Dysregulated Oxidative Stress Pathways in Schizophrenia: Integrating Single-Cell Transcriptomic and Human Biomarker Evidence
Abstract
1. Introduction
- To quantify key oxidative stress markers in the serum of schizophrenic patients;
- To assess liver function parameters in schizophrenic individuals;
- To compare oxidative stress markers and liver function test parameters between schizophrenic patients and healthy controls.
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Diagnosis and Blood Sample Collection
2.4. Determination of Oxidative Stress Markers
2.4.1. Single-Cell RNA Sequencing (scRNA-Seq) Data Analysis
2.4.2. Lipid Peroxidation (MDA) Assay
2.4.3. Advanced Protein Oxidation Product (APOP) Assay
2.4.4. Nitric Oxide (NO) Assay
2.4.5. Superoxide Dismutase (SOD) Activity
2.4.6. Catalase (CAT) Activity
2.4.7. Glutathione (GSH) Assay
2.5. Liver Function Tests (LFT)
2.6. Data Analysis
3. Result
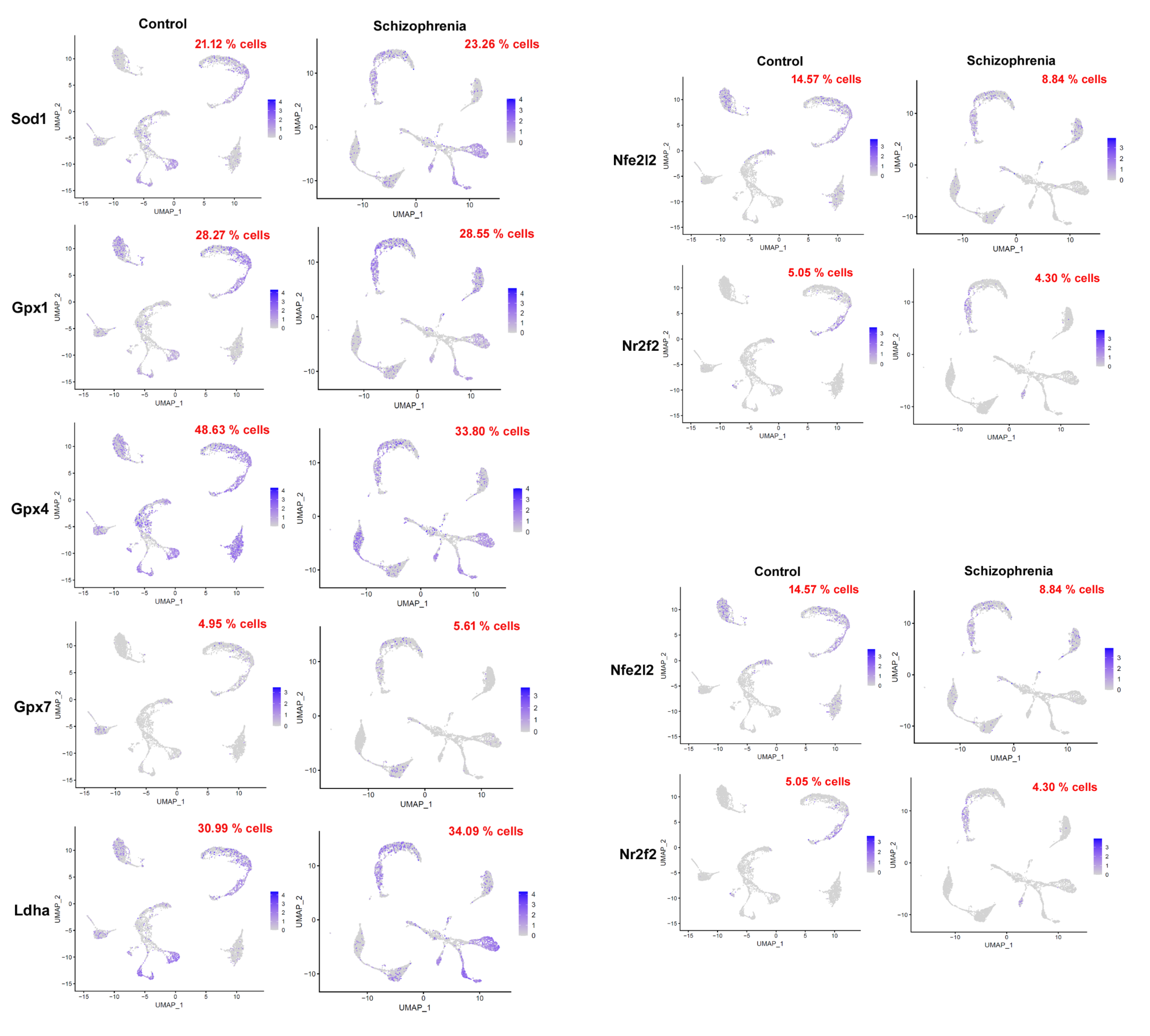
3.1. High Expression of the Oxidative Stress Biomarker (In Schizophrenia-Induced Mouse Single-Cell Data)
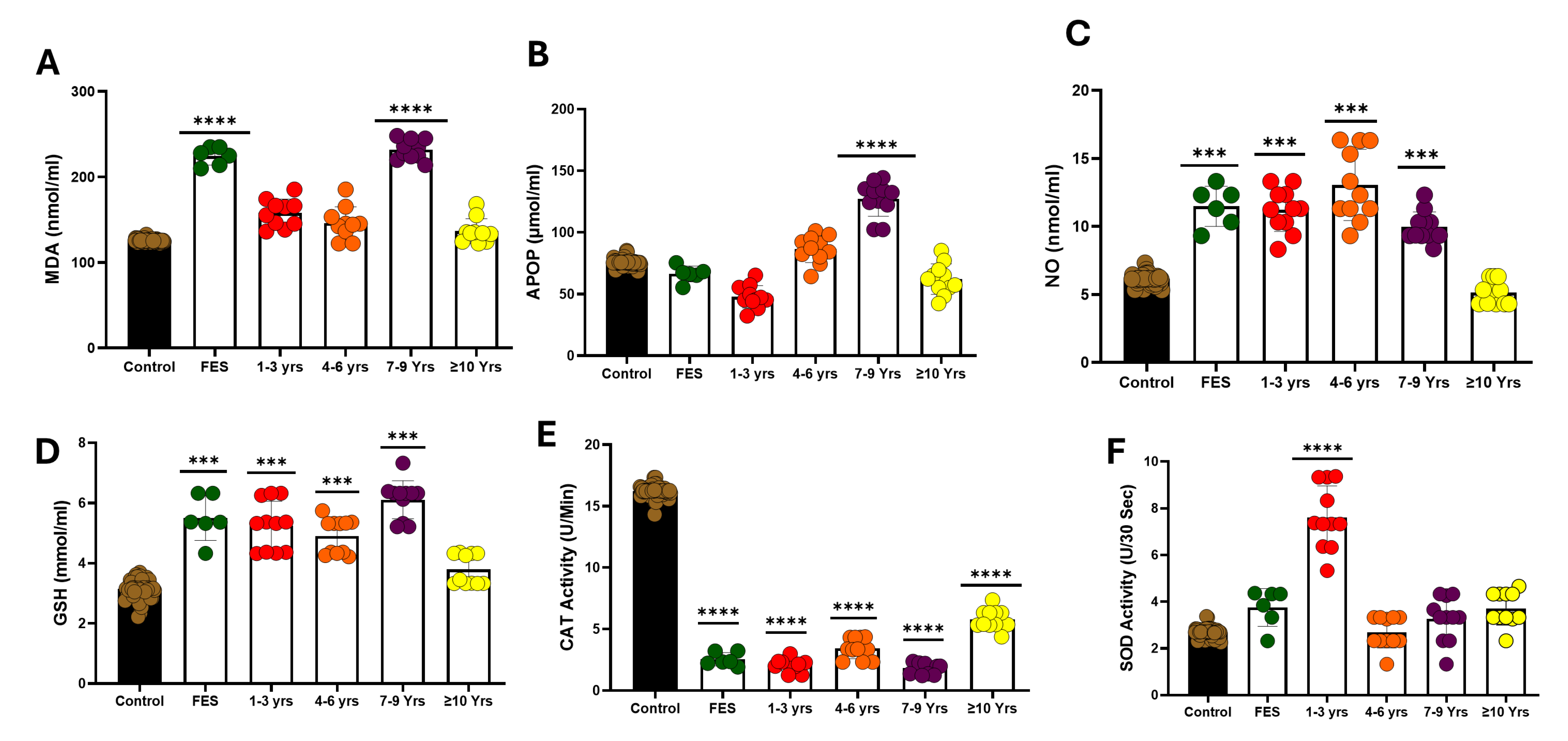
3.2. Serum Oxidative Stress
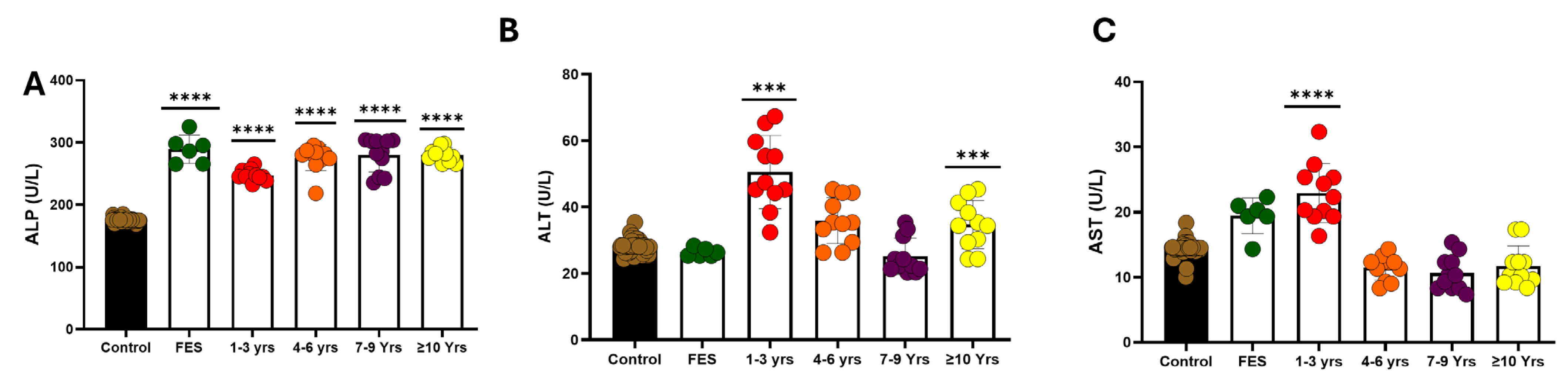
3.3. Liver Function Test
3.4. Serum Oxidative Stress in Various Lengths of Disease
3.5. Liver Function Test in Various Lengths of Disease
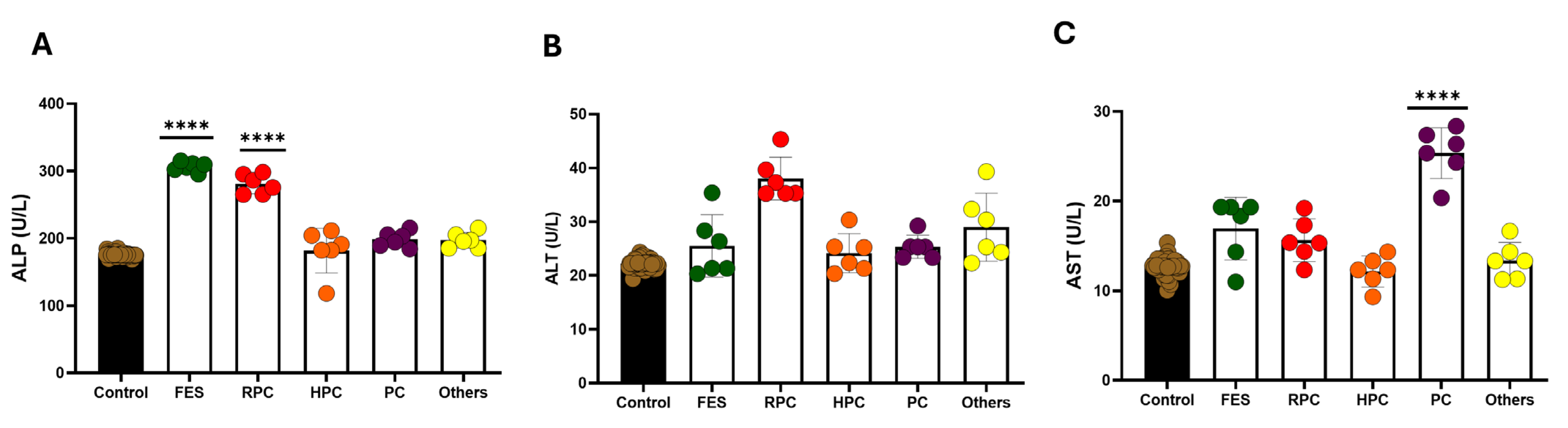
3.6. Effect of Treatment on Oxidative Stress Markers in Serum
3.7. Effect of Treatment on Liver Function
4. Discussion
5. Limitations of the Present Study
- Other parameters that influence oxidative stress and antioxidant enzyme levels were not included in the study, like dietary habits, smoking habits, lifestyles, etc.
- All the selected cases were acute, which might influence the oxidative stress and antioxidant enzyme levels. Comparisons needed to be made with chronic cases.
- Measurements of oxidative stress and antioxidant enzyme levels were performed before the treatment was started; no subsequent measurements were performed to verify the changes in these parameters with the treatment or improvement in the disease process.
- The deficit of glutathione may result in impairment of the myelination process in the brain’s white matter in schizophrenia [47].
- The result of the present study showed an alteration of liver function parameters, including AST, ALT, and ALP. The findings of the present study are consistent with past studies [68].
- We acknowledge that lifestyle-related variables such as smoking, diet, BMI, and antipsychotic medication use were not controlled for in this study and may act as confounders. Future studies should incorporate these factors to better isolate disease-related oxidative and hepatic changes.
6. Duration of Disease
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| MDA | malondialdehyde |
| NO | nitric oxide |
| GSH | glutathione |
| SOD | superoxide dismutase |
| CAT | catalase |
| APOP | advanced oxidation protein prod MDA and NO uct |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
References
- Schizophrenia—National Institute of Mental Health (NIMH). Available online: https://www.nimh.nih.gov/health/statistics/schizophrenia (accessed on 1 July 2025).
- Luvsannyam, E.; Jain, M.S.; Pormento, M.K.L.; Siddiqui, H.; Balagtas, A.R.A.; Emuze, B.O.; Poprawski, T. Neurobiology of Schizophrenia: A Comprehensive Review. Cureus 2022, 14, e23959. [Google Scholar] [CrossRef]
- Strassnig, M.; Signorile, J.; Gonzalez, C.; Harvey, P.D. Physical Performance and Disability in Schizophrenia. Schizophr. Res. Cogn. 2014, 1, 112–121. [Google Scholar] [CrossRef]
- Sultana, A.; Jahan, S.S.; Jakanta Faika, M.; Islam, T.; Ferdous, N.-E.; Nessa, A. Bangladeshi parents’ knowledge and awareness about cervical cancer and willingness to vaccinate female family members against human papilloma virus: A cross sectional study. Int. J. Community Med. Public Health 2023, 10, 3446–3453. [Google Scholar] [CrossRef]
- Administration, S.A. and M.H.S. Table 3.22, DSM-IV to DSM-5 Schizophrenia Comparison. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519704/table/ch3.t22/ (accessed on 1 July 2025).
- Rahman, T.; Lauriello, J. Schizophrenia: An Overview. Focus 2016, 14, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Shimu, S.J.; Patil, S.M.; Dadzie, E.; Tesfaye, T.; Alag, P.; Więckiewicz, G. Exploring Health Informatics in the Battle against Drug Addiction: Digital Solutions for the Rising Concern. J. Pers. Med. 2024, 14, 556. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D. The Neurodevelopmental Hypothesis of Schizophrenia, Revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef]
- Voineskos, A.N.; Hawco, C.; Neufeld, N.H.; Turner, J.A.; Ameis, S.H.; Anticevic, A.; Buchanan, R.W.; Cadenhead, K.; Dazzan, P.; Dickie, E.W.; et al. Functional Magnetic Resonance Imaging in Schizophrenia: Current Evidence, Methodological Advances, Limitations and Future Directions. World Psychiatry 2024, 23, 26–51. [Google Scholar] [CrossRef]
- MacDonald, H.J.; Kleppe, R.; Szigetvari, P.D.; Haavik, J. The Dopamine Hypothesis for ADHD: An Evaluation of Evidence Accumulated from Human Studies and Animal Models. Front. Psychiatry 2024, 15, 1492126. [Google Scholar] [CrossRef]
- Schoretsanitis, G. The Role of Neurotransmitters in Schizophrenia. Neurosci. Psychiatry Open Access 2024, 7, 239–241. [Google Scholar] [CrossRef]
- Paul, T.; See, J.W.; Vijayakumar, V.; Njideaka-Kevin, T.; Loh, H.; Lee, V.J.Q.; Dogrul, B.N. Neurostructural Changes in Schizophrenia and Treatment-Resistance: A Narrative Review. Psychoradiology 2024, 4, kkae015. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain Structural and Functional Abnormalities in Mood Disorders: Implications for Neurocircuitry Models of Depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Young, K.A.; Manaye, K.F.; Liang, C.-L.; Hicks, P.B.; German, D.C. Reduced Number of Mediodorsal and Anterior Thalamic Neurons in Schizophrenia. Biol. Psychiatry 2000, 47, 944–953. [Google Scholar] [CrossRef]
- Geva, S.; Jentschke, S.; Argyropoulos, G.P.D.; Chong, W.K.; Gadian, D.G.; Vargha-Khadem, F. Volume Reduction of Caudate Nucleus Is Associated with Movement Coordination Deficits in Patients with Hippocampal Atrophy Due to Perinatal Hypoxia-Ischaemia. Neuroimage Clin. 2020, 28, 102429. [Google Scholar] [CrossRef]
- Laricchiuta, D.; Papi, M.; Decandia, D.; Panuccio, A.; Cutuli, D.; Peciccia, M.; Mazzeschi, C.; Petrosini, L. The Role of Glial Cells in Mental Illness: A Systematic Review on Astroglia and Microglia as Potential Players in Schizophrenia and Its Cognitive and Emotional Aspects. Front. Cell. Neurosci. 2024, 18, 1358450. [Google Scholar] [CrossRef]
- Dietz, A.G.; Goldman, S.A.; Nedergaard, M. Glial Cells in Schizophrenia: A Unified Hypothesis. Lancet Psychiatry 2020, 7, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L.; D’Angelo, M.; Monaco, F.; Di Stefano, V.; Steardo, L. Decoding Neural Circuit Dysregulation in Bipolar Disorder: Toward an Advanced Paradigm for Multidimensional Cognitive, Emotional, and Psychomotor Treatment. Neurosci. Biobehav. Rev. 2025, 169, 106030. [Google Scholar] [CrossRef]
- Poggi, G.; Klaus, F.; Pryce, C.R. Pathophysiology in Cortico-Amygdala Circuits and Excessive Aversion Processing: The Role of Oligodendrocytes and Myelination. Brain Commun. 2024, 6, fcae140. [Google Scholar] [CrossRef]
- Bertocci, M.A.; Bergman, J.; Santos, J.P.L.; Iyengar, S.; Bonar, L.; Gill, M.K.; Abdul-Waalee, H.; Bebko, G.; Stiffler, R.; Lockovich, J.; et al. Emotional regulation neural circuitry abnormalities in adult bipolar disorder: Dissociating effects of long-term depression history from relationships with present symptoms. Transl. Psychiatry 2020, 10, 374. [Google Scholar] [CrossRef]
- Schneider, K.N.; Sciarillo, X.A.; Nudelman, J.L.; Cheer, J.F.; Roesch, M.R. Anterior Cingulate Cortex Signals Attention in a Social Paradigm That Manipulates Reward and Shock. Curr. Biol. 2020, 30, 3724–3735.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Lu, J.; Lei, Y.; Li, H. Social Exclusion Increases the Executive Function of Attention Networks. Sci. Rep. 2021, 11, 9494. [Google Scholar] [CrossRef] [PubMed]
- Molnar-Szakacs, I.; Uddin, L.Q. Anterior Insula as a Gatekeeper of Executive Control. Neurosci. Biobehav. Rev. 2022, 139, 104736. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Writer, S. Understanding Neurotransmitters in Schizophrenia Beyond Dopamine. Psychiatrist.com. 30 October 2024. Available online: https://www.psychiatrist.com/news/understanding-neurotransmitters-in-schizophrenia-beyond-dopamine/ (accessed on 20 May 2024).
- Guidara, W.; Messedi, M.; Naifar, M.; Maalej, M.; Grayaa, S.; Omri, S.; Ben Thabet, J.; Maalej, M.; Charfi, N.; Ayadi, F. Predictive value of oxidative stress biomarkers in drug-free patients with schizophrenia and schizo-affective disorder. Psychiatry Res. 2020, 293, 113467. [Google Scholar] [CrossRef] [PubMed]
- Cuenod, M.; Steullet, P.; Cabungcal, J.-H.; Dwir, D.; Khadimallah, I.; Klauser, P.; Conus, P.; Do, K.Q. Caught in Vicious Circles: A Perspective on Dynamic Feed-Forward Loops Driving Oxidative Stress in Schizophrenia. Mol. Psychiatry 2022, 27, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Chen, J.; Ba, H.J.; Yang, C.; Liu, J.W.; Guo, R.; Li, S.L.; Huang, P.; Li, C.T.; Zhang, S.H. Revealing the Oxidative Stress-Related Molecular Characteristics and Potential Therapeutic Targets of Schizophrenia through Integrated Gene Expression Data Analysis. Mol. Neurobiol. 2025, 62, 10484–10498. [Google Scholar] [CrossRef] [PubMed]
- Sertan Copoglu, U.; Virit, O.; Hanifi Kokacya, M.; Orkmez, M.; Bulbul, F.; Binnur Erbagci, A.; Semiz, M.; Alpak, G.; Unal, A.; Ari, M.; et al. Increased Oxidative Stress and Oxidative DNA Damage in Non-Remission Schizophrenia Patients. Psychiatry Res. 2015, 229, 200–205. [Google Scholar] [CrossRef]
- Ruan, Y.; Cheng, J.; Dai, J.; Ma, Z.; Luo, S.; Yan, R.; Wang, L.; Zhou, J.; Yu, B.; Tong, X.; et al. Chronic stress hinders sensory axon regeneration via impairing mitochondrial cristae and OXPHOS. Sci. Adv. 2023, 9, eadh0183. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Zhang, Q.; Liu, Y.-F.; Zheng, W.-Y.; Si, T.L.; Su, Z.; Cheung, T.; Jackson, T.; Li, X.-H.; Xiang, Y.-T. Schizophrenia and Oxidative Stress from the Perspective of Bibliometric Analysis. Front. Psychiatry 2023, 14, 1145409. [Google Scholar] [CrossRef]
- Peng, Z.; Jia, Q.; Mao, J.; Jiang, S.; Ma, Q.; Luo, X.; An, Z.; Huang, A.; Ma, C.; Yi, Q. The Role of Ferroptosis and Oxidative Stress in Cognitive Deficits among Chronic Schizophrenia Patients: A Multicenter Investigation. Schizophrenia 2025, 11, 4. [Google Scholar] [CrossRef]
- Huang, D.; Liu, S. Oxidative Stress and Schizophrenia. J. Psychiatry Brain Sci. 2017, 2, 4. [Google Scholar] [CrossRef]
- Rawani, N.S.; Chan, A.W.; Dursun, S.M.; Baker, G.B. The Underlying Neurobiological Mechanisms of Psychosis: Focus on Neurotransmission Dysregulation, Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction. Antioxidants 2024, 13, 709. [Google Scholar] [CrossRef]
- Ahmed, G.K.; Ramadan, H.K.-A.; Elbeh, K.; Haridy, N.A. The Role of Infections and Inflammation in Schizophrenia: Review of the Evidence. Middle East Curr. Psychiatry 2024, 31, 9. [Google Scholar] [CrossRef]
- Maas, D.A.; Vallès, A.; Martens, G.J.M. Oxidative Stress, Prefrontal Cortex Hypomyelination and Cognitive Symptoms in Schizophrenia. Transl. Psychiatry 2017, 7, e1171. [Google Scholar] [CrossRef]
- Więdłocha, M.; Zborowska, N.; Marcinowicz, P.; Dębowska, W.; Dębowska, M.; Zalewska, A.; Maciejczyk, M.; Waszkiewicz, N.; Szulc, A. Oxidative Stress Biomarkers among Schizophrenia Inpatients. Brain Sciences 2023, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Loven, D.P.; James, J.F.; Biggs, L.; Little, K.Y. Increased Manganese-Superoxide Dismutase Activity in Postmortem Brain from Neuroleptic-Treated Psychotic Patients. Biol. Psychiatry 1996, 40, 230–232. [Google Scholar] [CrossRef]
- Murray, A.J.; Rogers, J.C.; Katshu, M.Z.U.H.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, L.; Cai, Y. Progress in the Study of Oxidative Stress Damage in Patients with Schizophrenia: Challenges and Opportunities. Front. Psychiatry 2025, 16, 1505397. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.M.; Kurtulmus, A.; Gul, A.Z.; Kocyigit, A.; Kirpinar, I. Oxidative Stress and Schizophrenia: A Comparative Cross-Sectional Study of Multiple Oxidative Markers in Patients and Their First-Degree Relatives. Int. J. Clin. Pract. 2021, 75, e14711. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, U.J.; Lee, B.H.; Cha, M. Safeguarding the Brain from Oxidative Damage. Free Radic. Biol. Med. 2025, 226, 143–157. [Google Scholar] [CrossRef]
- Oxidative Stress—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/psychology/oxidative-stress (accessed on 1 July 2025).
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 552535. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Fizíková, I.; Dragašek, J.; Račay, P. Mitochondrial Dysfunction, Altered Mitochondrial Oxygen, and Energy Metabolism Associated with the Pathogenesis of Schizophrenia. Int. J. Mol. Sci. 2023, 24, 7991. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.; Wu, A.; Laksono, I.; Prce, I.; Maheandiran, M.; Kiang, M.; Andreazza, A.C.; Mizrahi, R. Mitochondrial Function in Individuals at Clinical High Risk for Psychosis. Sci. Rep. 2018, 8, 6216. [Google Scholar] [CrossRef]
- Xu, H.; Yang, F. The Interplay of Dopamine Metabolism Abnormalities and Mitochondrial Defects in the Pathogenesis of Schizophrenia. Transl. Psychiatry 2022, 12, 464. [Google Scholar] [CrossRef]
- Sumadewi, K.T.; Harkitasari, S.; Tjandra, D.C. Biomolecular Mechanisms of Epileptic Seizures and Epilepsy: A Review. Acta Epileptol. 2023, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Almohmadi, N.H.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Abdelaziz, A.M.; Jabir, M.S.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Glutamatergic Dysfunction in Neurodegenerative Diseases Focusing on Parkinson’s Disease: Role of Glutamate Modulators. Brain Res. Bull. 2025, 225, 111349. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Fan, G.; Liu, M.; Liu, J.; Huang, Y. The Initiator of Neuroexcitotoxicity and Ferroptosis in Ischemic Stroke: Glutamate Accumulation. Front. Mol. Neurosci. 2023, 16, 1113081. [Google Scholar] [CrossRef]
- Magdaleno Roman, J.Y.; Chapa González, C. Glutamate and Excitotoxicity in Central Nervous System Disorders: Ionotropic Glutamate Receptors as a Target for Neuroprotection. Neuroprotection 2024, 2, 137–150. [Google Scholar] [CrossRef]
- Nicosia, N.; Giovenzana, M.; Misztak, P.; Mingardi, J.; Musazzi, L. Glutamate-Mediated Excitotoxicity in the Pathogenesis and Treatment of Neurodevelopmental and Adult Mental Disorders. Int. J. Mol. Sci. 2024, 25, 6521. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Gong, X.X.; Qin, Z.H.; Wang, Y. Molecular mechanisms of excitotoxicity and their relevance to the pathogenesis of neurodegenerative diseases-an update. Acta Pharmacol. Sin. 2025, in press. [Google Scholar] [CrossRef]
- Korczowska-Łącka, I.; Słowikowski, B.; Piekut, T.; Hurła, M.; Banaszek, N.; Szymanowicz, O.; Jagodziński, P.P.; Kozubski, W.; Permoda-Pachuta, A.; Dorszewska, J. Disorders of Endogenous and Exogenous Antioxidants in Neurological Diseases. Antioxidants 2023, 12, 1811. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Ezema, B.O.; Eze, C.N.; Ayoka, T.O.; Nnadi, C.O. Antioxidant-Enzyme Interaction in Non-Communicable Diseases. J. Explor. Res. Pharmacol. 2024, 9, 262–275. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Obeme-Nmom, J.I.; Abioye, R.O.; Flores, S.S.R.; Udenigwe, C.C. Regulation of Redox Enzymes by Nutraceuticals: A Review of the Roles of Antioxidant Polyphenols and Peptides. Food Funct. 2024, 15, 10956–10980. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Hernández, D.E.; Salvadores, N.A.; Moya-Alvarado, G.; Catalán, R.J.; Bronfman, F.C.; Court, F.A. Axonal Degeneration Induced by Glutamate Excitotoxicity Is Mediated by Necroptosis. J. Cell Sci. 2018, 131, jcs214684. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.-Y.; Wang, L.; Li, Y.; Xiao, X. Genetic Insights of Schizophrenia via Single Cell RNA-Sequencing Analyses. Schizophr. Bull. 2023, 49, 914–922. [Google Scholar] [CrossRef]
- Blog, R.-S. Single-Cell RNA Sequencing Uncovers Cell Type-Specific Genetic Insights Underlying Schizophrenia|RNA-Seq Blog 2024. Available online: https://www.rna-seqblog.com/single-cell-rna-sequencing-uncovers-cell-type-specific-genetic-insights-underlying-schizophrenia/ (accessed on 30 May 2024).
- Shimu, S.J. A 10-Year Retrospective Quantitative Analysis of The CDC Database: Examining the Prevalence of Depression in the Us Adult Urban Population. Front. Health Inform. 2025, 13, 6053–6059. [Google Scholar] [CrossRef]
- Piwecka, M.; Rajewsky, N.; Rybak-Wolf, A. Single-Cell and Spatial Transcriptomics: Deciphering Brain Complexity in Health and Disease. Nat. Rev. Neurol. 2023, 19, 346–362. [Google Scholar] [CrossRef]
- Du, X.; Qi, R.; Li, C.; Zhou, W.; Wang, S.; Wei, S.; Wu, R.; Yin, Y.; Li, T.; Zhu, X. Directed and efficient generation of calretinin interneurons from human pluripotent stem cells. Stem Cell Res. Ther. 2025, 16, 465. [Google Scholar] [CrossRef]
- Chehimi, S.N.; Crist, R.C.; Reiner, B.C. Unraveling Psychiatric Disorders through Neural Single-Cell Transcriptomics Approaches. Genes 2023, 14, 771. [Google Scholar] [CrossRef]
- Ma, G.; Wu, J.; Wu, Y.; Yang, J.; Zhang, J.; Huang, Y.; Tian, R.; Zhou, X.; Tan, X.; Li, Y.; et al. Single-cell transcriptomics reveals Sox6 positive interneurons enriched in the prefrontal cortex of female mice vulnerable to chronic social stress. Mol. Psychiatry 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Raabe, F.J.; Galinski, S.; Papiol, S.; Falkai, P.G.; Schmitt, A.; Rossner, M.J. Studying and Modulating Schizophrenia-Associated Dysfunctions of Oligodendrocytes with Patient-Specific Cell Systems. npj Schizophr. 2018, 4, 23. [Google Scholar] [CrossRef]
- D’Angelo, M.; Di Stefano, V.; Pullano, I.; Monaco, F.; Steardo, L. Psychiatric Implications of Genetic Variations in Oligodendrocytes: Insights from hiPSC Models. Life 2025, 15, 921. [Google Scholar] [CrossRef]
- Kondo, R.; Kimura, H.; Ikeda, M. Genetic Association between Gene Expression Profiles in Oligodendrocyte Precursor Cells and Psychiatric Disorders. Front. Psychiatry 2025, 16, 1566155. [Google Scholar] [CrossRef] [PubMed]
- Dal Santo, F.; García-Portilla, M.P.; Fernández-Egea, E.; González-Blanco, L.; Sáiz, P.A.; Giordano, G.M.; Galderisi, S.; Bobes, J. The Dimensional Structure of the Positive and Negative Syndrome Scale in First-Episode Schizophrenia Spectrum Disorders: An Exploratory Graph Analysis from the OPTiMiSE Trial. Schizophrenia 2024, 10, 81. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Y.; Djekidel, M.N.; Chen, W.; Bhattacherjee, A.; Chen, Z.; Scolnick, E.; Zhang, Y. Cell type-specific mechanism of Setd1a heterozygosity in schizophrenia pathogenesis. Sci Adv. 2022, 8, eabm1077. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Zorlu, M.E.; Uygur, K.K.; Yılmaz, N.S.; Demirel, Ö.Ö.; Aydil, U.; Kızıl, Y.; Uslu, S. Evaluation of Advanced Oxidation Protein Products (AOPP) and Superoxide Dismutase (SOD) Tissue Levels in Patients with Nasal Polyps. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 4824–4830. [Google Scholar] [CrossRef]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and Nitrate Measurement by Griess Reagent in Human Plasma: Evaluation of Interferences and Standardization. Methods Enzym. 2008, 440, 361–380. [Google Scholar] [CrossRef]
- Durak, I.; Yurtarslanl, Z.; Canbolat, O.; Akyol, O. A Methodological Approach to Superoxide Dismutase (SOD) Activity Assay Based on Inhibition of Nitroblue Tetrazolium (NBT) Reduction. Clin. Chim. Acta 1993, 214, 103–104. [Google Scholar] [CrossRef]
- Grilo, L.F.; Martins, J.D.; Cavallaro, C.H.; Nathanielsz, P.W.; Oliveira, P.J.; Pereira, S.P. Development of a 96-Well Based Assay for Kinetic Determination of Catalase Enzymatic-Activity in Biological Samples. Toxicol. Vitr. 2020, 69, 104996. [Google Scholar] [CrossRef]
- Tipple, T.E.; Rogers, L.K. Methods for the Determination of Plasma or Tissue Glutathione Levels. Methods Mol. Biol. 2012, 889, 315–324. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries Following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Yang, M.; Liu, J.; Zhang, Y.; Liu, D.; Zhang, X. Variations of Plasma Oxidative Stress Levels in Male Patients with Chronic Schizophrenia. Correlations with Psychopathology and Matrix Metalloproteinase-9: A Case-Control Study. BMC Psychiatry 2024, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Dahake, H.S.; Warade, J.; Kansara, G.S.; Pawade, Y.; Ghangle, S. Study of Malondialdehyde as an Oxidative Stress Marker in Schizophrenia. Int. J. Res. Med. Sci. 2016, 4, 4730–4734. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Carletti, B.; Banaj, N.; Piras, F.; Bossù, P. Schizophrenia and Glutathione: A Challenging Story. J. Pers. Med. 2023, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, P.; Yang, H.; Huang, C.; Sun, W.; Zhang, X.; Tang, X. Altered Serum Glutathione Disulfide Levels in Acute Relapsed Schizophrenia Are Associated with Clinical Symptoms and Response to Electroconvulsive Therapy. BMC Psychiatry 2025, 25, 242. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Weng, Y.-H.; He, J.; Chen, M.-R.; Chen, Y.; Qi, Z.-Q.; Zhang, Z.-M. A Genetic Variant of the Glutamate-Cysteine Ligase Catalytic Subunit Is Associated with Susceptibility to Ischemic Stroke in the Chinese Population. Eur. J. Med. Res. 2025, 30, 421. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Micó, J.A.; Rojas-Corrales, M.O.; Gibert-Rahola, J.; Parellada, M.; Moreno, D.; Fraguas, D.; Graell, M.; Gil, J.; Irazusta, J.; Castro-Fornieles, J.; et al. Reduced antioxidant defense in early onset first-episode psychosis: A case-control study. BMC Psychiatry 2011, 11, 26. [Google Scholar] [CrossRef]
- Chen, P.; Yao, L.; Yuan, M.; Wang, Z.; Zhang, Q.; Jiang, Y.; Li, L. Mitochondrial Dysfunction: A Promising Therapeutic Target for Liver Diseases. Genes Dis. 2024, 11, 101115. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, L.; van der Laan, L.J.W.; Pan, Q.; Verstegen, M.M.A. Mitochondrial Dysfunction and Oxidative Stress in Liver Transplantation and Underlying Diseases: New Insights and Therapeutics. Transplantation 2021, 105, 2362–2373. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Rojano-Alfonso, C.; Micó-Carnero, M.; Caballeria-Casals, A.; Peralta, C.; Casillas-Ramírez, A. New Insights Into the Role of Autophagy in Liver Surgery in the Setting of Metabolic Syndrome and Related Diseases. Front. Cell Dev. Biol. 2021, 9, 670273. [Google Scholar] [CrossRef]
- Darmanto, A.G.; Yen, T.-L.; Jan, J.-S.; Linh, T.T.D.; Taliyan, R.; Yang, C.-H.; Sheu, J.-R. Beyond Metabolic Messengers: Bile Acids and TGR5 as Pharmacotherapeutic Intervention for Psychiatric Disorders. Pharmacol. Res. 2025, 211, 107564. [Google Scholar] [CrossRef]
- Grant, R.K.; Brindle, W.M.; Donnelly, M.C.; McConville, P.M.; Stroud, T.G.; Bandieri, L.; Plevris, J.N. Gastrointestinal and Liver Disease in Patients with Schizophrenia: A Narrative Review. World J. Gastroenterol. 2022, 28, 5515–5529. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Ibrahim, R.; Theberge, K.; May, M.; Houseknecht, K.L. Non-Alcoholic Fatty Liver Disease (NAFLD) and Mental Illness: Mechanisms Linking Mood, Metabolism and Medicines. Front. Neurosci. 2022, 16, 1042442. [Google Scholar] [CrossRef]
- Soto-Angona, Ó.; Anmella, G.; Valdés-Florido, M.J.; De Uribe-Viloria, N.; Carvalho, A.F.; Penninx, B.W.J.H.; Berk, M. Non-Alcoholic Fatty Liver Disease (NAFLD) as a Neglected Metabolic Companion of Psychiatric Disorders: Common Pathways and Future Approaches. BMC Med. 2020, 18, 261. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Siracusano, M.; Savino, R.; Saso, L.; Scapagnini, G.; Mazzone, L. Oxidative Stress Response and NRF2 Signaling Pathway in Autism Spectrum Disorder. Redox Biol. 2025, 83, 103661. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. Blood-Based Oxidation Markers in Medicated and Unmedicated Schizophrenia Patients: A Meta-Analysis. Asian J. Psychiatry 2022, 67, 102932. [Google Scholar] [CrossRef]
- Kuloglu, M.; Ustundag, B.; Atmaca, M.; Canatan, H.; Tezcan, A.E.; Cinkilinc, N. Lipid Peroxidation and Antioxidant Enzyme Levels in Patients with Schizophrenia and Bipolar Disorder. Cell Biochem. Funct. 2002, 20, 171–175. [Google Scholar] [CrossRef]
- Grignon, S.; Chianetta, J.M. Assessment of Malondialdehyde Levels in Schizophrenia: A Meta-Analysis and Some Methodological Considerations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 365–369. [Google Scholar] [CrossRef]
- Chiti, H.; Hosseini, E.; Ebrahimi, V.; Mousavi, S.N. Correlation of Dietary Macro- and Micro-Mineral Intake with Seminal Plasma Quality/Quantity and Oxidant/Antioxidant Status in Infertile Compared to the Normal Men: A Case-Control Study. Biol. Trace Elem. Res. 2024, 202, 1991–1997. [Google Scholar] [CrossRef]
- Gunes, M.; Camkurt, M.A.; Demir, S.; Ibiloglu, A.; Kaya, M.C.; Bulut, M.; Atli, A. Serum Malonyldialdehyde Levels of Patients with Schizophrenia. Klin. Psikofarmakol. Bul. 2015, 25. Available online: http://psychiatry-psychopharmacology.com/en/serum-malonyldialdehyde-levels-of-patients-with-schizophrenia-13652 (accessed on 2 July 2025).
- Uddin, S.M.N.; Sultana, F.; Uddin, M.G.; Dewan, S.M.R.; Hossain, M.K.; Islam, M.S. Effect of Antioxidant, Malondialdehyde, Macro-Mineral, and Trace Element Serum Concentrations in Bangladeshi Patients with Schizophrenia: A Case-Control Study. Health Sci. Rep. 2021, 4, e291. [Google Scholar] [CrossRef]
- Chien, Y.-L.; Hwu, H.-G.; Hwang, T.-J.; Hsieh, M.H.; Liu, C.-C.; Lin-Shiau, S.-Y.; Liu, C.-M. Clinical Implications of Oxidative Stress in Schizophrenia: Acute Relapse and Chronic Stable Phase. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109868. [Google Scholar] [CrossRef] [PubMed]
- Raffa, M.; Atig, F.; Mhalla, A.; Kerkeni, A.; Mechri, A. Decreased Glutathione Levels and Impaired Antioxidant Enzyme Activities in Drug-Naive First-Episode Schizophrenic Patients. BMC Psychiatry 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Mednova, I.A.; Smirnova, L.P.; Vasilieva, A.R.; Kazantseva, D.V.; Epimakhova, E.V.; Krotenko, N.M.; Semke, A.V.; Ivanova, S.A. Immunoglobulins G of Patients with Schizophrenia Protects from Superoxide: Pilot Results. J. Pers. Med. 2022, 12, 1449. [Google Scholar] [CrossRef]
- Ansari, Z.; Pawar, S.; Seetharaman, R. Neuroinflammation and oxidative stress in schizophrenia: Are these opportunities for repurposing? Postgrad Med. 2022, 134, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Buosi, P.; Borghi, F.A.; Lopes, A.M.; Facincani, I.d.S.; Fernandes-Ferreira, R.; Oliveira-Brancati, C.I.F.; do Carmo, T.S.; Souza, D.R.S.; da Silva, D.G.H.; de Almeida, E.A.; et al. Oxidative Stress Biomarkers in Treatment-Responsive and Treatment-Resistant Schizophrenia Patients. Trends Psychiatry Psychother. 2021, 43, 278–285. [Google Scholar] [CrossRef]
- Rambaud, V.; Marzo, A.; Chaumette, B. Oxidative Stress and Emergence of Psychosis. Antioxidants 2022, 11, 1870. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, V.V.; Kostić, J.; Krivokapić, Ž.; Krtinić, D.; Ranković, M.; Petković, M.; Ćosić, V. Decreased Activity of Erythrocyte Catalase and Glutathione Peroxidase in Patients with Schizophrenia. Medicina 2022, 58, 1491. [Google Scholar] [CrossRef]
- Orrico, F.; Laurance, S.; Lopez, A.C.; Lefevre, S.D.; Thomson, L.; Möller, M.N.; Ostuni, M.A. Oxidative Stress in Healthy and Pathological Red Blood Cells. Biomolecules 2023, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free. Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohib, M.M.; Uddin, M.B.; Rahman, M.M.; Tirumalasetty, M.B.; Al-Amin, M.M.; Shimu, S.J.; Alam, M.F.; Arbee, S.; Munmun, A.R.; Akhtar, A.; et al. Dysregulated Oxidative Stress Pathways in Schizophrenia: Integrating Single-Cell Transcriptomic and Human Biomarker Evidence. Psychiatry Int. 2025, 6, 104. https://doi.org/10.3390/psychiatryint6030104
Mohib MM, Uddin MB, Rahman MM, Tirumalasetty MB, Al-Amin MM, Shimu SJ, Alam MF, Arbee S, Munmun AR, Akhtar A, et al. Dysregulated Oxidative Stress Pathways in Schizophrenia: Integrating Single-Cell Transcriptomic and Human Biomarker Evidence. Psychiatry International. 2025; 6(3):104. https://doi.org/10.3390/psychiatryint6030104
Chicago/Turabian StyleMohib, Mohammad Mohabbulla, Mohammad Borhan Uddin, Md Majedur Rahman, Munichandra Babu Tirumalasetty, Md. Mamun Al-Amin, Shakila Jahan Shimu, Md. Faruk Alam, Shahida Arbee, Afsana R. Munmun, Asif Akhtar, and et al. 2025. "Dysregulated Oxidative Stress Pathways in Schizophrenia: Integrating Single-Cell Transcriptomic and Human Biomarker Evidence" Psychiatry International 6, no. 3: 104. https://doi.org/10.3390/psychiatryint6030104
APA StyleMohib, M. M., Uddin, M. B., Rahman, M. M., Tirumalasetty, M. B., Al-Amin, M. M., Shimu, S. J., Alam, M. F., Arbee, S., Munmun, A. R., Akhtar, A., & Mohiuddin, M. S. (2025). Dysregulated Oxidative Stress Pathways in Schizophrenia: Integrating Single-Cell Transcriptomic and Human Biomarker Evidence. Psychiatry International, 6(3), 104. https://doi.org/10.3390/psychiatryint6030104






