1. Updates on Imaging
Initial investigations for an unexplained unilateral pleural effusion involve radiological evaluation. The 2010 and 2023 guidelines stressed that thoracic ultrasound (TUS) increases the likelihood of pleural fluid aspiration and reduces the risk of organ puncture [
1,
2]. Different imaging techniques are employed in diagnosing malignant pleural effusions, including thoracic ultrasound (TUS), Computed Tomography (CT), Positron Emission Tomography (PET)-CT, and Magnetic Resonance Imaging (MRI). Evidence suggests that TUS and CT share similar capabilities in identifying malignancy; however, TUS provides enhanced visualisation of visceral pleura abnormalities and diaphragmatic nodules. In contrast, CT offers a broader assessment, particularly for mediastinal involvement and distant disease sites. While MRI is rarely used, it has demonstrated comparable accuracy to CT, especially for detecting subtle chest wall or diaphragm infiltration [
3,
4].
The morphological assessment of the pleura using MRI was comparable to CT assessment and MRI was better able to detect subtle chest wall and/or diaphragmatic infiltration compared to CT [
5]. MRI can assist in distinguishing malignant pleural disease from benign conditions by identifying hyperintense areas in T2-weighted and gadolinium-enhanced T1-weighted images. The detection of multiple hyperintense spots on the pleura and early contrast enhancements in dynamic imaging may serve as indicators of malignancy, particularly when assessed alongside other established morphological features [
6,
7]. PET-CT is sometimes used as part of the radiological assessment of pleural malignancy. Whilst fluorodeoxyglucose (FDG) uptake is not limited to malignant tissue and may be found in regions of inflammation or infection, integration of morphological data and PET-CT can increase the sensitivity and specificity for detecting pleural malignancy [
8,
9]. However, the only randomised controlled trial (RCT) in this field comparing PET-CT-guided versus CT-guided biopsy in suspected malignant pleural thickening did not support the use of PET in this cohort in a study based in the United Kingdom [
10].
When evaluating the diagnostic accuracy of various imaging techniques, thoracic ultrasound (TUS) shows moderate sensitivity and high specificity, while CT scans demonstrate moderate sensitivity and specificity. PET-CT, though highly sensitive and specific, is limited by a low evidence base, and MRI offers high sensitivity and specificity for detecting pleural malignancies, although its use remains limited. The BTS pleural guideline recommends that CT should be used to assess the entire thorax and positive findings may support a clinical diagnosis of malignancy when biopsy is not an option (conditional); however, a negative CT does not exclude malignancy (Strong—by consensus).
Table 1 shows the features that increase the possibility of malignancy. TUS may be useful at presentation to support a diagnosis of pleural malignancy in the context of a pleural effusion (conditional). PET-CT can be considered to support a diagnosis of pleural malignancy when there are suspicious CT or clinical features and negative histological results, or when invasive sampling is not an option (conditional). Whilst MRI may be a potential diagnostic tool in pleural malignancy in the future, there is not enough evidence to recommend its routine use in diagnosing pleural malignancy at present.
2. Updates on Indwelling Pleural Catheters and Systemic Anti-Cancer Treatment
Malignant pleural effusions (MPEs) are indicative of advanced-stage disease, typically carrying a poor prognosis. As a result, treatment strategies focus primarily on symptom relief and improving the patient’s quality of life (QoL). Definitive interventions such as pleurodesis or the insertion of an indwelling pleural catheter (IPC) are often employed to minimise the necessity for repeated pleural procedures. Recent updates suggest that IPCs may be considered as a first-line approach, especially in cases where pleurodesis may not be feasible. One of the main differences between the 2010 guideline and the updated guidance is the role of indwelling pleural catheters (IPCs) as a first line management option in MPE [
1,
2]. The previous guidance recommended IPCs as a second-line intervention in patients not known to have non-re-expandable lung (NEL), with chest drain and pleurodesis being recommended as the first-line intervention in patients. Patients were also required to have a prognosis of more than a month.
The 2023 update of the BTS guidelines examines whether systemic anti-cancer treatment (SACT) for responsive tumours can reduce the necessity for pleural interventions. Research suggests that while systemic therapies may improve outcomes, they do not eliminate the need for definitive pleural procedures, as fluid management is often required to alleviate symptoms and maintain quality of life. Mitchell et al. assessed the impact of systemic oncological therapy against no systemic treatment on the removal of existing IPCs in patients with MPE from breast cancer; there was a trend towards a higher rate of IPC removal in patients receiving first-line chemotherapy compared with third line chemotherapy, but the study was not powered for this subgroup analysis [
11]. A study evaluating breast cancer patients with MPE for pleural progression-free survival (PPFS) after undergoing drainage and pleurodesis or therapeutic aspiration found that 49 of 78 of the patients who received systemic treatment following pleural aspiration went on to achieve pleurodesis, suggesting that chemotherapy alone did not control the effusion [
12]. PPFS was defined as the time from systemic therapy in one group and from pleurodesis and systemic therapy in the second group to pleural progression or to death arising from any cause.
A study assessing the response of MPE secondary to non-squamous non-small-cell lung cancer to bevacizumab combination chemotherapy found that 12 of the 15 responders reported effusion recurrence after cessation of systemic treatment, suggesting that systemic bevacizumab was effective in controlling pleural fluid for the duration of treatment [
13]. Jiang et al. reported on patients with epidermal growth factor receptor (EGFR)-mutated NSCLC and acquired tyrosine kinase inhibitor (TKI) resistance that were treated with bevacizumab and either a continuation of EGFR-TKI or switch chemotherapy [
14]. This study found that patients in the continuation of EGFR-TKI group had significantly greater PPFS and complete/partial pleural effusion response compared to those who switched to chemotherapy.
Overall, there was insufficient evidence to determine if SACT reduces the need for definitive pleural procedures in MPE. The BTS recommendation is that definitive pleural intervention should not be deferred until after SACT (conditional—by consensus).
3. Updates on Talc Pleurodesis Versus Pleural Aspiration
Malignant pleural effusions (MPEs) frequently recur following initial aspiration. For more lasting management, talc pleurodesis via chest drain is often used. This method works by permanently adhering the visceral and parietal pleura, which prevents fluid from accumulating again. However, the procedure requires hospitalisation. As an alternative, pleural aspiration can be performed without pleurodesis, offering the advantage of avoiding hospital stays, although the likelihood of fluid recurrence is higher. In the BTS 2010 pleural guidelines, only therapeutic pleural aspiration was recommended in patients who had a life expectancy of less than one month. The updated BTS guidelines reviewed the available evidence comparing talc pleurodesis with pleural aspiration to determine which procedure would have the most benefit for patients with MPE.
Sorenson et al. conducted a randomised controlled trial to determine whether pleural drainage with talc instillation was better than pleural drainage alone in the management of MPE [
15]. This study reported a pleurodesis success rate of 100% in those treated with talc slurry (9/9) and 58% in those treated with pleural aspiration alone (4/7); however, this study did not reflect normal practice as patients in the ‘pleural aspiration’ arm were post-thoracoscopy with a chest drain in situ for 72 h rather than more simple aspiration. The study did find that all patients (9/9) treated with talc slurry had subjectively improved breathlessness compared with 86% (6/7) of those treated with pleural aspiration [
16]. A retrospective analysis of the ‘Surveillance, Epidemiology and End Results’ (SEER) database found that patients who underwent talc slurry pleurodesis had a longer hospital stay than patients who had a pleural aspiration [
15]. This study also found that 55% of patients who had an initial pleural aspiration required a second procedure, of which 32% occurred within 14 days, and reported similar pneumothorax rates using pleural aspiration and talc slurry. Patients treated with pleural aspiration required more pleural procedures than talc slurry and those treated with repeated pleural aspiration suffered pneumothorax more than with talc slurry [
15].
Based on this limited evidence, the BTS concludes that talc slurry pleurodesis may be associated with a longer hospital stay than pleural aspiration (ungraded), talc slurry pleurodesis appears to reduce the need for re-intervention and reduces the overall number of complications compared with pleural aspiration alone (ungraded), and that patients undergoing pleural aspiration as the first intervention will often require a second procedure, with approximately one-third requiring this within two weeks (ungraded). The BTS recommends that management of MPE using talc pleurodesis (or another method) is recommended in preference to repeated aspiration, especially in those with a better prognosis, but the relative risks and benefits should be discussed with the patient (conditional—by consensus).
4. Update on Indwelling Pleural Catheters as First Line Management
Chest drain insertion with talc pleurodesis and IPCs provide definitive treatment options in the management of MPEs. Talc pleurodesis has long been considered the standard of care for managing MPE, as described above. Clinical practice has evolved and IPCs are increasingly used as first-line management of MPE. The 2023 BTS guidelines reviewed available evidence to determine if IPCs are superior to talc slurry pleurodesis at improving clinical outcomes. The main outcomes addressed in the evidence reviewed were quality of life, length of hospital stay, need for re-intervention, symptoms (breathlessness and chest pain), complications, and pleurodesis rates. Three studies comparing quality of life for patients with MPE treated with an IPC or talc slurry pleurodesis found no statistically significant difference in quality of life between the two treatment options [
17,
18,
19]. Fysh et al. found that a higher proportion of patients achieved more than one minimally important improvement in quality of life (measures by the visual analogue score) with IPC compared to talc slurry pleurodesis (93% vs. 50%,
p = 0.002), but this only included 15 and 12 patients, respectively, and was a secondary outcome in a non-randomised study [
20]. Five studies reported on length of hospital stay [
17,
18,
19,
21,
22] and four studies reported on total number of inpatient days from intervention to death or completion of follow-up [
16,
18,
20,
22].
Overall, patients managed by IPC spent less time in hospital for both the initial procedure and total inpatient days compared to those managed with talc slurry pleurodesis. Three studies showed that the pleural re-intervention rate was higher with talc slurry than with IPC [
17,
18,
21]. There were significant improvements in dyspnoea following either IPC insertion or talc pleurodesis, with no significant difference between the two groups [
17,
18,
19,
20,
21,
23]. Meta-analysis of the four studies reporting on complications and adverse events found that there were fewer complications in the talc slurry group compared to the IPC group, but this did not reach statistical significance [
17,
18,
20,
21]. Due to the varying definition of pleurodesis across the studies identified, it was not possible to undertake a meta-analysis on the pleurodesis rates of the two interventions. One randomised trial of IPC vs talc slurry pleurodesis reported a specific pleurodesis outcome rather than re-intervention rate, but did not provide the definition of pleurodesis [
23]; it reported a pleurodesis success rate of 86% in the talc slurry pleurodesis group and 68% in the IPC group (
p = 0.19).
According to the evidence presented in the updated guidelines, both talc slurry pleurodesis and IPCs effectively alleviate symptoms of dyspnoea and enhance quality of life. However, there appears to be no significant difference in clinical outcomes between the two treatment methods. IPC insertion appears to be associated with a shorter length of initial hospital stay at the time of intervention and fewer subsequent inpatient days (ungraded). IPCs appear to be associated with a reduced need for further pleural intervention (defined as requirement for a further pleural breaching procedure) when compared with talc slurry pleurodesis (Moderate). There appears to be no difference in the number of adverse events experienced by patients treated with talc slurry pleurodesis or IPC (Very Low).
5. Update on Combined Procedures with IPCs
Pleurodesis can be achieved either by administering talc via talc slurry (emulsification of talc in normal saline which is then administered via a chest drain) or poudrage (administration of talc powder as an aerosol during thoracoscopy). It has been postulated that talc poudrage may allow better coverage of the pleural space as the talc is directly visualised and may be associated with shorter length of stay as the talc is delivered at the same procedure as fluid drainage. The updated BTS pleural guideline reviewed the available evidence to address the clinical question of whether thoracoscopy and talc poudrage pleurodesis is superior to chest drain and talc slurry pleurodesis. From the literature review, five studies were identified as relevant to the clinical question [
19,
24,
25,
26,
27]. Bhatnagar et al. and Walker et al. found that there was no significant difference in quality of life between patients who received talc slurry and those that received talc poudrage [
19,
24]. Of the studies reporting on length of hospital stay, none showed a significant difference in length of hospital stay between the two procedures [
19,
24,
26].
The need for re-intervention and pleurodesis rate data were usually combined. Four studies reported on pleurodesis rate, but different definitions were used between studies [
25,
26,
27,
28]. Meta-analysis of the pleurodesis failure rate at day 30 showed that pleurodesis failure was higher in the talc slurry group (206 per 1000) than the talc poudrage group (138 per 1000 (103 to 189)), although this did not reach statistical significance [
24,
25,
26,
27].
Bhatnagar et al. and Walker et al. found that whilst there was an overall improvement in breathlessness in patients who received either talc slurry or talc poudrage, there were no significant differences between the two treatment groups [
20,
25]. Bhatnagar et al. found that there was no significant difference in perceived thoracic pain between the two treatment groups [
24]. Conversely, Dresler et al. found that 10% of the patients who had received talc slurry experienced chest pain, whilst only 5% of those in the thoracic poudrage arm reported a similar level of chest pain [
25]. However, overall the incidence of pain did not differ between the two study arms.
Four studies reported the total number of complications per treatment group. Although the meta-analysis suggests that the likelihood of experiencing complications following talc slurry or talc poudrage is very similar, the number of complications per patient appears to be higher in the talc poudrage group [
24,
25,
26,
27].
Overall, there is no significant difference between quality of life, length of hospital stay, and symptoms (specifically chest pain and breathlessness) between patients with MPE treated with chest drain and talc slurry or those who had thoracoscopy and talc poudrage (ungraded) [
1]. Although there appears to be no significant difference in the occurrence of one or more complications following either chest drain and talc slurry or thoracoscopy and talc poudrage, patients who undergo thoracoscopy and talc poudrage may have an increased number of complications (ungraded) [
1]. The pleurodesis failure rate may be lower in patients with MPE treated by thoracoscopy and talc poudrage when compared to chest drain and talc slurry (Low). In light of this, the BTS recommends that patients with MPE should be offered either talc slurry or talc poudrage to reduce the need of recurrent intervention (conditional). In situations where a diagnostic procedure such as pleural biopsies are being performed at thoracoscopy and pleurodesis is desirable, talc poudrage should be conducted during the same procedure to reduce the need for repeated interventions [
1].
IPCs are being increasingly used in patients with MPE. As such, there may be a role for administering a pleurodesis agent through a functioning IPC after a sufficient period of drainage [
1]. The BTS reviewed the available evidence to determine if administering intrapleural agents (such as talc or other pleurodesis agents) through an IPC improves clinical outcomes in patients with MPE.
The literature review identified one study by Bhatnagar et al., which compared pleurodesis rates in patients IPCs and an expandable lung, who were randomised to receive either talc or a placebo (0.9% sodium chloride) instilled into their IPC after 10 days of drainage [
28]. This study found that patients who received talc slurry reported an improved quality of life compared to the group who received the placebo. Chest pain and breathlessness were reported as improved in both groups throughout the trial with chest pain being significantly lower in the talc group across the duration of the study, whilst breathlessness was only reported as significantly lower in the talc group at day 56. This study also found that there was a statistically significant difference in successful pleurodesis between the two groups, with 51% of the participants in the talc group achieving pleurodesis at day 70, compared with 27% in the placebo group. There was no significant difference in complications (underlying disease progression, fluid accumulation, IPC blockage, and infection) between the talc slurry and placebo groups [
28].
This review found that pleurodesis rates and quality of life may be improved in malignant pleural effusion patients with expandable lung (defined as >75% of hemithorax) who have talc instilled via an indwelling pleural catheter (ungraded). Chest pain and breathlessness may be reduced in malignant pleural effusion patients with expandable lung (defined as >75% of hemithorax) who have talc instilled via an indwelling pleural catheter (ungraded). Complication rates do not appear to differ between malignant pleural effusion patients treated with an indwelling pleural catheter and talc or placebo (ungraded). The BTS recommends that instillation of talc via an IPC should be offered to patients with expandable lung where the clinician or patient deems achieving pleurodesis and IPC removal to be important (conditional—by consensus).
6. Update on Non-Expandable Lung and Surgical Management of Malignant Pleural Effusion
Surgical intervention may be a viable treatment option for MPE in patients able to tolerate surgery. The BTS reviewed the available evidence on using a surgical approach to manage MPE to determine if surgical pleurodesis or decortication is better than talc slurry pleurodesis at improving clinical outcomes. One prospective (non-randomised) cohort study compared quality-of-life scores in patients managed by video-assisted thoracoscopic surgery (VATS) plus talc pleurodesis, VATS plus IPC, IPC only, or talc slurry via chest drain [
19]. This study found that there was no significant difference in quality of life between the different groups.
Three studies reported on length of hospital stay, but no study directly compared surgical management against talc slurry pleurodesis [
29,
30,
31]. Overall, patients who underwent a VATS plus talc pleurodesis had a longer average stay than patients who had talc slurry pleurodesis. Meta-analysis was not possible on the few studies reporting on the need for re-intervention. Cardillo et al. reported that VATS was repeated in two subjects at 12+ months following the initial VATS and talc pleurodesis procedures [
31]. Trotter et al. reported that one patient required thoracotomy with decortication following VATS pleurodesis, but no timeframe was provided [
29].
Brega-Massone et al. reported on pain during the first 3 days post-thoracotomy vs post-VATS, using the WHO three-step cancer pain relief scale [
30]. This study found that all the VATS patients experienced mild-to-moderate pain, whilst 70% of thoracotomy patients experienced moderate-to-severe pain. Walker et al. used the Eastern Cooperative Oncology Group (ECOG) pain score to assess the pain experienced by patients with IPC, those who had a VATS plus IPC and those who underwent talc slurry via a chest drain [
19]. Data from this study showed that 60% of patients with IPC only and 75% of patients who had VATS plus IPC had an ECOG pain score of four or less. Conversely, 60% of the patients who underwent talc slurry via a chest drain reported an ECOG pain score of five or greater [
19]. Walker et al. also assessed breathlessness across the different treatment groups (VATS plus talc pleurodesis, VATS plus IPC, IPC only, or talc slurry via chest drain); there were no statistically significant differences in breathlessness reported between groups [
19].
Three studies reported on complications experienced with surgical management of MPE. Brega-Massone et al. reported that 2% of patients who underwent VATS and chemical pleurodesis experienced complications, whereas this increased to 7% in those who underwent thoracotomy and chemical pleurodesis [
30]. Cardillo et al. and Trotter et al. both focused on patients undergoing VATS plus talc pleurodesis and reported that 3% and 15% of their participants, respectively, experienced complications [
29,
31]. Three studies reported on pleurodesis success rates. All three studies reported a ≥88% success rate with VATS and talc pleurodesis [
29,
30,
31]. One study reported a lower recurrence in the VATS and talc group, with 88% having no evidence of effusion at five months compared to 75% in the minimal lateral thoracotomy and talc pleurodesis group [
30].
Overall, there was insufficient evidence to determine if surgical management of MPE is superior to talc slurry pleurodesis. Whilst both surgical and non-surgical intervention for MPE may improve quality of life and reduce breathlessness, surgical intervention may require a longer hospital stay compared to conventional talc slurry pleurodesis (ungraded) [
1]. In MPE patients with NEL, the pleurodesis failure rate may be higher if thoracoscopic decortication is not performed (ungraded) [
1]. In summary, patients with MPE who are fit enough for surgery can be offered either surgical talc pleurodesis or talc slurry pleurodesis, after a discussion of the risks versus benefits of either procedure with the patient.
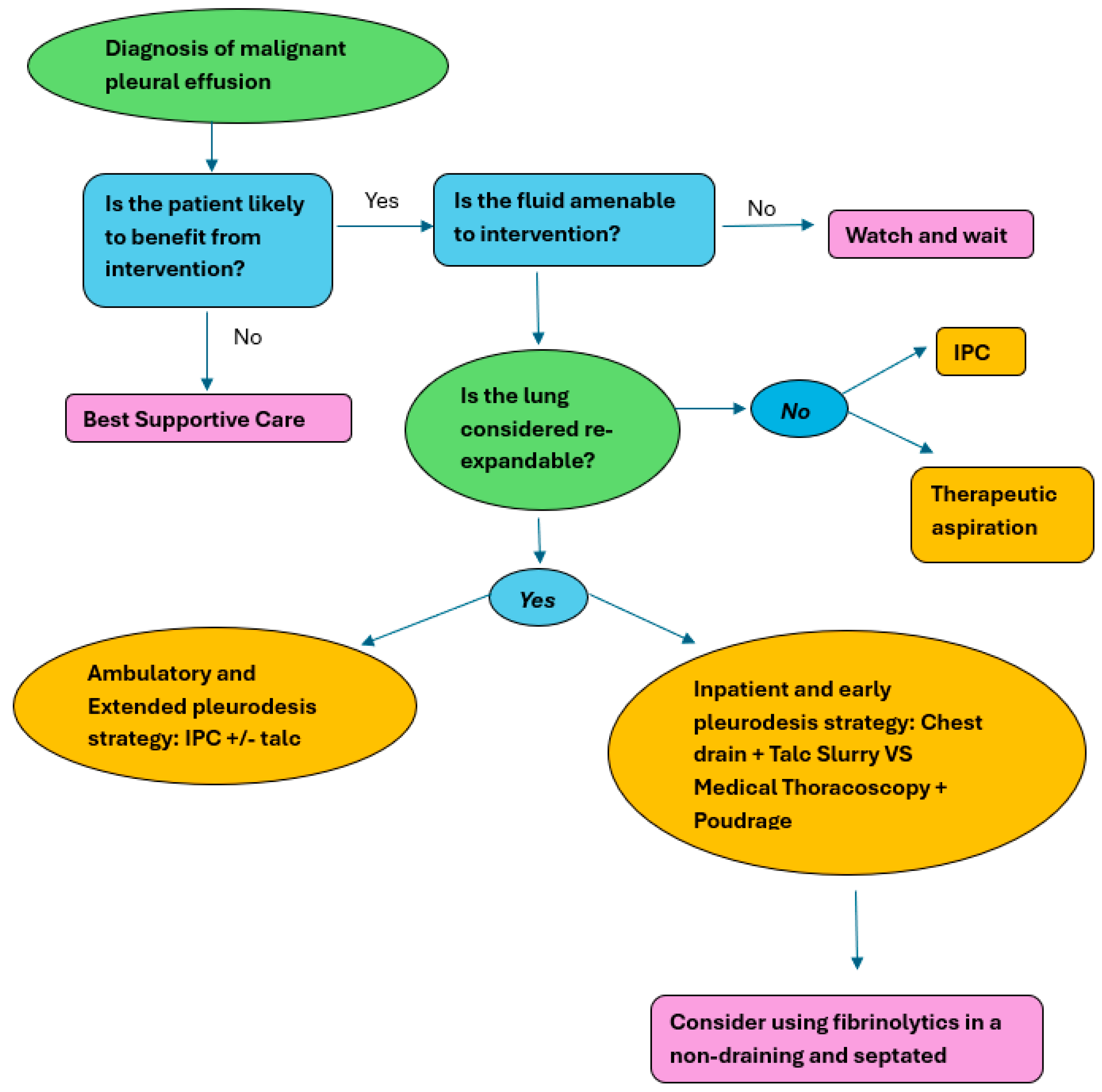
The 2023 BTS pleural guidelines recommend that patients without known NEL should be offered a choice of IPC or pleurodesis as first-line intervention in the management of malignant pleural effusion. The relative risks and benefits should be discussed with patients to individualise treatment choice (conditional).
IPCs are the preferred management technique for patients with an MPE who have a NEL. The updated BTS pleural guidelines addresses the usefulness of using alternative techniques (pleural aspiration, talc slurry pleurodesis, talc poudrage pleurodesis, and decortication surgery) to manage non-expandable lung in MPE. For the purpose of this review, NEL has been defined as more than 25% of the lung not being opposed to the chest wall on chest radiograph, accepting that there may be significant inter-observer variation in chest radiograph interpretation of the presence of NEL. NEL may occur because the visceral pleura is thickened, thus limiting re-expansion, or if there is an endobronchial obstruction preventing re-expansion.
Efthymiou et al. retrospectively identified 116 patients undergoing IPC insertion for NEL diagnosed intra-operatively during video-assisted thoracoscopic surgery (VATS) [
32]. Of the 48 patients able to complete a questionnaire following IPC insertion, 65% were at least ‘moderately’ satisfied in the improvement in their mobility post-procedure and 48% reported an at least ‘moderate’ improvement in their breathlessness [
32]. A retrospective review of 231 IPC procedures (including 28 patients with NEL) found that the presence of NEL was associated with a significantly higher chance of the IPC being in situ for >100 days; the rate of pleural infection was 4.9%, with 94% of the infections being controlled with antibiotics [
33]. Qureshi et al. retrospectively reviewed 52 patients undergoing IPC insertion for trapped lung and found that spontaneous pleurodesis occurred in 48% [
34]. This study also found that the average length of time the IPC was in situ was 94 days (range 30–255), with 94% of patients reporting a symptomatic improvement. No study specifically investigated whether surgical decortication was better at improving clinical outcomes than IPC; however, Cardillo et al. reported 29 patients who underwent decortication surgery, prior to talc pleurodesis, for NEL and had a pleurodesis success rate of 97% [
31]. Conversely, a further 15 patients with NEL who did not undergo decortication had a pleurodesis success rate of 13%.
It can be surmised that, despite the small risk of infection associated with IPCs, they have an important role in improving quality of life and reducing breathlessness in patients with MPE and NEL (ungraded) [
1]. In patients with MPE and less than 25% NEL, talc slurry pleurodesis can be considered as it may improve quality of life and pleurodesis rates (ungraded) [
1].
Due to the limited evidence available, no recommendations can be made on the use of pleural aspiration, talc slurry pleurodesis, talc poudrage pleurodesis, or decortication surgery versus an IPC to control symptoms in patients with MPE and NEL.
7. Update on Septated Malignant Pleural Effusions
Patients with MPE and septated effusions are less likely to benefit from pleural fluid drainage because percutaneous drainage alone cannot effectively drain the effusion in the presence of multiple septations. This cohort of patients tend to have a worse prognosis than other patients with MPE [
35]. Effective drainage of the pleural space, either by decortication or by intrapleural enzymes, may improve symptoms. The available evidence was reviewed to determine if intrapleural enzymes are better than surgical intervention or no treatment at improving clinical outcomes in septated MPE.
The literature search did not identify any studies assessing the role of surgery in patients with septated MPE, supporting the view that surgery is rarely used for these patients. Mishra et al. looked at quality of life as a secondary outcome and reported no difference between the intervention group (intrapleural enzymes) and the placebo group (no treatment) in inpatients with chest drains [
35]. This study also found that patients treated with intrapleural enzymes had a significantly shorter hospital stay compared to the no-treatment group.
Whilst the trials included in this review used different definitions for the need of re-intervention and pleurodesis rates, the meta-analysis of these RCTs showed that overall, the pleurodesis failure rate was lower in the intrapleural enzyme-treated group (287 per 1000 (177 to 464)) when compared to the no-treatment group (377 per 1000) [
36,
37,
38]. Meta-analysis of the studies conducted by Mishra et al. and Saydam et al. showed a decrease in breathlessness in the intrapleural enzyme group when compared to the no-treatment group [
35,
36].
In summary, there may be a role for intra-pleural fibrinolytics in reducing pleurodesis failure rates (Very Low), reducing length of hospital stay (ungraded) and improving breathlessness (Very Low) in selected patients with MPE and septated effusions when compared to no treatment [
1]. However, there is insufficient evidence for the BTS 2023 Pleural Guidelines to make a recommendation on the use of either intra-pleural fibrinolytics or surgery to improve clinical outcomes in this cohort of patients [
1].
This differs from the 2010 guidelines, which recommended intrapleural instillation of fibrinolytic drugs for the relief of distressing dyspnoea due to multiloculated malignant effusion resistant to simple drainage.
IPCs offer an ambulatory management pathway for patients with MPE. The second therapeutic intervention in malignant effusion (TIME2) and The Australasian Malignant Pleural Effusion (AMPLE) trials used regimes of alternate day drainages, which has become routine practice. The updated BTS guidelines reviewed the available evidence to determine if the type of drainage regime (daily drainage, alternate day drainage, or symptom-based/conservative drainage) has an impact on clinical outcomes in MPE.
Wahidi et al. found that there was no statistically significant difference in quality of life between patients who had symptom-based drainage and those who had daily drainage [
38]. Conversely, the AMPLE-2 trial reported that patient-reported quality-of-life (QoL) measures were better in the aggressive daily drainage group that the symptom-guided drainage group [
39]. This study also found that there were no reported differences in total episodes of hospital admission, total days in hospital, or effusion-related bed days between the ‘symptom-guided’ drainage and daily drainage groups [
40]. Both the AMPLE-2 and ASAP trials found that there was no statistically significant difference in pain between the symptom-based drainage group and the daily drainage group, with similar adverse events in both groups [
38,
39]. The AMPLE-2 trial, which also reported on breathlessness, did not find any statistically significant difference in breathlessness between these two drainage strategies [
40].
Pleurodesis rates were reported in both studies and meta-analysis revealed that spontaneous pleurodesis by 60 days (AMPLE-2) or autopleurodesis by 12 weeks (Impact of Aggressive versus Standard Drainage Regimen Using a Long-Term Indwelling Pleural CatheterASAP) was less frequent in the symptom-based drainage group (190 patients per 1000 compared with 431 per 1000 in the daily drainage group) [
38,
40].
In summary, symptoms, complications, and length of hospital stay appear to be the same for daily drainage, symptom-guided drainage, or alternate daily drainage (ungraded). There appear to be no differences in the occurrence of complications between daily drainage and symptom-based/conservative drainage regimes (Low). Daily drainage appears to increase pleurodesis rates when compared to alternate drainage or symptom-based drainage regimes (Low). Daily drainage may improve quality of life when compared to a symptom-based/conservative drainage approach, but there is no current evidence that daily drainage improves quality of life when compared to alternate daily drainages (ungraded). The BTS recommends that where IPC removal is a priority, daily IPC drainages are recommended to offer increased rates of pleurodesis when compared with less frequent drainages of symptom-guided or alternate drainage regimes (conditional). Patients should be advised that daily drainage does not offer any advantages over degree of breathless or chest pain and the often-preferred thrice-a-week drainage works just as well (Strong—by consensus).
8. Update on Intra-Pleural Chemotherapy
If patients’ fitness allows, systemic anti-cancer treatment (SACT) can allow disease control and the preservation of quality of life [
41,
42]. Agents given as SACT have been historically used for pleurodesis of MPE. With the growing number of novel anti-cancer treatments, there has been interest in whether delivery of intrapleural anti-cancer treatments can improve clinical outcomes over systemic treatments.
Du et al. and Jie Wang et al. compared the intrapleural administration of chemotherapy alone against combination therapy with chemotherapy plus vascular endothelial growth factor (VEGF) inhibitor [
42,
43]. For Du et al., progression was defined as the accumulation of pleural fluid and for Jie Wang et al. progressive disease was defined as pleural effusion increasing more than 25% from the initial effusion along with other signs of metastatic disease, although how this was measured is not clear. Jie Wang et al. found a statistically significant improvement in quality of life in the intrapleural combination therapy group compared to the intrapleural chemotherapy group [
43]. Zhao et al. compared intracavitary (mixed intrapleural and intra-abdominal) chemotherapy with intracavitary combination therapy (with chemotherapy plus VEGF inhibitor or angiogenesis inhibitor) [
44]. This study also found a statistically significant improvement in quality of life in the group that received combination therapy.
Jie Wang et al. reported substantially improved breathlessness and reduced chest pain with intrapleural chemotherapy and intrapleural combination therapy, but there was no significance between the groups (
p > 0.05) [
43]. This study found that neither treatment group resulted in respiratory complications (haemopneumothorax or pneumothorax). Jie Wang et al. also reported a trend toward reduced effusion relapse in patients who received intrapleural combination therapy (10% effusion relapse rate in the intrapleural combination therapy group compared to 31% relapse rate in the intrapleural chemotherapy group) [
43].
Similarly, Du et al. suggest that patients who received intrapleural combination therapy had increased pleural effusion control compared to those who received intrapleural chemotherapy (83% increased pleural effusion control in the intrapleural combination group compared to 50% in the intrapleural chemotherapy group) [
42]. Although no study directly compared intrapleural chemotherapy against systemic anti-cancer therapies, the limited data suggest that progression-free survival and overall survival time may be improved with combination intrapleural treatments over intrapleural chemotherapy alone [
43,
44].
There is no direct evidence to determine if intrapleural chemotherapy is superior to systemic treatment in treating pleural malignancy. Based on the very limited evidence, intrapleural combination therapies (chemotherapy plus VEGF inhibitor or angiogenesis inhibitor) may improve effusion control and increase quality of life, progression-free survival, and survival time when compared with chemotherapy alone (ungraded). The BTS recommends that intrapleural chemotherapy should not be routinely used for the treatment of malignant pleural effusion (conditional—by consensus).