Miyawaki and Urban Tiny Forests in Italy
Abstract
1. Introduction
2. Tiny Forests
2.1. Quantitative Features
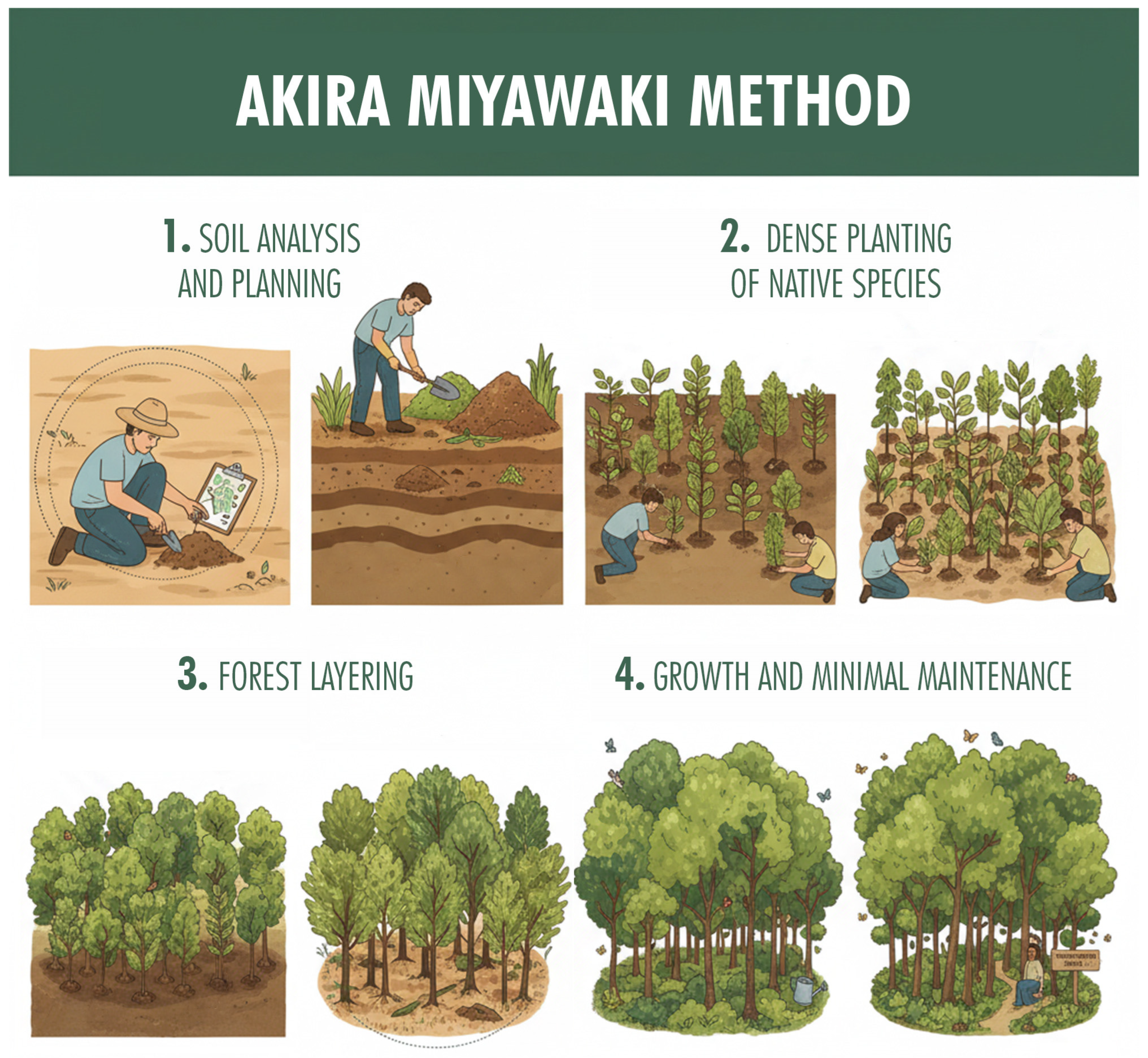
2.2. Qualitative Features and the Miyawaki Method
- (a)
- Site assessment and reference vegetation.
- (b)
- Species pool selection and stratification.
- (c)
- Planting density and spacing.
- (d)
- Mulching.
3. The Evolution of Tiny Forests
4. The Utility of Tiny Forests
- Cool cities by two to six degrees.
- Reduce air pollution, dust, and act as air filters.
- Lower rates of cardiac diseases, stroke, and asthma.
- Increase physical activity.
- Protect biodiversity by providing habitats.
- Act as carbon sinks.
- Support social cohesion.
- Reduce stress and crime.
- Produce oxygen and reduce carbon.
- Assist in active stormwater management.
- The recovery of naturalistic intelligence.
- A reconnection with natural time.
- A “spontaneous” understanding of concepts like adaptation, succession, and evolution.
- A “spontaneous” grasp of competition and cooperation dynamics.
- Recognition of taxa and the distinction between flora and vegetation.
- Study and comprehension of plant/vegetation–climate relationships.
- Study and understanding of soil-flora-fauna interactions.
- Application of modern technologies to studying natural environments (including the use of smartphones).
- And much more.
5. Recovery of Naturalistic Intelligence
- The method by which the cognitive process is structured and proposed.
- The object of the cognitive process.
- Other children, who are the first educators.
- Parents and teachers, who act as privileged interlocutors.
- The environment, the social and physical context in which the child grows.
5.1. The Object of the Cognitive Process
5.2. What Characteristics Should a Forest Have to Stimulate Naturalistic Intelligence?
6. The Recovery of Natural Time
7. The Application of Modern Technologies
8. The Italian Tiny Forest Project: Some Examples
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Bank Group. Urban Development Overview. Available online: https://www.worldbank.org/en/topic/urbandevelopment/overview#1 (accessed on 3 April 2025).
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Cormier, L.; De Lajartre, A.; Carcaud, N. La Planification des Trames Vertes, du Global au Local: Réalités et Limites. Cybergeo: European Journal of Geography 2010, Aménagement, Urbanisme, Document 504. Available online: http://cybergeo.revues.org/23187 (accessed on 30 June 2025).
- Konijnendijk, C.; Ricard, R.M.; Kenney, A.; Randrup, T.B. Defining urban forestry: A comparative perspective of North America and Europe. Urban For. Urban Green. 2006, 4, 93–103. [Google Scholar] [CrossRef]
- Konijnendijk, C. The 3-30-300 rule for urban forestry and greener cities. Biophilic Cities J. 2021, 4, 2. [Google Scholar]
- Browning, M.H.E.M.; Locke, D.H.; Konijnendijk, C.; Labib, S.M.; Rigolon, A.; Yeager, R.; Bardhan, M.; Berland, A.; Dadvand, P.; Helbich, M.; et al. Measuring the 3-30-300 rule to help cities meet nature access thresholds. Sci. Total Environ. 2024, 907, 167739. [Google Scholar] [CrossRef]
- Ministry of Ecological Transition, Biodiversity, Forestry, the Sea and Fisheries. Green and Blue Frame. 2023. Available online: https://www.ecologie.gouv.fr/politiques-publiques/trame-verte-bleue (accessed on 30 June 2025).
- FAO. Guidelines on Urban and Peri-Urban Forestry; Salbitano, F., Borelli, S., Conigliaro, M., Chen, Y., Eds.; FAO Forestry Paper No. 178; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- O’Brien, L.E.; Urbanek, R.E.; Gregory, J.D. Ecological functions and human benefits of urban forests. Urban For. Urban Green. 2022, 75, 127707. [Google Scholar] [CrossRef]
- Cárdenas, M.L.; Pudifoot, B.; Narraway, C.L.; Pilat, C.; Beumer, V.; Hayhow, D.B. Nature-based solutions building urban resilience for people and the environment: Tiny Forest as a case study. Q. J. For. 2022, 116, 27–37. [Google Scholar]
- Lewis, H. Mini-Forest Revolution: Using the Miyawaki Method to Rapidly Rewild the World; Chelsea Green Publishing: White River Junction, VT, USA, 2022. [Google Scholar]
- Egerer, M.; Suda, M. Designing “Tiny Forests” as a lesson for transdisciplinary urban ecology learning. Urban Ecosyst. 2023, 26, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F. The eco-pedagogical microforest: A shared oasis of proximity. A cutting-edge project at the intersection of ecology, urbanism and pedagogy. TeMA J. Land Use Mobil. Environ. 2023, 2, 33–54. [Google Scholar]
- Morino Project. Tiny Forests, Japan. Available online: https://morinoproject.com/english/ (accessed on 14 September 2025).
- Urban Tiny Forest, USA. Available online: https://www.urbantinyforest.com/ (accessed on 14 September 2025).
- Plant, Earth & Forest, India. Available online: https://www.pef-india.org/ (accessed on 14 September 2025).
- Nacons, India. Plantation with Afforestation. Available online: https://nacons.org/plantation-with-afforestation/ (accessed on 14 September 2025).
- SayTrees, India. Urban Forestry. Available online: https://www.saytrees.org/urban-forestry (accessed on 14 September 2025).
- Sowoods, Belgium. Available online: https://sowoods.be/projet/la-micro-foret-euroclear/ (accessed on 14 September 2025).
- Urban Forests. Available online: https://www.urbanforest.be/ (accessed on 14 September 2025).
- ConsciousNet & Mini Big Forest, France. Available online: https://www.consciousnet.org/ (accessed on 14 September 2025).
- MiniBigForest. Available online: https://www.minibigforest.com/ (accessed on 14 September 2025).
- Biggest Mini Forest, Portugal. Available online: https://www.biggestminiforest.com/ (accessed on 14 September 2025).
- IVN, Netherlands. Tiny Forest Program. Available online: https://www.ivn.nl/aanbod/tiny-forest/ (accessed on 14 September 2025).
- Sugi Project, Spain. Available online: https://www.sugiproject.com/forests/makers/minibig-forest-espana (accessed on 14 September 2025).
- Miya Forest, Germany. Tiny Forest Initiative. Available online: https://www.miya-forest.de/en/tiny-forest (accessed on 14 September 2025).
- London Borough of Hammersmith & Fulham/Earthwatch, UK. Tiny Forests. Available online: https://www.lbhf.gov.uk/environment/climate-and-ecological-emergency/tiny-forests (accessed on 14 September 2025).
- Earthwatch Europe. Tiny Forest. Available online: https://tinyforest.earthwatch.org.uk/ (accessed on 14 September 2025).
- FAO-FRA. On Definitions of Forest and Forest Change; Working Paper 33; FAO Forest Resources Assessment: Rome, Italy, 2000. [Google Scholar]
- Bischoff, N. Selvicoltura di Montagna; Ufficio federale svizzero dell’Ambiente, delle foreste e del paesaggio: Berna, Switzerland, 1994. [Google Scholar]
- Bernetti, G. Selvicoltura Speciale; UTET: Torino, Italy, 1995. [Google Scholar]
- Heylighen, F. The science of self-organization and adaptivity. In Knowledge Management, Organizational Intelligence and Learning, and Complexity; Kiel, L.D., Ed.; EOLSS Publishers Co., Ltd.: Oxford, UK, 2002; Available online: https://www.eolss.net/sample-chapters/c15/E1-29-01-05.pdf (accessed on 14 September 2025).
- Banzhaf, W. Self-organizing systems. In Encyclopedia of Complexity and Systems Science; Meyers, R., Ed.; Springer: New York, NY, USA, 2009; pp. 8040–8050. [Google Scholar] [CrossRef]
- Miyawaki, A. Restoration of evergreen broad-leaved forests in the Pacific region. In Ecosystem Rehabilitation: Ecosystem Analysis and Synthesis; Wali, M.K., Ed.; SPB Academic Publishing: The Hague, Netherlands, 1992; Volume 2. [Google Scholar]
- Butfoy, L. Miyawaki Method Handbook; Kent County Council: Kent, UK, 2023. Available online: https://www.kent.gov.uk (accessed on 14 September 2025).
- Daou, A.; Saliba, M.; Kallab, A. A review of the Miyawaki method. SSRN 2024. [Google Scholar] [CrossRef]
- Schirone, B.; Salis, A.; Vessella, F. Effectiveness of the Miyawaki method in Mediterranean forest restoration programs. Landsc. Ecol. Eng. 2011, 7, 81–92. [Google Scholar] [CrossRef]
- Hilmers, T.; Friess, N.; Bässler, C.; Heurich, M.; Brandl, R.; Pretzsch, H.; Seidl, R.; Müller, J. Biodiversity along temperate forest succession. J. Appl. Ecol. 2018, 55, 2756–2766. [Google Scholar] [CrossRef]
- Geng, Q.; Arif, M.; Yuan, Z.; Zheng, J.; He, X.; Ding, D.; Yin, F.; Li, C. Plant species composition and diversity along successional gradients in arid and semi-arid regions of China. For. Ecol. Manag. 2022, 524, 120542. [Google Scholar] [CrossRef]
- Šipek, M.; Ravnjak, T.; Šajna, N. Understorey species distinguish late successional and ancient forests after decades of minimum human intervention: A case study from Slovenia. For. Ecosyst. 2023, 10, 100096. [Google Scholar] [CrossRef]
- Oldeman, R.A.A. Forests: Elements of Silvology; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-75213-1. [Google Scholar]
- Qi, H.; Dempsey, N.; Cameron, R. Seeing the Forest for the Trees? An Exploration of the Miyawaki Forest Method in the UK. Arboric. J. 2024, 46, 292–304. [Google Scholar] [CrossRef]
- Manuel, C. The Miyawaki Method—Data & Concepts; Urban Forests, 2020. Available online: https://www.readkong.com/page/the-miyawaki-method-data-concepts-urban-forests-6923563 (accessed on 15 September 2025).
- University of Tuscia. The Botanical Garden “Angelo Rambelli”. Available online: https://www.ortobotanico.unitus.it/index.php/it/ (accessed on 10 February 2025).
- Ottburg, F.; Lammertsma, D.; Dimmers, W.; Lerink, B.; Schelhaas, M.-J.; Janssen, J. Tiny Forests: Groene Mini-Oases in de Stad: Monitoring van Biodiversiteit en Bijdragen aan CO2-Op-Slag, Wateropvang en Tegengaan Hittestress in Elf Tiny Forests; Rapport/Wageningen Environmental Research, No. 3189; Wageningen Environmental Research: Wageningen, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Akram, M.T.; Khan, M.M.; Taj, N.; Qadri, R.; Al-Maskri, A.; Khan, A.M. Miyawaki Technique for Sustainable Urban Greening and Ecological Restoration: A Review. CABI Rev. 2025, 20, 0028. [Google Scholar] [CrossRef]
- Rajadurai, J. Assessing the Sociological Impact of the Miyawaki Method on Urban Health and Environment. Int. J. Res. Innov. Soc. Sci. 2024, 8, 3360–3367. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Sagasaki, Y.; Nozaki, H.; Miyazaki, Y. Physiological Effects of a Small Green Space Installed on the Side of a Clinic for Outpatients with Depression. Front. Environ. Health 2025, 4, 1601838. [Google Scholar] [CrossRef]
- Arantes, B.L.; Locke, D.H.; Moreira, G.C.; Grove, J.M. The Relationships between Urban Tree Canopy Cover and Crime in São Paulo City, Brazil. Urban For. Urban Green. 2024, 101, 128497. [Google Scholar] [CrossRef]
- Gardner, H. Frames of Mind: The Theory of Multiple Intelligences, 2nd ed.; Basic Books: New York, NY, USA, 2011. [Google Scholar]
- Durrell, G. My Family and Other Animals; Originally published 1956; Open Road Integrated Media: New York, NY, USA, 2016. [Google Scholar]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Barbiero, G.; Berto, R. Introduzione alla Biofilia; Carocci Editore: Roma, Italy, 2019. [Google Scholar]
- Ceci, S.J. On Intelligence…More or Less: A Bio-Ecological Treatise on Intellectual Development; Prentice Hall: New York, NY, USA, 1990. [Google Scholar]
- Reggio Emilia Approach. 2025. Available online: https://www.reggiochildren.it/en/reggio-emilia-approach/ (accessed on 10 February 2025).
- Edwards, C.; Gandini, L.; Forman, G. (Eds.) I Cento Linguaggi dei Bambini. L’approccio di Reggio Emilia All’educazione Dell’infanzia; Edizioni Junior: Bergamo, Italy, 2014. [Google Scholar]
- Montessori, M. Il Metodo della Pedagogia Scientifica Applicato All’educazione Infantile nelle Case dei Bambini, 3rd ed.; Maglione & Strini/Loescher: Torino, Italy, 1935. [Google Scholar]
- Piaget, J. The Science of Education and the Psychology of the Child; Penguin Publishing Group: Harmondsworth, UK, 1976. [Google Scholar]
- Papert, S. The Connected Family: Bridging the Digital Generation Gap; Longstreet Press: Charlotte, NC, USA, 1996; ISBN 978-1-56352-335-9. [Google Scholar]
- Reggio Children. Atelier. 2025. Available online: https://www.reggiochildren.it/rc/atelier/ (accessed on 10 February 2025).
- Roth, T.L.; Sweatt, J.D. Annual research review: Epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J. Child Psychol. Psychiatry 2011, 52, 398–408. [Google Scholar] [CrossRef]
- McEwen, B.S.; Bulloch, K. Epigenetic impact of the social and physical environment on brain and body. Metabolism 2019, 100, 153941. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An overview of the epigenetic modifications in the brain under normal and pathological conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef]
- Weaver, I. Epigenetics in psychology. In Noba Textbook Series: Psychology; Biswas-Diener, R., Diener, E., Eds.; DEF publishers: Champaign, IL, USA, 2025; Available online: http://noba.to/37p5cb8v (accessed on 10 April 2025).
- Bacon, E.R.; Brinton, R.D. Epigenetics of the developing and aging brain: Mechanisms that regulate onset and outcomes of brain reorganization. Neurosci. Biobehav. Rev. 2021, 125, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, L.; Czamara, D. Epigenetics of prenatal stress in humans: The current research landscape. Clin. Epigenet. 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Reyes Gómez, U.; Hernández Rico, M.P.; Reyes Hernández, D.; Hernández, L.J.; Ortiz Martínez, M. La música de Mozart en el periodo prenatal [Mozart’s music in the prenatal period]. Ginecol. Obstet. Mex. 2006, 74, 424–428. [Google Scholar]
- Dastgheib, S.S.; Layegh, P.; Sadeghi, R.; Foroughipur, M.; Shoeibi, A.; Gorji, A. The effects of Mozart’s music on interictal activity in epileptic patients: Systematic review and meta-analysis of the literature. Curr. Neurol. Neurosci. Rep. 2014, 14, 420. [Google Scholar] [CrossRef]
- Sujatashamkuwar, A.; Ashokan, V.; Shrivas, Y.; Baghel, P.; Sujata, S. Effect of classical music on fetus: A review. J. Ayurveda Herb. Med. 2022, 8, 119–124. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Bonhoeffer, S. An evolutionary scenario for the transition to undifferentiated multicellularity. Proc. Natl. Acad. Sci. USA 2003, 100, 1095–1098. [Google Scholar] [CrossRef]
- Colizzi, E.S.; Vroomans, R.M.; Merks, R.M. Evolution of multicellularity by collective integration of spatial information. eLife 2020, 9, e56349. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Cell communications among microorganisms, plants, and animals: Origin, evolution, and interplays. Int. J. Mol. Sci. 2020, 21, 8052. [Google Scholar] [CrossRef]
- Trewavas, A.J. Aspects of plant intelligence. Ann. Bot. 2003, 91, 1–20. [Google Scholar] [CrossRef]
- Calvo, P.; Gagliano, M.; Souza, G.M.; Trewavas, A. Plants are intelligent, here’s how. Ann. Bot. 2020, 125, 11–28. [Google Scholar] [CrossRef]
- Castiello, U. Plant intelligence from a comparative psychology perspective. Biology 2023, 12, 819. [Google Scholar] [CrossRef]
- Lovelock, J.E. Gaia: A New Look at Life on Earth; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Wood, J.D.; Detto, M.; Browne, M.; Kraft, N.J.B.; Konings, A.G.; Fisher, J.B.; Quetin, G.R.; Trugman, A.T.; Magney, T.S.; Medeiros, C.D.; et al. The ecosystem as super-organism, revisited: Scaling hydraulics to forests under climate change. Integr. Comp. Biol. 2024, 64, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.W.; Perry, D.A.; Jones, M.D.; Myrold, D.D.; Durall, D.M.; Molina, R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 1997, 388, 579–582. [Google Scholar] [CrossRef]
- de la Cal, L.; Gloor, P.A.; Weinbeer, M. Can plants sense humans? Using plants as biosensors to detect the presence of eurythmic gestures. Sensors 2023, 23, 6971. [Google Scholar] [CrossRef]
- Gilsoul, M. Maria Montessori: Une vie au Service de L’enfant; Éditions Desclée de Brouwer: Paris, France, 2020. [Google Scholar]
- Piaget Org. About Piaget. 2025. Available online: https://piaget.org/about-piaget/ (accessed on 10 February 2025).
- Kohn, E. How Forests Think: Toward an Anthropology Beyond the Human; University of California Press: Berkeley, CA, USA, 2013. [Google Scholar]
- Coccia, E. La vie des Plantes. Une Métaphysique du Mélange; Editions Payot et Rivages: Paris, France, 2016. [Google Scholar]
- Rovelli, C. L’ordine del Tempo; Adelphi: Milano, Italy, 2017. [Google Scholar]
- Project Gutenberg. International Conference Held at Washington for the Purpose of Fixing a Prime Meridian and a Universal Day. October, 1884. Protocols of the Proceedings; Gibson Bros: Banbridge, UK, 1884; Available online: https://www.gutenberg.org (accessed on 12 February 2024).
- CGPM. Resolution 4 of the 27th CGPM (2022) on the Use and Future Development of UTC; BIPM, Pavillon de Breteuil: Sèvres Cedex, France, 2022. [Google Scholar] [CrossRef]
- Hut, R.A.; Beersma, D.G. Evolution of time-keeping mechanisms: Early emergence and adaptation to photoperiod. Phil. Trans. R. Soc. B 2011, 366, 2141–2154. [Google Scholar] [CrossRef]
- Morshedian, A.; Fain, G.L. Light adaptation and the evolution of vertebrate photoreceptors. J. Physiol. 2017, 595, 4947–4960. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Kaneko, M.; Allada, R. Biological clocks. Proc. Natl. Acad. Sci. USA 1999, 96, 8819–8820. [Google Scholar] [CrossRef]
- Foster, R.; Kreitzman, L. Rhythms of Life: The Biological Clocks That Control the Daily Lives of Every Living Thing; Yale University Press: New Haven, CT, USA, 2005. [Google Scholar]
- McClung, C.R. Plant circadian rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef]
- Harrison, E.M.; Gorman, M.R. Rapid adjustment of circadian clocks to simulated travel to time zones across the globe. J. Biol. Rhythms 2015, 30, 557–562. [Google Scholar] [CrossRef]
- Dunlap, J.C.; Loros, J.J. Making time: Conservation of biological clocks from fungi to animals. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Thanh Vo, B.; Mas, P.; Johannes, F. Time’s up: Epigenetic clocks in plants. Curr. Opin. Plant Biol. 2024, 81, 102602. [Google Scholar] [CrossRef]
- The Nobel Assembly at Karolinska Institutet. The 2017 Nobel Prize in Physiology or Medicine—Press Release. 2017, Announced 2 October 2017. Available online: https://www.nobelprize.org/prizes/medicine/2017/press-release/ (accessed on 30 July 2025).
- The European Commission. Influenced by Light, Biological Rhythms Say a Lot About Health. Horizon Magazine, 8 June 2023. Available online: https://projects.research-and-innovation.ec.europa.eu/en/horizon-magazine/influenced-light-biological-rhythms-say-lot-about-health (accessed on 30 July 2025).
- Roenneberg, T.; Merrow, M. The circadian clock and human health. Curr. Biol. 2016, 26, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef]
- Samanta, S.; Ali, S.A. Impact of circadian clock dysfunction on human health. Explor. Neurosci. 2022, 1, 4–30. [Google Scholar] [CrossRef]
- Ruff, B. How to Make Weather Instruments; WikiHow: Palo Alto, CA, USA, 2025; Available online: https://www.wikihow.com/Make-Weather-Instruments (accessed on 10 February 2025).
- Sciencing. Science Projects. 2025. Available online: https://sciencing.com/science-projects/ (accessed on 10 February 2025).
- Silvetti, F. BIO Sound Machine. Istituto di Istruzione Superiore “Cattaneo-Dall’Aglio”. 2021. Available online: https://www.cattaneodallaglio.edu.it/bio-sound-machine/ (accessed on 14 September 2025).
- Cesaretti, L. Green Robotic Challenge: Costruire un orto Automatico con il Lego Mindstorms EV3. 2025. Available online: https://www.weturtle.org/dettaglio-progetti/90/green-robotic-challenge-costruire-un-orto-automatico-con-il.html (accessed on 14 September 2025).
- European Environment Agency (EEA). Corine Biotopes. European Environment Agency. 2017. Available online: https://www.eea.europa.eu/en/datahub/datahubitem-view/d8dd768f-9bc4-4002-9386-0aef2c516f76 (accessed on 30 July 2025).
- Moss, D. EUNIS Habitat Classification—A Guide for Users; European Topic Centre on Biological Diversity: Paris, France, 2008. [Google Scholar]
- European Environment Agency (EEA). EUNIS Habitat Classification; European Environment Agency: Copenhagen, Denmark, 2025; Available online: https://eunis.eea.europa.eu/index.jsp (accessed on 5 March 2025).
- Rivas-Martínez, S. Global Bioclimatics (Clasificación Bioclimática de la Tierra); Phytosociological Research Center/Universidad Complutense de Madrid: Madrid, Spain, 2004. [Google Scholar]
- Blasi, C. Fitoclimatologia del Lazio; Università La Sapienza & Regione Lazio: Roma, Italy, 1994. [Google Scholar]
- Società Italiana di Restauro Forestale (SIRF). RIMM—Rete Italiana delle Microforeste Miyawaki. Restauro Forestale, 5 Giugno 2025. Available online: https://www.restauroforestale.it/2025/06/05/rimm-rete-italiana-delle-microforeste-miyawaki/ (accessed on 14 September 2025).
- Hindle, R.L. A Vertical Garden: Origins of the Vegetation-Bearing Architectonic Structure and System (1938). Stud. Hist. Gard. Des. Landsc. 2012, 32, 99–110. [Google Scholar] [CrossRef]
- Vertical Garden Patrick Blanc. Available online: https://www.verticalgardenpatrickblanc.com/ (accessed on 14 September 2025).
- Boeri, S. Bosco Verticale. Morphology of a Vertical Forest; Mondadori Electa: Milan, Italy, 2024; p. 240, ISBN 978-8891843074/ISBN 8891843075. [Google Scholar]
- “25 Verde. L’edificio-Foresta a Torino” Luciano Pia. Architettura Ecosostenibile. Available online: https://www.architetturaecosostenibile.it/architettura/progetti/25-verde-torino-294 (accessed on 14 September 2025).



| Type | Legislation ID | Name | Reference |
|---|---|---|---|
| European Legislation | Regulation (EU) 2024/1991 of the European Parliament and of the Council of 24 June 2024 on nature restoration and amending Regulation (EU) 2022/869—Nature Restoration Law. | Nature Restoration Law | https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32024R1991&qid=1722240349976 (accessed on 30 June 2025) |
| Italian Legislation | Law of 14 January 2013, No. 10—Rules for the development of urban green spaces. | Rules for the development of urban green spaces | https://www.normattiva.it/atto/caricaDettaglioAtto?atto.dataPubblicazioneGazzetta=2013-02-01&atto.codiceRedazionale=13G00031&tipoDettaglio=originario&qId= (accessed on 30 June 2025) |
| Italian Legislation | Decree of the Ministry of the Environment and the Protection of Land and Sea of 9 October 2020 (Official Gazette No. 281 of 11 November 2020) | Procedures for the design of reforestation interventions pursuant to Article 4 of Decree-Law of 14 October 2019, No. 111, converted, with amendments, by Law of 12 December 2019, No. 141 | https://www.reteambiente.it/normativa/43092/dm-ambiente-9-ottobre-2020/ (accessed on 30 June 2025) |
| Italian Legislation | Legislative Decree of 3 April 2018, No. 34—Consolidated Forestry and Forest Supply Chains Act (TUFF) | Consolidated Forestry and Forest Supply Chains Act (TUFF) | https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:decreto.legislativo:2018;34~art10-com4 (accessed on 30 June 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirone, B.; Pica, A.; Fratini, F.; Menegoni, P.; Cianfaglione, K. Miyawaki and Urban Tiny Forests in Italy. Earth 2025, 6, 116. https://doi.org/10.3390/earth6040116
Schirone B, Pica A, Fratini F, Menegoni P, Cianfaglione K. Miyawaki and Urban Tiny Forests in Italy. Earth. 2025; 6(4):116. https://doi.org/10.3390/earth6040116
Chicago/Turabian StyleSchirone, Bartolomeo, Antonio Pica, Fabiola Fratini, Patrizia Menegoni, and Kevin Cianfaglione. 2025. "Miyawaki and Urban Tiny Forests in Italy" Earth 6, no. 4: 116. https://doi.org/10.3390/earth6040116
APA StyleSchirone, B., Pica, A., Fratini, F., Menegoni, P., & Cianfaglione, K. (2025). Miyawaki and Urban Tiny Forests in Italy. Earth, 6(4), 116. https://doi.org/10.3390/earth6040116








