Abstract
Man-made deposits of fly ash (FA)—a solid by-product of coal combustion—accompany practically every thermal power station and many industrial plants. The total annual production of FA worldwide is about seven–eight hundred million tons, of which less than one third is recycled. The accumulated FA has become a problem for the environment due to its heavy metal content, which can be leached out. The mineral composition of FA is mainly represented by the glass phase and also by quartz, mullite, magnetite and other minerals. In the last decade, intensive studies have been carried out on the use of FA for the preparation of geopolymer materials. Due to their energy savings, environmentally friendly processing and high physical-mechanical properties, geopolymers are gaining attention in the construction industry as a potential replacement for Portland cement. In this work, we focused on the effect of natural dolomite addition to FA and mechanical activation of this blend on the geopolymerization process. The influence of dolomite dosage and duration of mechanical activation in a planetary mill on the reactivity of the blend in relation to NaOH solution and on the geopolymer compressive strength was studied.
1. Introduction
Man-made deposits of coal fly ash—a solid by-product of coal combustion—accompany practically every thermal power station and many industrial plants. The total annual production of coal ash worldwide, mainly fly ash (FA), is about seven–eight hundred million tons, of which less than one third is recycled. The accumulated FA has become a problem for the environment due to its heavy metal content, which can be leached out. The mineral composition of FA is mainly represented by the glass phase and also by quartz, mullite, magnetite and other minerals. In the last decade, intensive studies have been carried out on the use of FA for the preparation of geopolymer materials. Geopolymers are a subclass of alkali-activated materials synthesized by the reaction of low-calcium, natural and industrially produced aluminosilicate raw materials, with an alkaline agent (for example, NaOH solution or water glass) at temperatures close to ambient temperature. [1,2,3,4,5]. Due to their energy savings, environmentally friendly processing and high physical-mechanical properties, geopolymers are gaining attention in the construction industry as a potential replacement for Portland cement. In addition, geopolymers possess a complex of valuable physicochemical properties, and thus, based on them, it is possible to create environmentally friendly materials for fire and heat protection, wastewater treatment, matrices for the immobilization of heavy metals and radioactive waste, and so on [6,7,8]. In previous work, we studied the geopolymerization of FA blended with natural calcite [9].
To increase the reactivity of the raw material, mechanical activation (MA) of the (FA + calcite) blends was carried out. The addition of calcite to FA was found to improve the compressive strength of the geopolymers, especially at an early age. In this work, we focus on the effect of natural dolomite addition to the same FA using the same experimental program. The dolomite content in the mixtures was 0, 1, 3, 5, and 10 wt.%. The influence of dolomite dosage and MA time in a planetary mill on the reactivity of the raw material in relation to NaOH solution, and on the geopolymer compressive strength, was studied.
2. Materials and Methods
2.1. Materials
Low-calcium FA (Class F) was sampled from the Apatity thermal power plant (Murmansk region, Russia). Quartz and mullite are the main crystalline phases in the FA (Figure 1). Natural dolomite was taken from Titan deposit, Murmansk Region, Russia. About 4–5% of quartz and a minor amount of calcite (Figure 1) are present in dolomite as admixtures. The dolomite was milled in a ball mill and sieved with a 300 µm mesh. Table 1 shows the chemical compositions of the FA and dolomite.
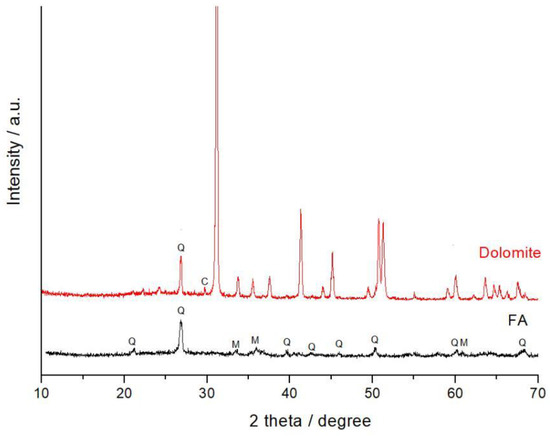
Figure 1.
The XRD patterns of the FA and natural dolomite. Phases marked are: Q—quartz, M—mullite, C—calcite.

Table 1.
Chemical composition of the FA and dolomite, wt.%.
2.2. Mechanical Activation
MA was carried out in an AGO-2 laboratory planetary mill (Novic, Novosibirsk, Russia) in air, at a centrifugal force of 40 g. Steel vials and steel balls 8 mm in diameter were used as the milling bodies. The details of MA were described in [9].
In this study, the terms “milling” and “mechanical activation” (MA) refer to the same process of mechanical treatment using a laboratory planetary mill.
2.3. Synthesis of Geopolymers
Sodium hydroxide solution (8.3 M) was used as the alkaline agent. The mass ratio of Na2O (present in NaOH solution) to the mechanically activated (FA + dolomite) mixtures in the paste was equal to 0.06. The water content was adjusted to give the same workability of the pastes, therefore, the water content varies. The water to solid ratio (w/s) was defined considering the amount of water present in the NaOH solution. Table 2 shows the composition of the blends used for preparation of the pastes. The unreacted (FA + dolomite) blends are denoted as FAXD, where X is the dolomite content in the blend, wt.%. For example, FA10D denotes the blend containing 90% FA and 10% dolomite. The geopolymers, synthesized using these mixtures, are referred to as GFAXD.

Table 2.
Composition of blends used for preparation of pastes.
The pastes were cast into 1.41 × 1.41 × 1.41 cm cubic molds. As with the (FA + calcite) blends [9], specimens were cured in a relative humidity of 95 ± 5% at 22 ± 2 °C for 24 h. After demolding, the specimens were further cured to testing time in the same conditions as applied in the first 24 h. Compressive strength data were obtained from an average of 3 samples after 7, 28, 180, and 360 days.
2.4. Characterization Methods
The specific surface area of the powders was measured using the nitrogen BET method with a Flow-Sorb II 2300 instrument (Micromeritics). Powder X-ray diffraction (XRD) patterns were recorded on a Shimadzu XRD-6000 instrument using Cu–Kα radiation. Scanning was carried out with a step of 0.02° (2 theta) and the dwell time was 1 s. FTIR spectra were recorded with a Nicolet 6700 FTIR spectrometer using potassium bromide tablets. Geopolymer microstructure and morphology were studied using a LEO 420 scanning electron microscope (Zeiss) operated at 10 kV after gold coating on the fractured surface.
3. Results and Discussions
3.1. Effect of Mechanical Activation
The BET-specific surface area (SSA) of the initial FA was 2.5 ± 0.1 m2·g−1. MA of the (FA + dolomite) blends for 30–400 s resulted in a continuous increase in the surface area. With a fixed duration of milling, the dolomite content in the blend had little effect on the SSA of the blend. The SSA of the (FA + dolomite) blends milled for 30, 180, and 400 s were 2.9 ± 0.1, 5.3 ± 0.2, and 6.5 ± 0.3 m2·g−1, respectively.
Figure 2 shows representative XRD patterns of the FA10D mixture milled for 30 s and 180 s. It is clear from comparison of the peak intensities that disorder of the mineral lattice and/or decrease of crystallite size induced by milling, for dolomite, were considerably more pronounced than those for mullite and quartz, which are present in the FA. Obviously, this is due the difference in hardness of the minerals. The hardness on the Mohs scale for quartz, mullite, and dolomite is 7, 6.3–7.5, and 3.5–4.0, respectively.
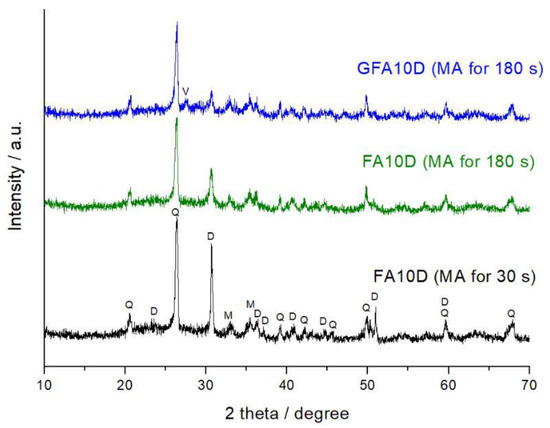
Figure 2.
The XRD patterns of the FA10D mixture mechanically activated for 30 s and 180 s, and of the geopolymer synthesized using the mixture mechanically activated for 180 s after 360 d of curing (GFA10B). Phases marked are: Q—quartz, M—mullite, D—dolomite, V—vaterite.
3.2. Mechanical Properties
Figure 3 shows the compressive strength of geopolymers measured after 7, 28, 180, and 360 days of curing. As in the case of (FA + calcite) mixtures [9], blending the FA with dolomite results in an increase in compressive strength with an increase in the proportion of the added carbonate mineral. The positive effect of adding dolomite to the FA is more pronounced for the early age of curing. For example, the GFA10D geopolymer cured for 7 d showed a strength 8.2, 2.3 and 1.4-fold higher than the GFA0D geopolymer for 30, 180 and 400 s milling time, respectively (Figure 3). For the same geopolymers cured for 360 d, the corresponding strength increased 1.5, 1.2 and 1.1-fold, respectively.
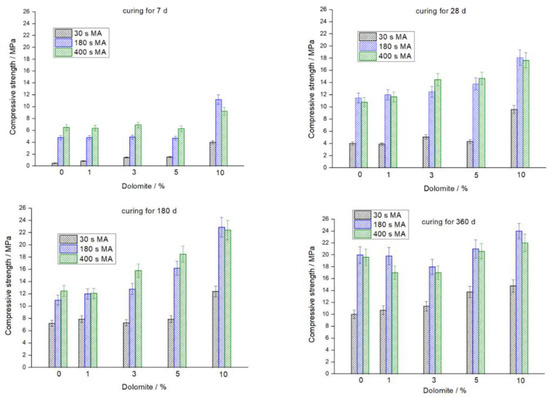
Figure 3.
Effect of dolomite content and MA time on the 7, 28, 180, and 360 day compressive strength of geopolymers.
Comparing the addition of calcite or dolomite to the FA, it should be noted that calcite is more preferable in terms of the strength of geopolymers. For the blends (FA + calcite) containing 1, 3, 5, and 10% calcite milled for 180 s, the compressive strength of the geopolymers cured for 7 d was 7.4, 11.2, 13.0, and 16.8 MPa, respectively [9]. For the blends (FA + dolomite), the corresponding strength was 4.8, 4.9, 4.7, and 11.2 MPa, respectively (Figure 3). With an increase in the curing time, the difference in the strength of geopolymers prepared using calcite and dolomite decreases. For the blends (FA + calcite) containing 1, 3, 5, and 10% calcite milled for 180 s, the compressive strength of the geopolymers cured for 28 d was 12.7, 16.6, 19.5, and 22.4 MPa, respectively [9]. For the blends (FA + dolomite), the corresponding strength was 12.0, 12.5, 13.8, and 18.1 MPa, respectively.
In agreement with the SSA of the (FA + dolomite) blends, MA for 180 s compared to MA for 30 s, leads to a significant increase in compressive strength. The effect of further MA from 180 to 400 s is either very small positive or even deleterious (Figure 3). However, as can be expected, the finer fly ash should show a better solubility in NaOH solution to produce a higher amount of geopolymer gel and, therefore, a higher compressive strength. A similar effect of “over grinding” on the strength was observed for geopolymers prepared using (FA + calcite) blends [9]. This can be explained by the optimal particle size distribution, which is another key factor affecting the geopolymers’ strength [10].
3.3. XRD and FTIR Spectroscopy Analysis
Figure 2 shows the XRD pattern of the GFA10D specimen (milling for 180 s, curing for 360 d). The formation of a binder product, amorphous sodium containing aluminosilicate hydrogel (N-A-S-H gel), is confirmed by the appearance of a halo in the 2θ = 25–35° region.
It should be noted that the main dolomite reflection, at about 2θ = 30.8° in the XRD pattern of the GFA10D, slightly decreased in intensity compared to that of the FA10D mixture (Figure 2). Along with this, the XRD pattern of the GFA10D shows a small peak, which can be attributed to a newly formed phase–vaterite, a calcium carbonate polymorph. The formation of vaterite was revealed also in geopolymers prepared using a mechanically activated mixture containing 90% FA and 10% calcite [9]. This transformation can proceed through recrystallization in sodium hydroxide solution according to the Ostwald step rule [11].
FTIR spectra of the blend containing 10% dolomite (FA10D) milled for 400 s, as well as the geopolymers prepared using this blend in 7 and 180 d ages, are presented in Figure 4. The main absorption band at 1089 cm−1 is due to the asymmetric stretching Si-O-T (T = Si, Al) vibrations. The IR peaks in the 800–600 cm−1 region are related to the presence of quartz and mullite in the FA. The broad band in the 3700–3100 cm–1 region (O-H stretching vibration) in the spectrum of FA10D is due to the H2O adsorbed by the mixture from air in the course of MA. The band at 1456 cm−1 corresponds to the asymmetric stretching vibrations of the carbonate group in dolomite.
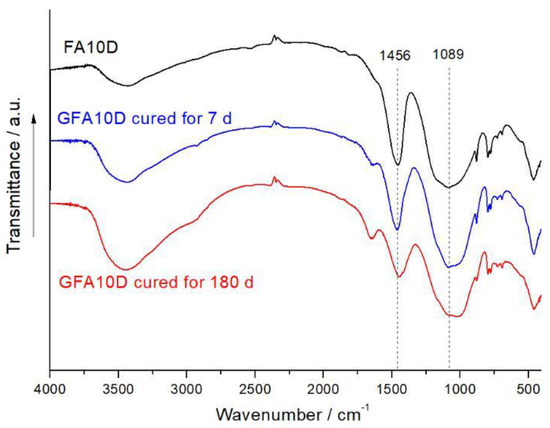
Figure 4.
FTIR spectra of the mixture containing 10% dolomite (FA10D) and the corresponding geopolymer GFA10D cured for 7 and 180 d. Milling of the blend was carried out for 400 s.
The main peak associated with the Si-O-T asymmetric stretching vibrations in the spectra of GFA10D geopolymer shifted towards a lower wavenumber in comparison to that of the unreacted blend (Figure 4). This indicates the formation of a binder product, N-A-S-H gel, and can be explained by the substitution of silicon for aluminum in the SiO4 tetrahedra, and a decrease in the degree of polymerization of the aluminosilicate framework of the FA.
The main peak of dolomite for the GFA10D, at 1456 cm−1, slightly broadened and decreased in intensity in comparison to that for the FA10D (Figure 4). In agreement with the XRD data (Figure 2), this is consistent with the partial transformation of dolomite to vaterite. In the IR spectrum of vaterite, the main band of the CO3 group is split (1420 and 1490 cm–1) due to the reduced symmetry of the carbonate ion in the structure of this mineral [12]. The broadening of the band in the 1500–1350 cm−1 region in the spectra of GFA10D (Figure 4) may be related also to the formation of small amounts of sodium carbonates and hydrocarbonates, as a result of the atmospheric carbonation of unreacted sodium hydroxide.
3.4. Microstructural Studies
Figure 5 displays the microstructures of selected geopolymer specimens prepared using the (FA + dolomite) blends mechanically activated for 30 and 180 s and cured for 360 d. The GFA10D geopolymers prepared using a blend containing 10% dolomite, milled for 180 and 30 s, are characterized by compressive strength of 24.0 and 14.8 MPa, respectively (Figure 3). In accordance with the strength data, the former geopolymer (Figure 5 a) shows a more uniform dense microstructure in comparison to the latter (Figure 5 b)."

Figure 5.
SEM images of the geopolymers cured for 360 d: GFA10D, 180 s MA (a), GFA10D, 30 s MA (b) and GFA1D, 30 s MA (c).
The GFA1D geopolymer based on the (99% FA + 1% dolomite) blend milled for 30 s, which is characterized by compressive strength of 10.8 MPa (Figure 3), contains a noticeable amount of unreacted FA spherical particles and has a more porous structure (Figure 5c) compared to the structure of the GFA10D geopolymers (Figure 5a,b).
4. Conclusions
The room temperature curing geopolymers were synthesized using the mechanically activated blends of FA and natural dolomite. The dolomite content in the blends was 0, 1, 3, 5, and 10 wt.%. NaOH solution was used as alkaline agent.
Milling of the blends in a planetary mill considerably enhanced their reactivity with respect to the sodium hydroxide solution, as was observed using FTIR spectroscopy and SEM. Blending of the FA with dolomite improved the geopolymer strength, especially during the early age of curing. For geopolymers prepared using the (90% fly ash + 10% dolomite) mixture cured for 7 d, the strength was 8.2, 2.3 and 1.4-fold higher than that for the geopolymers prepared using the 100% FA for 30 s, 180 s and 400 s milling time, respectively.
The main geopolymerization product of the mechanically activated (FA + dolomite) mixtures was X-ray amorphous sodium containing aluminosilicate hydrogel. For the geopolymer prepared using the mixture containing 10% dolomite, the partial transformation of dolomite to vaterite was revealed by X-ray diffraction.
From the point of view of the strength of geopolymers, the blending of the FA with dolomite is less preferable than that with calcite. For the blends (FA + dolomite) containing 1, 3, 5, and 10% dolomite milled for 180 s the compressive strength of the geopolymers cured for 7 d was 4.8, 4.9, 4.7, and 11.2 MPa, respectively. For the blends (FA + calcite) the corresponding strength was 7.4, 11.2, 13.0, and 16.8 MPa, respectively. With an increase in the curing time, the difference in the strength of geopolymers prepared using calcite and dolomite decreases.
Funding
The reported study was funded by RFBR, project number 20-03-00486.
Acknowledgments
The authors acknowledge support from the Centre for Thermogravimetric and Calorimetric Research of the Research Park of St. Petersburg State University where the thermal analysis was performed. V.V. Semushin is acknowledged for his help in SEM/EDS studies.
References
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–349. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Alkali-Activated Materials: State of the Art Report of RILEM TC 224-AAM; Springer Science & Business Media: Dordrecht, Holland, 2014. [Google Scholar]
- Krivenko, P. Why alkaline activation–60 years of the theory and practice of alkali-activated materials. J. Ceram. Sci. Technol. 2017, 8, 323–333. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendor, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Hu, Y.; Zhou, J.L.; Tam, V.W.Y. Review on designs and properties of multifunctional alkali-activated materials (AAMs). Constr. Build. Mater. 2019, 200, 474–489. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Biotechnol. 2019, 18, 271–297. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Gurevich, B.I.; Myshenkov, M.S.; Chislov, M.V.; Kalinkina, E.V.; Zvereva, I.A.; Cherkezova-Zheleva, Z.; Paneva, D.; Petkova, V. Synthesis of fly ash-based geopolymers: Effect of calcite addition and mechanical activation. Minerals 2020, 10, 827. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Mádai, F.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Min. Proc. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- White, W.B. The carbonate minerals. In The Infrared Spectra of Minerals; Farmer, W.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 227–284. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).