opportunities. Cancer 2017, 123, 2154–2162. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Gupta, A.; Cripe, T.P. Immunotherapies for Pediatric Solid Tumors: A Targeted Update. Pediatr. Drugs 2021, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ligon, J.A.; Wessel, K.M.; Shah, N.N.; Glod, J. Adoptive Cell Therapy in Pediatric and Young Adult Solid Tumors: Current Status and Future Directions. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef]
- Straathof, K.; Flutter, B.; Wallace, R.; Jain, N.; Loka, T.; Depani, S.; Wright, G.; Thomas, S.; Cheung, G.W.-K.; Gileadi, T.; et al. Antitumor activity without on-target off-tumor toxicity of GD2–chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: An interim analysis. Nat. Med. 2020, 26, 1686–1690. [Google Scholar] [CrossRef]
- Xu, X.; Huang, W.; Heczey, A.; Liu, D.; Guo, L.; Wood, M.; Jin, J.; Courtney, A.; Liu, B.; Di Pierro, E.; et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin. Cancer Res. 2019, 25, 7126–7138. [Google Scholar] [CrossRef]
- Tumino, N.; Weber, G.; Besi, F.; Del Bufalo, F.; Bertaina, V.; Paci, P.; Quatrini, L.; Antonucci, L.; Sinibaldi, M.; Quintarelli, C.; et al. Polymorphonuclear myeloid-derived suppressor cells impair the anti-tumor efficacy of GD2.CAR T-cells in patients with neuroblastoma. J. Hematol. Oncol. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Hegde, M.; Joseph, S.K.; Pashankar, F.; DeRenzo, C.; Sanber, K.; Navai, S.; Byrd, T.T.; Hicks, J.; Xu, M.L.; Gerken, C.; et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Johnson, A.J.; Wilson, A.L.; Brown, C.; Yokoyama, J.K.; Künkele, A.; Chang, C.A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: An interim analysis. Nat. Med. 2021, 27, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced With a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Cohen, A.R. Brain Tumors in Children. N. Engl. J. Med. 2022, 386, 1922–1931. [Google Scholar] [CrossRef]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Roos, D.E.; Smith, J.G. Randomized trial on radiotherapy for paediatric diffuse intrinsic pontine glioma (DIPG). Radiother. Oncol. 2014, 113, 425. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Wilson, A.L.; Huang, W.; Seidel, K.; Brown, C.; Gustafson, J.A.; Yokoyama, J.K.; Johnson, A.J.; Baxter, B.A.; Koning, R.W.; et al. Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov. 2022, 13, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662, Erratum in Nat. Rev. Drug Discov. 2016, 15, 660. [Google Scholar] [CrossRef]
- Lichty, B.D.; Breitbach, C.J.; Stojdl, D.F.; Bell, J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 559–567. [Google Scholar] [CrossRef]
- Nguyen, T.; Avci, N.G.; Shin, D.H.; Martinez-Velez, N.; Jiang, H. Tune Up In Situ Autovaccination against Solid Tumors with Oncolytic Viruses. Cancers 2018, 10, 171. [Google Scholar] [CrossRef]
- de la Nava, D.; Selvi, K.M.; Alonso, M.M. Immunovirotherapy for Pediatric Solid Tumors: A Promising Treatment That is Becoming a Reality. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Louten, J. Virus Structure and Classification. Essential Human Virol. 2016, 19–29. [Google Scholar] [CrossRef]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.; Cho, C.; Chiocca, E. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur. J. Pharmacol. 2018, 837, 117–126. [Google Scholar] [CrossRef]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Malogolovkin, A.; Gasanov, N.; Egorov, A.; Weener, M.; Ivanov, R.; Karabelsky, A. Combinatorial Approaches for Cancer Treatment Using Oncolytic Viruses: Projecting the Perspectives through Clinical Trials Outcomes. Viruses 2021, 13, 1271. [Google Scholar] [CrossRef]
- Martinez-Quintanilla, J.; Seah, I.; Chua, M.; Shah, K. Oncolytic viruses: Overcoming translational challenges. J. Clin. Investig. 2019, 129, 1407–1418. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Sherif, S.; Mall, R.; Almeer, H.; Naik, A.; Al Homaid, A.; Thomas, R.; Roelands, J.; Narayanan, S.; Mohamed, M.G.; Bedri, S.; et al. Immune-related 3-lncRNA signature with prognostic connotation in a multi-cancer setting. J. Transl. Med. 2022, 20, 1–20. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Molinari, V.; Jones, D.T.; Izquierdo, E.; Brouwer-Visser, J.; Giangaspero, F.; Haberler, C.; Pietsch, T.; Jacques, T.S.; et al. Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial. Cancer Cell 2018, 33, 829–842.e5. [Google Scholar] [CrossRef]
- Casey, D.L.; Cheung, N.-K.V. Immunotherapy of Pediatric Solid Tumors: Treatments at a Crossroads, with an Emphasis on Antibodies. Cancer Immunol. Res. 2020, 8, 161–166. [Google Scholar] [CrossRef]
- Lieberman, N.A.P.; DeGolier, K.; Kovar, H.M.; Davis, A.; Hoglund, V.; Stevens, J.; Winter, C.; Deutsch, G.; Furlan, S.N.; Vitanza, N.A.; et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: Implications for development of immunotherapy. Neuro-Oncology 2018, 21, 83–94. [Google Scholar] [CrossRef]
- Jiang, H.; Clise-Dwyer, K.; Ruisaard, K.E.; Fan, X.; Tian, W.; Gumin, J.; Lamfers, M.L.; Kleijn, A.; Lang, F.F.; Yung, W.-K.A.; et al. Delta-24-RGD Oncolytic Adenovirus Elicits Anti-Glioma Immunity in an Immunocompetent Mouse Model. PLoS ONE 2014, 9, e97407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Rivera-Molina, Y.; Gomez-Manzano, C.; Clise-Dwyer, K.; Bover, L.; Vence, L.M.; Yuan, Y.; Lang, F.F.; Toniatti, C.; Hossain, M.B.; et al. Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Res 2017, 77, 3894–3907. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef] [PubMed]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Ahern, C.; Weigel, B.; Poirier, J.; Rudin, C.; Chen, Y.; Cripe, T.; Bernhardt, M.; Blaney, S. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: A report of the Children′s Oncology Group. Pediatr. Blood Cancer 2015, 62, 743–750. [Google Scholar] [CrossRef]
- Schenk, E.L.; Mandrekar, S.J.; Dy, G.K.; Aubry, M.C.; Tan, A.D.; Dakhil, S.R.; Sachs, B.A.; Nieva, J.J.; Bertino, E.; Hann, C.L.; et al. A Randomized Double-Blind Phase II Study of the Seneca Valley Virus (NTX-010) versus Placebo for Patients with Extensive-Stage SCLC (ES SCLC) Who Were Stable or Responding after at Least Four Cycles of Platinum-Based Chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J. Thorac. Oncol. 2019, 15, 110–119. [Google Scholar] [CrossRef]
- Streby, K.A.; Geller, J.; Currier, M.; Warren, P.; Racadio, J.; Towbin, A.; Vaughan, M.; Triplet, M.; Ott-Napier, K.; Dishman, D.; et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer PatientsPhase I Trial of Oncolytic HSV in Children and Young Adults. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef]
- Streby, K.A.; Currier, M.A.; Triplet, M.; Ott, K.; Dishman, D.J.; Vaughan, M.R.; Ranalli, M.A.; Setty, B.; Skeens, M.A.; Whiteside, S.; et al. First-in-Human Intravenous Seprehvir in Young Cancer Patients: A Phase 1 Clinical Trial. Mol. Ther. 2019, 27, 1930–1938. [Google Scholar] [CrossRef]
- Fueyo, J.; Alemany, R.; Gomez-Manzano, C.; Fuller, G.; Khan, A.; Conrad, C.A.; Liu, T.-J.; Jiang, H.; Lemoine, M.G.; Suzuki, K.; et al. Preclinical Characterization of the Antiglioma Activity of a Tropism-Enhanced Adenovirus Targeted to the Retinoblastoma Pathway. Gynecol. Oncol. 2003, 95, 652–660. [Google Scholar] [CrossRef]
- Sherif, S.; Roelands, J.; Mifsud, W.; Ahmed, E.I.; Raynaud, C.M.; Rinchai, D.; Sathappan, A.; Maaz, A.; Saleh, A.; Ozer, E.; et al. The immune landscape of solid pediatric tumors. J. Exp. Clin. Cancer Res. 2022, 41, 1–18. [Google Scholar] [CrossRef]
- Sadozai, H.; Gruber, T.; Hunger, R.E.; Schenk, M. Recent Successes and Future Directions in Immunotherapy of Cutaneous Melanoma. Front. Immunol. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shin, D.H.; Nguyen, T.T.; Fueyo, J.; Fan, X.; Henry, V.; Carrillo, C.C.; Yi, Y.; Alonso, M.M.; Collier, T.L.; et al. Localized Treatment with Oncolytic Adenovirus Delta-24-RGDOX Induces Systemic Immunity against Disseminated Subcutaneous and Intracranial Melanomas. Clin. Cancer Res. 2019, 25, 6801–6814. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Clements, D.R.; Sterea, A.M.; Jang, H.W.; Gujar, S.A.; Lee, P.W.K. Dendritic Cells in Oncolytic Virus-Based Anti-Cancer Therapy. Viruses 2015, 7, 6506–6525. [Google Scholar] [CrossRef] [PubMed]
- Hofman, L.; Lawler, S.; Lamfers, M. The Multifaceted Role of Macrophages in Oncolytic Virotherapy. Viruses 2021, 13, 1570. [Google Scholar] [CrossRef] [PubMed]
- Früh, K.; Yang, Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr. Opin. Immunol. 1999, 11, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Tumor immunotherapy: The tumor cell as an antigen-presenting cell. Curr. Opin. Immunol. 1994, 6, 722–727. [Google Scholar] [CrossRef]
- Nishio, N.; Diaconu, I.; Liu, H.; Cerullo, V.; Caruana, I.; Hoyos, V.; Bouchier-Hayes, L.; Savoldo, B.; Dotti, G. Armed Oncolytic Virus Enhances Immune Functions of Chimeric Antigen Receptor–Modified T Cells in Solid Tumors. Cancer Res 2014, 74, 5195–5205. [Google Scholar] [CrossRef]
- Rosewell Shaw, A.; Porter, C.E.; Watanabe, N.; Tanoue, K.; Sikora, A.; Gottschalk, S.; Brenner, M.K.; Suzuki, M. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol. Ther. 2017, 25, 2440–2451. [Google Scholar] [CrossRef]
- Watanabe, N.; McKenna, M.; Shaw, A.R.; Suzuki, M. Clinical CAR-T Cell and Oncolytic Virotherapy for Cancer Treatment. Mol. Ther. 2021, 29, 505–520. [Google Scholar] [CrossRef]
- Moon, E.K.; Wang, L.-C.S.; Bekdache, K.; Lynn, R.C.; Lo, A.; Thorne, S.H.; Albelda, S.M. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 2017, 7, e1395997. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.E.; Shaw, A.R.; Jung, Y.; Yip, T.; Castro, P.D.; Sandulache, V.C.; Sikora, A.; Gottschalk, S.; Ittman, M.M.; Brenner, M.K.; et al. Oncolytic Adenovirus Armed with BiTE, Cytokine, and Checkpoint Inhibitor Enables CAR T Cells to Control the Growth of Heterogeneous Tumors. Mol. Ther. 2020, 28, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, F.; Zhang, A.; Zhang, D.; Nie, W.; Xu, T.; Han, B.; Seth, P.; Wang, H.; Yang, Y.; et al. Oncolytic adenovirus targeting TGF-β enhances anti-tumor responses of mesothelin-targeted chimeric antigen receptor T cell therapy against breast cancer. Cell. Immunol. 2020, 348, 104041. [Google Scholar] [CrossRef]
- Chen, T.; Ding, X.; Liao, Q.; Gao, N.; Chen, Y.; Zhao, C.; Zhang, X.; Xu, J. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J. Immunother. Cancer 2021, 9, e001647. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.R.; Porter, C.E.; Yip, T.; Mah, W.-C.; McKenna, M.K.; Dysthe, M.; Jung, Y.; Parihar, R.; Brenner, M.K.; Suzuki, M. Oncolytic adeno-immunotherapy modulates the immune system enabling CAR T-cells to cure pancreatic tumors. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Tanoue, K.; Shaw, A.R.; Watanabe, N.; Porter, C.; Rana, B.; Gottschalk, S.; Brenner, M.; Suzuki, M. Armed Oncolytic Adenovirus–Expressing PD-L1 Mini-Body Enhances Antitumor Effects of Chimeric Antigen Receptor T Cells in Solid Tumors. Cancer Res. 2017, 77, 2040–2051. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef]
- Aalipour, A.; Le Boeuf, F.; Tang, M.; Murty, S.; Simonetta, F.; Lozano, A.X.; Shaffer, T.M.; Bell, J.C.; Gambhir, S.S. Viral Delivery of CAR Targets to Solid Tumors Enables Effective Cell Therapy. Mol. Ther. Oncolytics 2020, 17, 232–240. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Ma, J.; Wang, X.; Zhao, W.; Hossain, A.; Yang, Y. Adenovirus-mediated specific tumor tagging facilitates CAR-T therapy against antigen-mismatched solid tumors. Cancer Lett. 2020, 487, 1–9. [Google Scholar] [CrossRef]
- Park, A.K.; Fong, Y.; Kim, S.-I.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.-C.; Chen, N.G.; Thomas, S.H.; et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Wing, A.; Fajardo, C.A.; Posey, A.D.; Shaw, C.; Da, T.; Young, R.M.; Alemany, R.; June, C.H.; Guedan, S. Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus–Driven Production of a Bispecific T-cell Engager. Cancer Immunol. Res. 2018, 6, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Luo, Y.; Da, T.; Guedan, S.; Ruella, M.; Scholler, J.; Keith, B.; Young, R.; Engels, B.; Sorsa, S.; et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 2018, 3, e99573. [Google Scholar] [CrossRef]
- Evgin, L.; Kottke, T.; Tonne, J.; Thompson, J.; Huff, A.L.; van Vloten, J.; Moore, M.; Michael, J.; Driscoll, C.; Pulido, J.; et al. Oncolytic virus–mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gottschalk, S.; Song, X.-T. Synergistic Antitumor Effects of Chimeric Antigen Receptor-Modified T Cells and Oncolytic Virotherapy. Blood 2014, 124, 5808. [Google Scholar] [CrossRef]
- Slaney, C.Y.; von Scheidt, B.; Davenport, A.J.; Beavis, P.A.; Westwood, J.A.; Mardiana, S.; Tscharke, D.C.; Ellis, S.; Prince, H.M.; Trapani, J.A.; et al. Dual-specific Chimeric Antigen Receptor T Cells and an Indirect Vaccine Eradicate a Variety of Large Solid Tumors in an Immunocompetent, Self-antigen Setting. Clin. Cancer Res. 2017, 23, 2478–2490. [Google Scholar] [CrossRef]
- Wenthe, J.; Naseri, S.; Labani-Motlagh, A.; Enblad, G.; Wikström, K.; Eriksson, E.; Loskog, A.; Lövgren, T. Boosting CAR T-cell responses in lymphoma by simultaneous targeting of CD40/4-1BB using oncolytic viral gene therapy. Cancer Immunol. Immunother. 2021, 70, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, J.; Zhang, Q.; Jin, G.; Su, X.; Liu, S.; Liu, F. Enhancement of CD70-specific CAR T treatment by IFN-γ released from oHSV-1-infected glioblastoma. Cancer Immunol. Immunother. 2022, 71, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Chalise, L.; Kato, A.; Ohno, M.; Maeda, S.; Yamamichi, A.; Kuramitsu, S.; Shiina, S.; Takahashi, H.; Ozone, S.; Yamaguchi, J.; et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Δ combination therapy against glioblastoma. Mol. Ther. Oncolytics 2022, 26, 265–274. [Google Scholar] [CrossRef]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.-E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Michieletto, D.; Lusic, M.; Marenduzzo, D.; Orlandini, E. Physical principles of retroviral integration in the human genome. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Russo-Carbolante, E.M.; Picanço-Castro, V.; Alves, D.; Fernandes, A.; Almeida-Porada, G.; Tonn, T.; Covas, D. Integration pattern of HIV-1 based lentiviral vector carrying recombinant coagulation factor VIII in Sk-Hep and 293T cells. Biotechnol. Lett. 2011, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Barber, G.N. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018, 33, 599–605. [Google Scholar] [CrossRef]
- Kurts, C.; Robinson, B.W.S.; Knolle, P.A. Cross-priming in health and disease. Nat. Rev. Immunol. 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Siegler, E.L.; Kenderian, S.S. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Decker, T.; Stockinger, S.; Karaghiosoff, M.; Muller, M.; Kovarik, P. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest. 2002, 109, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef]
- Zamarin, D.; Holmgaard, R.B.; Ricca, J.; Plitt, T.; Palese, P.; Sharma, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 2017, 8, 14340. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.M.; Zarozinski, C.C.; Lin, M.-Y.; Brehm, M.A.; Chen, H.D.; Welsh, R.M. Attrition of Bystander CD8 T Cells during Virus-Induced T-Cell and Interferon Responses. J. Virol. 2001, 75, 5965–5976. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Kim, S.-K.; Calcagno, C.; Ghersi, D.; Puzone, R.; Celada, F.; Selin, L.K.; Welsh, R.M. IFN-Induced Attrition of CD8 T Cells in the Presence or Absence of Cognate Antigen during the Early Stages of Viral Infections. J. Immunol. 2006, 176, 4284–4295. [Google Scholar] [CrossRef] [PubMed]
- Evgin, L.; Huff, A.L.; Wongthida, P.; Thompson, J.; Kottke, T.; Tonne, J.; Schuelke, M.; Ayasoufi, K.; Driscoll, C.B.; Shim, K.G.; et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Breitbach, C.J.; De Silva, N.S.; Falls, T.; Aladl, U.; Evgin, L.; Paterson, J.; Sun, Y.Y.; Roy, D.; Rintoul, J.L.; Daneshmand, M.; et al. Targeting Tumor Vasculature With an Oncolytic Virus. Mol. Ther. 2011, 19, 886–894. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.-H.; et al. Oncolytic Vaccinia Virus Disrupts Tumor-Associated Vasculature in Humans. Cancer Res 2013, 73, 1265–1275. [Google Scholar] [CrossRef]
- Matuszewska, K.; Santry, L.A.; Van Vloten, J.P.; Auyeung, A.W.K.; Major, P.P.; Lawler, J.; Wootton, S.K.; Bridle, B.W.; Petrik, J. Combining Vascular Normalization with an Oncolytic Virus Enhances Immunotherapy in a Preclinical Model of Advanced-Stage Ovarian Cancer. Clin. Cancer Res. 2019, 25, 1624–1638. [Google Scholar] [CrossRef]
| Cancer Type | Phase | NCT | Age Range, Years | Cell Target | Route of Delivery for CAR T Cell | CAR T Cell Therapy | Cotherapy | Status |
|---|---|---|---|---|---|---|---|---|
| Solid tumors | I/II | NCT04432649 | 1–75 | B7-H3 (CD267) | Intravenous | Anti-CD267 4S CAR T cells | N/A | Recruiting |
| Relapsed/refractory non-CNS solid tumors | I | NCT04483778 | 0–26 | B7-H3 (CD267) | Intravenous | 4-1BBζ B7H3-EGFRt-DHFR; 4-1BBζ CD19-Her2tG | N/A | Recruiting |
| CD267-positive advanced solid tumors | I | NCT04864821 | 1–70 | B7-H3 (CD267) | Intravenous | Anti-CD267 CAR T cells | N/A | Not yet Recruiting |
| Relapsed/refractory CD267-positive solid tumors | I | NCT04897321 | 0–21 | B7-H3 (CD267) | Intravenous | Anti-CD267 CAR T cells | Lymphodepletion with cyclophosphamide and fludarabine | Recruiting |
| Solid tumors | N/A | NCT04691713 | 3–70 | B7-H3 (CD267) | Intravenous | Anti-CD267 CAR T cells | N/A | Recruiting |
| DIPG and relapse/refractory brain tumors | I | NCT04185038 | 1–26 | B7-H3 (CD267) | Locoregional | Anti-CD267 CAR T cells | N/A | Recruiting |
| Non-CNS solid tumors | I | NCT03618381 | 1–30 | EGFR | Intravenous | 4-1BBζ EGFR806-EGFRt; 4 1BBζ CD19-Her2tG | N/A | Recruiting |
| Relapsed/refractory brain tumors | I | NCT03638167 | 1–26 | EGFR806 | Locoregional | Anti EGFR806-specific CAR T cells | N/A | Recruiting |
| High risk and/or relapsed/refractory NB or other GD2-positive solid tumors | I/II | NCT03373097 [42] | 1–25 | GD2 | Intravenous | Anti-GD2 CAR T cells | N/A | Recruiting |
| Relapsed/refractory NB | I | NCT02761915 [39] | 0–1 | GD2 | Intravenous | Anti-GD2 CAR T cells | Lymphodepletion with leukapheresis, cyclophosphimide, fludarabine | Complete |
| GD2-positive OS, NB, or melanoma | I | NCT02107963 | 1–35 | GD2 | Intravenous | Anti-GD2 CAR T cells | Lymphodepletion cyclophosphamide and AP1903 | Complete |
| Relapsed/refractory solid tumors | I/II | NCT02992210 | 1–65 | GD2 | Intravenous | Anti-GD2 4S CAR T cells | N/A | Unknown |
| NB | I | NCT01822652 | All ages | GD2 | Intravenous | iC9-GD2 CAR T Cells | Lymphodepletion with Cyclophosphamide, fludarabine, pembrolizumab, other PD-1 inhibitors | Active not recruiting |
| NB | I | NCT00085930 | 1–21 | GD2 | Intravenous | Anti-GD2 CAR EBV-specific CTLs | N/A | Active not recruiting |
| DIPG or spinal DMG | I | NCT04196413 | 2–30 | GD2 | Intravenous | Anti-GD2 CAR T cells | Lymphodeption with fludarabineand cyclophosphamide | Recruiting |
| GD2-postive brain tumors | I | NCT04099797 | 1–21 | GD2 | Intravenous | C7R-GD2.CAR T cells | lymphodepletion chemotherapy | Recruiting |
| NB | I | NCT03294954 [40] | 1–21 | GD2 | Intravenous | Anti-GD2CAR NKT cells expressing IL-15 | Lymphodepletion with cyclophosphamide and fludarabine | Recruiting |
| Solid tumors | I/II | NCT05437315 | 1–75 | GD2 PSMA | Intravenous | Bi-4SCAR GD2/PSMA T cells | N/A | Recruiting |
| NB | I/II | NCT04637503 | 1–65 | GD2, CD276 (B7-H3), PSMA | Intravenous | Anti-GD2, PSMA, and CD276 CAR-T cells | N/A | Recruiting |
| GPC3-positive solid tumors | I | NCT04377932 | 1–21 | GPC3 | Intravenous | Anti-GPC3 CAR T cells | Lymphodeption with fludarabineand cyclophosphamide | Recruiting |
| GPC3-positive solid tumors | I | NCT04715191 | 1–21 | GPC3 | Intravenous | 15.21.GPC3-CAR T cells | Lymphodeption with fludarabineand cyclophosphamide | Not yet recruiting |
| Liver cancer | I | NCT02932956 | 1–21 | GPC3 | Intravenous | Anti-GPC3 CAR T cells | Lymphodeption with fludarabineand cyclophosphamide | Active not recruiting |
| Advanced sarcomas | I | NCT00902044 [43] | All ages | HER2 | Intravenous | Anti-HER2 CAR T cells | Lymphodeption with fludarabineand cyclophosphamide | Active not recruiting |
| Brain tumors | I | NCT03500991 [44] | 1–26 | HER2 | Locoregional | Anti-HER2 CAR T cells | N/A | Recruiting |
| GBM | I | NCT01109095 | All ages | HER2 | Intravenous | Anti-HER 2 CAR CMV-specific CTLs | N/A | Complete |
| Relapsed/refractory Brain tumors | I | NCT02442297 | >3 | HER2 | Locoregional | Anti-HER2 CAR T cells | N/A | Recruiting |
| Relapsed/refractory IL13Rα2-positive malignant glioma | I | NCT02208362 [45] | 12–75 | IL13Rα2 | Locoregional | IL13(EQ)BBzeta/CD19t+ TCM-enriched T cells | N/A | Active |
| Relapsed/refractory IL13Rα2-positive malignant glioma | I | NCT04510051 | 4–25 | IL13Rα2 | intraventricularly | IL13(EQ)BBzeta/CD19t+ TCM-enriched T Cells | Lymphodepletion | Recruiting |
| Sarcoma, osteosarcoma, or Ewing sarcoma | I/II | NCT03356782 | 1–75 | Sarcoma cell surface antigens | IV | Sarcoma-specific CAR T cells | N/A | Recruiting |
| Cancer Type | Phase | NCT | Age Range, Years | Virus Name | Virus Type/Family | Route of Delivery | Cotherapy | Status |
|---|---|---|---|---|---|---|---|---|
| Treatment-naïve DIPG or DMG | I | NCT03178032 [18] | 1–18 | Adenovirus (DNX-2401) | Adenoviridae | Intratumoral injection | Neoadjuvant therapy | Complete |
| Refractory retinoblastoma | I | NCT03284268 | 1–12 | Adenovirus (VCN-01) | Adenoviridae | Intravitreal injection | Systemic intraarterial or intravitreal chemotherapy or radiotherapy | Recruiting |
| Brain tumors | I/II | NCT03330197 | 0–21 | Adenovirus (Ad-RTS-hIL-12) | Adenoviridae | Intratumoral injection | Oral Vekedimex | Terminated |
| Recurrent high-grade gliomas | II | NCT04482933 | 3–21 | HSV G207 | Herpesviridae | Intratumoral injection | Radiation | Not yet R |
| Recurrent cerebellar solid tumors | I | NCT03911388 | 3–18 | HSV G207 | Herpesviridae | Intratumoral injection | - | Recruiting |
| Recurrent CNS supratentorial neoplasms | I | NCT02457845 | 3–18 | HSV G207 | Herpesviridae | Intratumoral injection | Radiation | Active, not yet recruiting |
| Non-CNS solid tumors | I | NCT00931931 | 7–30 | HSV1716 | Herpesviridae | Intratumoral injection or intravenous | - | Complete |
| Recurrent childhood CNS solid tumors that can be removed by surgery | I | NCT02031965 | 12–21 | HSV-1716 | Herpesviridae | Intratumoral injection | Dexamethasone, conventional surgery/resection | Terminated |
| Recurrent MB or recurrent ATRT | NCT02962167 | 1–39 | Modified Measles Virus (MV-NIS) | Paramyxoviridae | Intratumoral injection or intrathecal | - | Recruiting | |
| GBM, NB, or sarcoma | I/II | NCT01174537 | 3–75 | NDV | Paramyxoviridae | Intravenous | - | Withdrawn |
| Metastatic cancers resistant to conventional anticancer treatments | II | NCT00348842 | All ages | NDV | Paramyxoviridae | Intratumoral injection or intravenous | - | Withdrawn |
| Recurrent malignant gliomas | Ib | NCT03043391 | 12–21 | Polio/Rhinovirus Recombinant; PVS-RIPO | Picornaviridae | Intratumoral injection | - | Active, not yet recruiting |
| Non-CNS solid tumors | I | NCT01169584 | 2–21 | Recombinant Vaccinia Virus | Poxviridae | Intratumoral injection | - | Complete |
| Non-CNS bone and soft tissue sarcomas metastatic to the lung | II | NCT00503295 | >16 | Reovirus (REOLYSIN®) | Reoviridae | Intravenous | - | Complete |
| Relapsed/refractory ST with neuroendocrine features | I | NCT01048892 | 3–21 | Seneca Valley virus-001 | Picoranviridea | intravenous | Cyclophosphamide | Complete |
| Cancer Type | Study Year/Author | CAR T Cell Target | Oncolytic Agent | Route of Delivery |
|---|---|---|---|---|
| Neuroblastoma | 2014/Nishio [89] | GD2 | Onc.Ad-Rantes/IL-15 | Intravenous CART. Intertumoral OAdV |
| Lung cancer | 2014/Wang [105] | HER2 | EphA2-TEA-VV | N/A |
| Breast or liver tumor | 2016/Slaney [106] | HER2, melanocyte protein (gp100) | VV-gp100 | Intravenous |
| Head and neck squamous cell carcinoma | 2017/Rosewell [90] | HER2 | CAdVECIL12p70/aPDL1 | Intravenous CART. Intertumoral CAdV |
| Prostate cancer or squamous cell carcinoma | 2017/Tanoue [97] | HER2 | CAdVEC-aPDL1 | Intravenous CART. Intertumoral CAdV |
| Pancreatic ductal carcinoma or colorectal carcinoma | 2018/Wing [102] | Folate receptor alpha | Onc.Ad-EGFR BiTE | Intravenous CART. Intertumoral OAd-BiTE |
| Pancreatic ductal carcinoma | 2018/Watanabe [103] | Mesothelin | Onc.Ad-TNFa/IL-2 | Intravenous CART. Intertumoral/Intravenous OV |
| Lung cancer | 2018/Moon [92] | Mesothelin | VV.CXCL-11 | Intravenous CART. Intertumoral/Intravenous OV |
| Breast cancer | 2019/Park [101] | CD19 | OV19t | Intravenous CART. Intertumoral OV19t |
| PDAC or squamous cell carcinoma | 2020/Porter [93] | HER2 | CAdTrio | Intravenous CART, Intertumoral CAdTrio |
| Breast cancer | 2020/Li [94] | Mesothelin | rAd.sT | N/A |
| Melanoma | 2020/Aalipour [99] | CD19 | mCD19VV | Intertumoral |
| Liver cancer or hepatocellular carcinoma | 2020/Tang [100] | CD19 | AdC68-TMC-tCD19 | N/A |
| B cell lymphoma | 2021/Wenthe [107] | CD19 | LOAd703 | Intravenous CART. Intertumoral LOAd703 |
| PDAC | 2021/Rosewell [96] | HER2 | CAdTrio | Intravenous CART, Intertumoral CAdTrio |
| GBM | 2021/Huang [98] | B7H3 | oAD-IL7 | Intravenous CART, Intertumoral oAD-IL7 |
| Solid tumor | 2021/Chen [95] | CD19 | rTTVΔTK-IL21 | Intravenous CART, Intertumoral rTTVΔTK-IL21 |
| Subcutaneous melanoma or intracranial glioma tumor | 2022/Evgin [104] | EGFRvIII | VSIV-mIFN β | Intravenous |
| GBM | 2022/Zhu [108] | CD70 | oHSV-1 | Intertumoral |
| GBM | 2022/Chalise [109] | LpMab-2 | G47 Δ (third-generation oncolytic HSV-1) | Intravenous CART. Intertumoral G45 Δ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Munir, F.; Ragoonanan, D.; Zaky, W.; Khazal, S.J.; Tewari, P.; Fueyo, J.; Gomez-Manzano, C.; Jiang, H. Combining CAR T Cell Therapy and Oncolytic Virotherapy for Pediatric Solid Tumors: A Promising Option. Immuno 2023, 3, 37-56. https://doi.org/10.3390/immuno3010004
He J, Munir F, Ragoonanan D, Zaky W, Khazal SJ, Tewari P, Fueyo J, Gomez-Manzano C, Jiang H. Combining CAR T Cell Therapy and Oncolytic Virotherapy for Pediatric Solid Tumors: A Promising Option. Immuno. 2023; 3(1):37-56. https://doi.org/10.3390/immuno3010004
Chicago/Turabian StyleHe, Jiasen, Faryal Munir, Dristhi Ragoonanan, Wafik Zaky, Sajad J Khazal, Priti Tewari, Juan Fueyo, Candelaria Gomez-Manzano, and Hong Jiang. 2023. "Combining CAR T Cell Therapy and Oncolytic Virotherapy for Pediatric Solid Tumors: A Promising Option" Immuno 3, no. 1: 37-56. https://doi.org/10.3390/immuno3010004
APA StyleHe, J., Munir, F., Ragoonanan, D., Zaky, W., Khazal, S. J., Tewari, P., Fueyo, J., Gomez-Manzano, C., & Jiang, H. (2023). Combining CAR T Cell Therapy and Oncolytic Virotherapy for Pediatric Solid Tumors: A Promising Option. Immuno, 3(1), 37-56. https://doi.org/10.3390/immuno3010004







