Feasibility of the Bio-Mobilization of Rare Earth Elements from Bauxite Residual Red Mud †
Abstract
:1. Introduction
- To utilize the excreted metabolites of an isolated heterotrophic culture as a potential source for the extraction of REEs in an environmentally friendly manner;
- To investigate the amenability of bioleaching using glucose, molasses, and saw dust as the substrate.
- To conduct a comparative study of all leaching modes and elucidate the interplay between the biogenic metabolites and rare earth elements present in red mud.
2. Materials and Methods
2.1. Collection of Red Mud and a Fungal Sample
2.2. Pretreatment of the Substrate and Leaching Experiments
2.3. Analysis of Organic Acids
3. Results and Discussion
3.1. Characterization of Residual Waste
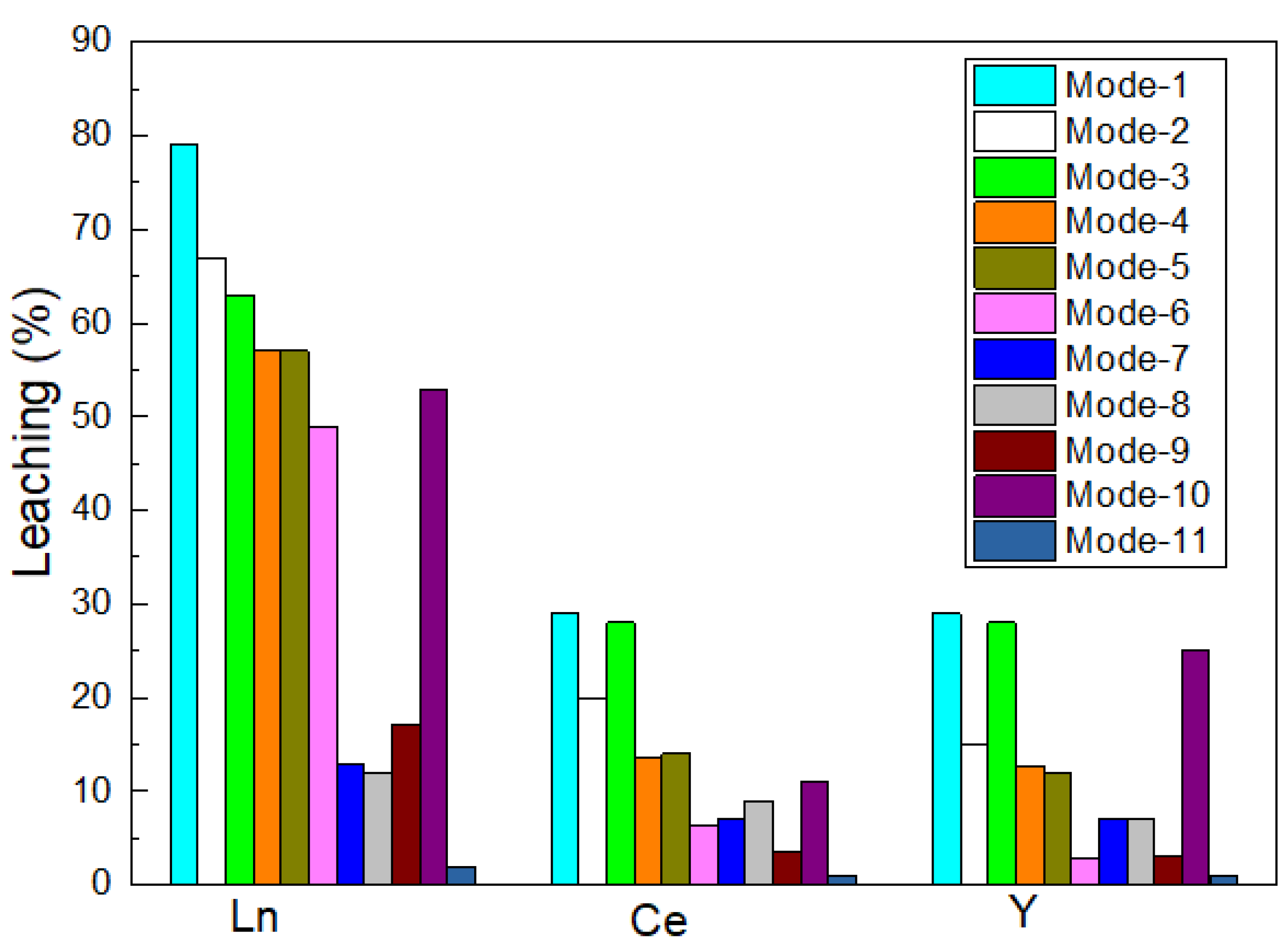
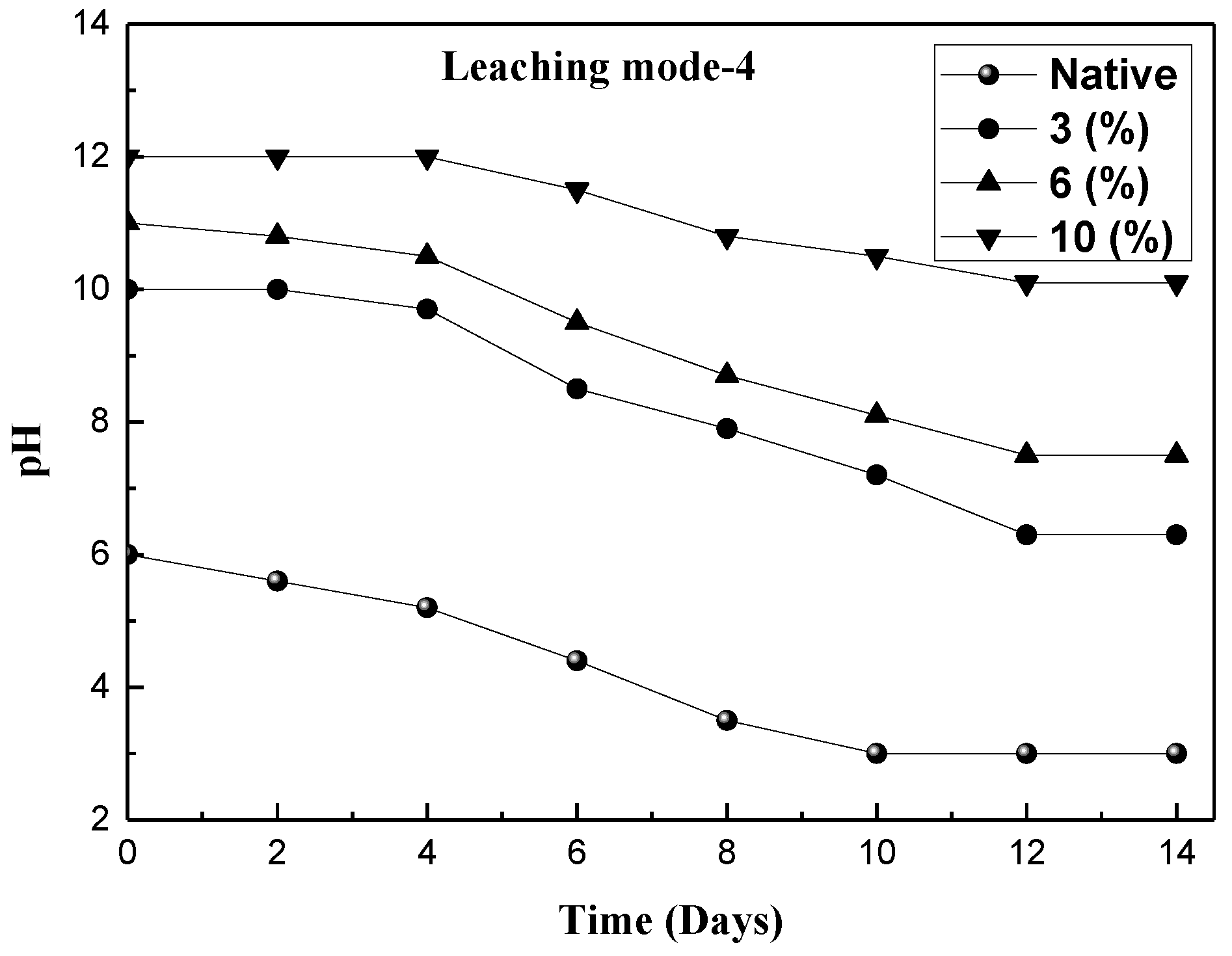
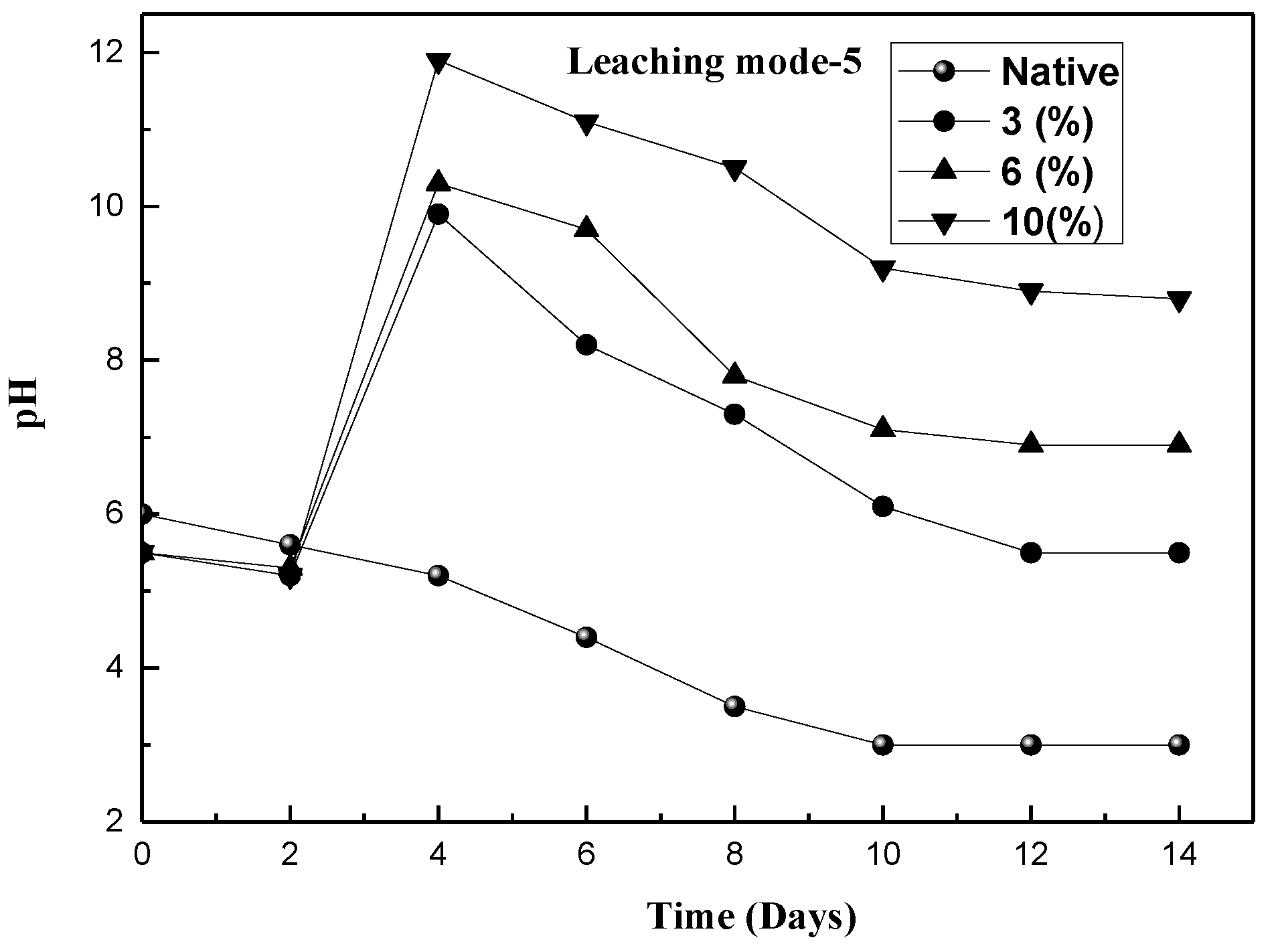
3.2. Extraction of Metals in Various Leaching Modes
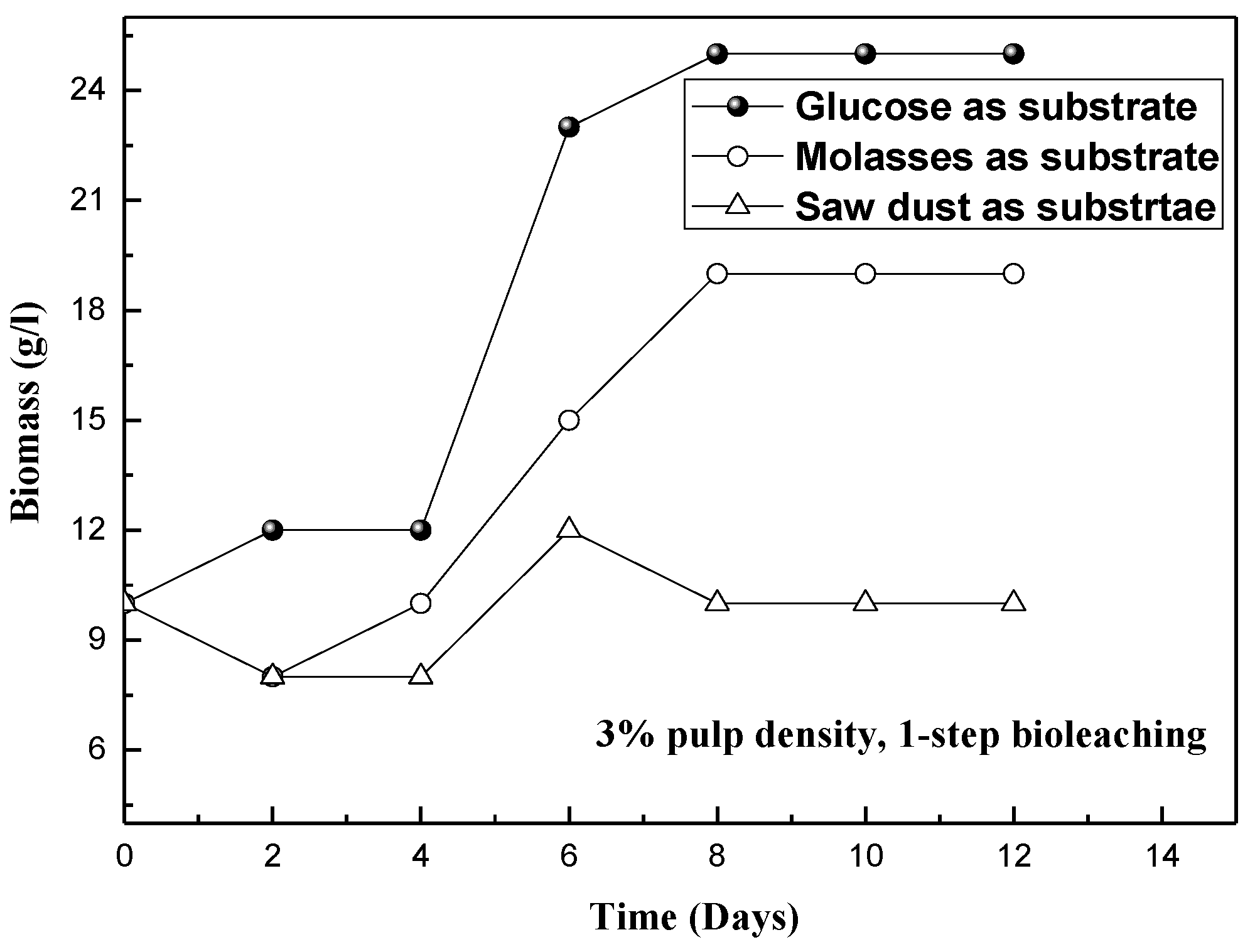
3.3. Production of Organic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Ilyas, S.; Kim, H.; Srivastava, R.R. Extraction equilibria of cerium(IV) with Cyanex 923 followed by precipitation kinetics of cerium(III) oxalate from sulfate solution. Sep. Pruif. Technol. 2021, 254, 117634. [Google Scholar] [CrossRef]
- Ilyas, S.; Kim, H.; Srivastava, R.R.; Choi, S. Cleaner production of rare earth elements from phosphorus-bearing sulfuric acid solution of vein deposit monazite. J. Clean. Prod. 2021, 278, 123435. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash, P.; Meshram, P. Overview on extraction and separation of rare earth elements from red mud: Focus on scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Mongelli, G. Ce-anomalies in the textural components of Upper Cretaceous karst bauxites from the Apulian carbonate platform (southern Italy). Chem. Geol. 1997, 140, 69–79. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Direct determination of landthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Ilyas, S.; Kim, H.; Choi, S.; Trinh, H.B.; Ghauri, M.A.; Ilyas, N. Biotechnological recycling of critical metals from waste printed circuit boards. J. Chem. Technol. Biotechnol. 2020, 95, 2796–2810. [Google Scholar] [CrossRef]
- Ilyas, S.; Bhatti, H.N.; Bhatti, I.A.; Sheikh, M.A.; Ghauri, M.A. Bioleaching of metal ions from low grade sulphide ore: Process optimization by using orthogonal experimental array design. Afr. J. Biotechnol. 2010, 9, 2801–2810. [Google Scholar]
- Ghorbani, Y.; Oliazadeh, M.; Shahvedi, A. Aluminum solubilization from red mud by some indigenous fungi in Iran. J. Appl. Biosci. 2008, 7, 207–213. [Google Scholar]
- Vachon, P.; Tyagi, R.D.; Auclair, J.C.; Wilkinson, K.J. Chemical and biological leaching of aluminum from red mud. Environ. Sci. Technol. 1994, 28, 26–30. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H. O2-enriched microbial activity with pH-sensitive solvo-chemical and electro-chlorination strategy to reclaim critical metals from the hazardous waste printed circuit boards. J. Hazard. Mater. 2021, 416, 125769. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Ting, Y.P. Metal extraction from municipal solid waste (MSW) incinerator fly ash—Chemical leaching and fungal bioleaching. Enzyme Microb. Technol. 2006, 38, 839–847. [Google Scholar] [CrossRef]
- Santhiya, D.; Ting, Y.P. Bioleaching of spent refinery processing catalyst using Aspergillus niger with high-yield oxalic acid. J. Biotechnol. 2005, 116, 171–184. [Google Scholar] [CrossRef]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J. Fungal leaching of metals from electronic scrap. Min. Metall. Explor. 2013, 30, 151–156. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolorRM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef]
- Escobal, A.; Gonzalez, J.; Iriondo, C.; Laborra, C. Liquid chromatographic determination of organic acids in txakoli from Bizkaia. Food Chem. 1997, 58, 381–384. [Google Scholar] [CrossRef]
- Burgstaller, W.; Schinner, F. Leaching of metals with fungi. J. Biotechnol. 1993, 27, 91–116. [Google Scholar] [CrossRef]
- d’Aquino, L.; Morgana, M.; Carboni, M.A.; Staiano, M.; Antisari, M.V.; Re, M.; Woo, S.L. Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol. Biochem. 2009, 41, 2406–2413. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]




| Substrates | Pretreatment | Quantity Used (g/L) |
|---|---|---|
| Glucose | Filter sterilized | 100 |
| Molasses | Autoclaved | 100 |
| Saw dust | Soaked in sulfuric acid, dried, and homogenized | 100 |
| Leaching Modes | Experimental Set Up |
|---|---|
| Mode-1 | Incubating the fungus with red mud using glucose as the substrate |
| Mode-2 | Pre-culturing the fungus using glucose as the substrate and adding the red mud after 3 days of incubation |
| Mode-3 | Using the cell-free spent medium, which was obtained after 10 days of fungal incubation using glucose as the substrate |
| Mode-4 | Incubating the fungus with red mud using pretreated molasses as the substrate |
| Mode-5 | Pre-culturing the fungus using pretreated molasses as the substrate and adding the red mud after 3 days of incubation |
| Mode-6 | Using the cell-free spent medium, which was obtained after 10 days of fungal incubation using pretreated molasses as the substrate |
| Mode-7 | Incubating the fungus with red mud using pretreated saw dust as the substrate |
| Mode-8 | Pre-culturing the fungus using pretreated saw dust as the substrate and adding the red mud after 3 days of incubation |
| Mode-9 | Using the cell-free spent medium, which was obtained after 10 days of fungal incubation using pretreated saw dust as the substrate |
| Mode-10 | Leaching with a synthetic acid mixture (equivalent to biogenic acids in optimal conditions) |
| Mode-11 | Leaching with sterile growth media |
| Organic Acid Production Using Glucose as the Substrate | |||
|---|---|---|---|
| Organic acid production (mM) | mode-1 | mode-2 | mode-3 |
| Citric acid | 12 | 15 | 63 |
| Oxalic acid | 2.5 | 1 | 29 |
| Tartaric acid | 1.8 | 0.5 | 24.5 |
| Gluconic acid | 1162 | 152 | 123 |
| Organic Acid Production Using Molasses as the Substrate | |||
| Organic acid production (mM) | mode-4 | mode-5 | mode-6 |
| Citric acid | 4.21 | 3.57 | 44.8 |
| Oxalic acid | 1.55 | 1.0 | 15.0 |
| Tartaric acid | 1.18 | 0.95 | 14.8 |
| Gluconic acid | 210.19 | 52.5 | 11 |
| Organic Acid Production in the Absence of Red Mud | |||
| Organic acid production (mM) | Glucose | Molasses | Saw dust |
| Citric acid | 63 | 45 | 0.67 |
| Oxalic acid | 28 | 15 | 07 |
| Tartaric acid | 25 | 15 | 03 |
| Gluconic acid | 122 | 11 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, S.; Kim, H.; Srivastava, R.R. Feasibility of the Bio-Mobilization of Rare Earth Elements from Bauxite Residual Red Mud. Environ. Sci. Proc. 2021, 6, 5. https://doi.org/10.3390/iecms2021-09334
Ilyas S, Kim H, Srivastava RR. Feasibility of the Bio-Mobilization of Rare Earth Elements from Bauxite Residual Red Mud. Environmental Sciences Proceedings. 2021; 6(1):5. https://doi.org/10.3390/iecms2021-09334
Chicago/Turabian StyleIlyas, Sadia, Hyunjung Kim, and Rajiv R. Srivastava. 2021. "Feasibility of the Bio-Mobilization of Rare Earth Elements from Bauxite Residual Red Mud" Environmental Sciences Proceedings 6, no. 1: 5. https://doi.org/10.3390/iecms2021-09334
APA StyleIlyas, S., Kim, H., & Srivastava, R. R. (2021). Feasibility of the Bio-Mobilization of Rare Earth Elements from Bauxite Residual Red Mud. Environmental Sciences Proceedings, 6(1), 5. https://doi.org/10.3390/iecms2021-09334








