Abstract
Mercury (Hg) pollution from artisanal and small-scale gold mining (ASGM) is a global environmental and public health concern. In Indonesia, ASGM remains widespread, yet assessments of multimedia contamination and health risks are limited. This study quantified Hg concentration in water, sediment, soil, fish, and cassava to evaluate environmental pollution and potential health risks in Waluran, Sukabumi, Indonesia. Mercury concentration in ASGM was higher than in the reference area, especially in fish (median: 4.76 mg/kg dw), cassava leaves (median: 15.7 mg/kg dw), and tailing sediments (median: 171 mg/kg dw). A remarkably high Hg concentration (9760 mg/kg dw) was detected in soil from amalgam-burning spots. An elevated Hg concentration was observed in the reference area, suggesting widespread contamination and potential for long-range dispersion. Over 85% of ASGM samples were categorized as heavily to extremely contaminated by the geo-accumulation index (Igeo). Bioaccumulation assessment indicated a high bioconcentration factor (BCF) in fish and moderate bioaccumulation factor (BAF) in cassava roots. Hazard Quotients (HQ) were greater than 1 for most exposure pathways in both adults and children, with the greatest risk deriving from cassava leaf consumption. These findings indicate severe Hg contamination within ASGM-affected communities and underscore the urgent need for public health interventions, environmental monitoring, and strengthened regulations to reduce Hg exposure in Indonesia.
1. Introduction
Mercury (Hg) is an extremely hazardous heavy metal to the environment and human health. It is among the chemicals of high public health concern, which pose a risk to global public health. Human exposure occurs mainly through the consumption of fish contaminated with methylmercury (MeHg), a potent neurotoxin. Additional pathways include ingestion or dermal contact with polluted soil and the inhalation of particulate matter or gaseous Hg0 [1].
Artisanal and small-scale gold mining (ASGM) provides livelihood for approximately millions of people throughout the world, especially in developing countries. ASGM relies on Hg for gold extraction, releasing large amounts of toxic pollutants. According to UNEP, it is the largest anthropogenic source of Hg worldwide, contributing to nearly 38% of global emissions [2]. Apart from this, Indonesia is also a significant center of ASGM, with more than 1200 activities scattered in 190 municipalities of 31 provinces, including 15 officially declared protected areas. Despite the ratification of the Minamata Convention by the country in 2017, Hg use in ASGM remains prevalent, contributing to 69.7% of Indonesia’s anthropogenic Hg emissions with an estimated amount of 307,125 kg per year [3].
The Indonesian government has been actively involved in the control of Hg since the Intergovernmental Negotiating Committee (INC) on the Legally Binding Instrument of Hg, conducted by the United Nations Environment Programme (UNEP). According to the RAN-PPM study, efforts to limit Hg usage in the ASGM sector resulted in a reduction of 10.45 tons. This was accomplished by limiting the ASGM sector’s usage of Hg and pursuing efforts to develop Hg-free gold processing methods [4]. The government continues to crack down on its usage in illicit mining, and various steps have been implemented to reduce its employment in unlawful mining [5].
Artisanal and small-scale gold mining in West Java is mostly centered in Gunung Pongkor, Tasikmalaya, and Sukabumi, while expert gold processors also operate in other parts of Indonesia. Despite the implementation of the 2020 Governor’s Regulation on Hg Reduction, Hg use in the ASGM sector remains significant, with the chemical being widely available at local kiosks [3]. The Waluran Subdistrict in southern Sukabumi, West Java, is a hotspot for illegal ASGM. The region’s gold-rich geology has attracted unregulated mining, with over 35 shafts, some deeper than 100 m, operating without environmental controls. Mercury-based amalgamation is commonly used, leading to serious Hg contamination of water, soil, and plants [6]. On the other hand, Waluran’s economy remains predominantly agricultural, with 78% of its population engaged in farming [7]. The subdistrict is a recognized producer of rice and cassava, with a strong presence of farmer cooperatives and small agro-industries. Waluran cultivated 10 hectares of cassava with a productivity of 260.76 quintals per hectare, resulting in a total yield of 260.76 tons, the highest productivity rate among neighboring subdistricts [8]. Notably, the Waluran subdistrict is home to several cassava-processing groups that produce traditional snacks such as “Keripik Enye”, which support local livelihoods and empower rural women.
Previous studies have reported elevated Hg levels in different environmental and biological samples in Sukabumi, Indonesia, particularly in ASGM-affected areas. Among food crops, cassava roots have been found to have the highest Hg levels of 31.07 ± 17.25 mg/kg ww, followed by cassava leaves, with levels of 4.61 ± 1.51 mg/kg ww, while papaya leaves and fruits showed lower concentrations of 1.53 ± 0.89 mg/kg ww and 0.1 mg/kg ww, respectively [9]. Rice grains contained 0.09 mg/kg ww of Hg, reflecting potential dietary exposure risks. Soils exhibited very high variability, and Hg levels ranged from 1.34 to 83.7 mg/kg dw in non-rhizosphere soil and from 0.63 to 88 mg/kg dw in rhizosphere soil, reflecting very high levels of contamination in farms [9]. Human biomonitoring also revealed severe Hg accumulation, particularly in hair samples: males averaged 3.27 ± 2.89 mg/kg, females 5.91 ± 4.69 mg/kg, and children 5.34 mg/kg [10]. A recent study in Gunung Pongkor, West Java, further strengthened these findings by demonstrating widespread Hg contamination across environmental compartments, including water, soil, sediment, fish, and cassava. These compartments showed concentrations of up to 4.49 μg/L in water, 144 mg/kg dw in soil, 150 mg/kg dw in sediment, 1.23 mg/kg dw in fish, and 5.09 mg/kg dw and 8.84 mg/kg dw in cassava roots and leaves, respectively [11].
Despite previous findings, a comprehensive study evaluating Hg contamination across multiple environmental media, water, sediment, soil, biota, and plants, and its associated human health risks, has not yet been conducted in Sukabumi. This study aims to assess the distribution of Hg, measure the levels of Hg accumulation, and examine the potential health risks associated with Hg exposure. Thorough assessments are crucial for gaining a deeper understanding of Hg contamination and its health effects in regions impacted by ASGM in Sukabumi.
2. Materials and Methods
2.1. Study Area
The study area is located in theWaluran sub-district, which is part of the Sukabumi regency, West Java, Indonesia (7°10′–7°13′ S, 106°35′–106°37′ E) and falls within the Ciletuh–Palabuhan Ratu Geopark [12]. The topography consists of hills with steep slopes forming a series of ridges interspersed with valleys, whose elevation ranges between 380 m and 800 m above sea level [13]. Precipitation averages between 2500 mm and 3500 mm annually. Primary rainfall occurs between December to February [14]. From an administrative viewpoint, Waluran comprises the following six villages: Waluran Mandiri, Mekar Mukti, Mangunjaya, Caringin, Nunggal, and Sukamukti [15].
This region is characterized by laterite soils that originate from intensely weathered Tertiary volcanic rocks consisting mainly of andesite, tuff, and volcanic breccia [16]. This setting can lead to agricultural production but simultaneously poses risks due to its irregular mining of meandering creeks, which contain mineralized strips of gold spots. As the study area is influenced by ASGM activities, an assessment of Hg contamination is crucial to understanding its broader environmental impact.
2.2. Sampling
Sampling was conducted at two ASGM-affected areas and one reference site to assess Hg contamination (Figure 1) in May 2024, capturing low-runoff conditions with minimal stormwater influence. A monthly reanalysis of weather data (Table S1) at the Waluran coordinates shows precipitation in May (143.2 mm) was among the lowest in 2024 (August 82.8 mm; October 72.2 mm), substantially lower than the wet-season months (e.g., January 475.8 mm; November 544.5 mm). May relative humidity (87.8%) and 10 m wind speed (1.87 m s−1) were within typical seasonal ranges, and surface solar radiation was stable (5.13 kWh m−2 day−1) [17,18]. This timing was chosen to minimize rainfall-runoff–driven variability (dilution and resuspension), thereby providing a representative background for Hg concentration. In Mangunjaya Village, located in an agricultural zone adjacent to known illegal gold mining shafts, soil and water samples were collected near cassava farms and local streams. The second site, Waluran Mandiri Village, is a recognized hotspot for illegal gold mining, locally known as Pertambangan tanpa Izin (PETI), where numerous vertical shafts, some exceeding 80 m in depth, are situated within or near community-managed farmlands. Sampling at this site focused on areas close to known amalgamation activities. In contrast, Mekar Mukti Village served as the reference site because of its lack of known mining activity and relatively undisturbed agricultural land. This reference site was used to provide a baseline Hg concentration for comparative risk analysis.
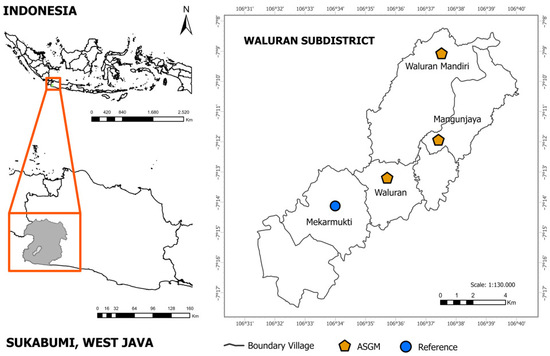
Figure 1.
Location of study area.
Sampling locations were chosen to represent areas near ASGM activities (impacted sites) and reference sites with minimal or no ASGM influence. A total of 48 environmental samples were collected from both ASGM-affected and reference areas in Waluran Subdistrict, Sukabumi Regency. From the ASGM area, the sampling included 11 water samples (pond water from artificial fish-farming structures, groundwater, and tailing water associated with ASGM effluent), 8 sediment samples (river, fish pond, and tailing), 7 soil samples (agriculture soil where cassava is cultivated and soil from burning amalgam sites), 10 tilapia fish species (Oreochromis niloticus) (three-point sampling includes 3–4 individuals from each point), and 4 samples each of cassava leaves and cassava roots. The reference site contributed 6 water samples (groundwater and pond water), 2 sediment samples (fish pond), 4 soil samples (agriculture), 6 fish (two-point sampling consisted of three individuals from each point), and 4 samples each of cassava leaves and roots. While there was a limited amount of river sediment in ASGM areas and fish pond sediment in reference areas, restricting statistical power, these samples were strategically collected from representative locations within each site, allowing for a preliminary yet informative assessment of Hg contamination patterns. All samples were rigorously gathered and handled following defined procedures to ensure the reliability and accuracy of the data for assessing the environmental impact of ASGM activities.
Water samples of approximately 100 mL were collected in plastic bottles. To reduce Hg adsorption and microbial activity, the samples were acidified to a pH level of less than 2 using nitric acid (HNO3). The acidic state also aids in maintaining Hg solubility so that the measurements obtained during analysis are more accurate. The samples were stored at a temperature below −4 °C for further analysis. Sediment and soil samples (approximately 200 g) were collected at a depth of 10–15 cm using a scoop and placed in zipper-locking plastic bags. Fish samples were collected by scooping them with a fish net from the fish pond. Immediately after capture, all fish were frozen to preserve tissue integrity. In the laboratory, the weight (Wt) and length (Lt) of each fish were recorded, and dorsal muscle tissue was carefully dissected for Hg analysis. Cassava samples were collected from the roots and leaves of the plant. The roots were scooped from a depth of 15 to 20 cm from the topsoil using a stainless-steel scoop. All samples were then placed in zipper-sealed plastic bags and frozen for storage until they were brought to the Prefectural University of Kumamoto in Japan.
2.3. Analytical Procedure
2.3.1. Sample Preparation
Water samples were collected and then filtered in the field using 0.2 μm syringe filters to remove fine particulates. Concerning sediment and soil, a freeze-drying technique was first used to preserve the sample’s structure, followed by sieving through a 2 mm mesh screen to remove larger fragments, such as roots, stones, and woody materials. Fish muscle was freeze-dried for 24 h before being milled into powder form to enhance analytical uniformity. For cassava, only the edible part requires peeling from the root, thorough washing, and dicing into small enough pieces for further processing. The roots and leaves were treated with tap water, followed by distilled water washes to allow for the detachment of soil while minimizing contamination from outside sources.
2.3.2. Analysis of Hg
Quantification of Hg in water was performed following the USEPA Method 245.1 [19], which, during the analysis, requires the reduction of total mercury into its elemental form (Hg0) using stannous chloride (SnCl2). Converting Hg ions to the vapor state allowed for detection through CV-AAS (cold vapor atomic absorption spectrometer) using the MA-3000 analyzer (Nippon Instruments Corporation, Tokyo, Japan). Detection was performed at a wavelength of 253.7 nm.
Soil, sediment, fish, and cassava samples were analyzed using the USEPA method 7473 [19], which is based on thermal decomposition and used the same MA-3000 analyzer. This technique involves thermally releasing Hg from the sample, which is then carried via an oxygen stream to a gold amalgamator, where it is captured. The spectrophotometric measurement determines the amount of vaporized Hg after heating the gold amalgamator filled with mercuric compounds, surpassing the desorption threshold temperatures. This method offers exceptional sensitivity, with a measurement accuracy of ≤0.001 μg/g, making it possible to measure mercury, even in soils, sediments, biological tissues, and cassava with complex compositions, without interference.
2.3.3. Quality Assurance and Quality Control for Hg Analysis
For consistency in analysis, all laboratory containers and glassware were thoroughly cleaned to eliminate any potential residues. The cleaning included a two-step washing with detergent, a rinse with tap water, and soaking in a 10% HNO3 solution, followed by overnight drying. Water sampling spike recovery tests showed retrieval rates of 97–108%. Moreover, CRMs from NMIJ AIST were used for validation through calibration. Specifically, the swordfish tissue CRM403-a for total Hg and sediment CRM7302-a for trace elements and methyl mercury were analyzed, yielding recovery rates of 93.8% to 97% and 92.3–95.0%, respectively, with CV below 5%. This demonstrated the method’s precision and reproducibility for determining Hg in samples.
2.4. Geo-Accumulation Index (Igeo) of Hg in Sediment and Soil
The Geo-accumulation Index (Igeo) was used to determine Hg contamination in sediment and soil by comparing metal enrichment to background levels. The Igeo was estimated using Equation (1) [20], and the Igeo classes are provided in Table S2.
where C represents the measured concentration of Hg in sediment and soil samples, while B denotes the background concentration of Hg, used as a reference to assess contamination levels. The background values applied were 0.023 mg/kg for sediment [21] and 0.020 mg/kg for soil [22], based on data from previous studies conducted in relatively uncontaminated areas in Indonesia. A constant factor of 1.5 is included as a corrective adjustment to account for natural lithological variations, thereby minimizing the risk of overestimating pollution levels due to inherent variations in the Earth’s crust.
2.5. Contamination Factor of Hg in Soil
The contamination factor (CF), a single metric, is widely regarded as a simple and effective instrument for monitoring heavy metal contamination [23]. The following equation is used to compute CF:
where C represents concentration of Hg in soil sample, and B is background concentration of Hg in soil (0.020 mg/kg dw [22]). Table S3 shows four classifications of soil quality based on the contamination factor.
2.6. Bioconcentration Factor (BCF) of Hg in Fish
Bioconcentration factor (BCF) determined the tendency of aquatic species to accumulate Hg from water. BCF is calculated as ratio of concentration of Hg in fish tissue to that in the surrounding water (3) [24]. Table S4 shows the classes of BCF [25].
where C in Fish represents concentration of Hg in fish (mg/kg ww) and C in Water represents concentration of Hg in water (mg/L).
2.7. Bioaccumulation Factor (BAF) of Hg in Cassava Root
Bioaccumulation factor (BAF) was used to assess cassava root’s ability to uptake Hg from in soil. BAF was assessed using Equation (4) [26].
where C in Cassava Root represents the concentration of Hg in cassava root (mg/kg dw) and C in Soil represents the concentration of Hg in soil (mg/kg dw).
2.8. Health Risk Assessment
The United States Environmental Protection Agency’s (USEPA) policy for chemical compounds’ health risk assessment [27] was used in this study to determine the health risk of Hg. Hg can enter the body through various pathways, including ingestion, dermal contact, and inhalation. Health risks have been evaluated for chronic daily intake (CDI). The CDI has been computed using the following equations:
where CDIing is CDI via ingestion, CDIinh is CDI via inhalation, and CDIder is CDI via dermal. The ingestion rate of water and fish, as well as cassava consumption, were used to calculate CDI for HQ and obtained directly from household surveys conducted among residents.
Hazard Quotient (HQ) (Equation (8)) assesses possible Hg exposure compared to a reference dose (RfD). An HQ greater than one suggests the probability of non-carcinogenic health impacts, whereas an HQ of one or less implies no risk. When multiple routes of exposure were investigated, Hazard Index (HI) was calculated (Equation (9)). An HI larger than one suggests the probability of unfavorable health consequences, whereas an HI of one or less implies a low risk. The input parameter values used in the human health risk assessment are presented in Table S5.
HI = HQing + HQinh + HQder
2.9. Statistical Analysis
The statistical analysis data from this study were analyzed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA). ArcGIS Pro, version 3.0.2 (Environmental Systems Research Institute (ESRI), Redlands, CA, USA) was used for spatial visualization and mapping. Normality of data was evaluated using the Kolmogorov–Smirnov test, which demonstrated a non-normal data distribution. Non-parametric analysis was subsequently employed to assess the relationships between variables with Spearman’s rank correlation coefficient. In addition, the Mann–Whitney U test was used to compare groups when the data were not normally distributed, while the t-test was applied for data with a normal distribution. The level of statistical significance in this research was p < 0.05.
3. Results and Discussion
3.1. Assessment of Hg Contamination
3.1.1. Water
The concentration of Hg in groundwater, pond water, and tailing water varied between ASGM and reference areas. Although the difference was not statistically significant (p = 0.167), the ASGM area’s mean Hg level (0.093 ± 0.064 µg/L) in groundwater was higher than the reference area (0.035 ± 0.015 µg/L) (Table 1). This suggests that Hg pollution may not be substantially leaching into groundwater. González-Fernández et al. [28] reported that, in some cases, soil characteristics can inhibit Hg from migrating into groundwater. However, a previous study showed that under certain environmental conditions, Hg can indeed leach into groundwater [29]. Groundwater from both areas was below the Indonesian standard (1 µg/L for drinking water [30]), suggesting the groundwater is within acceptable limits for consumption.

Table 1.
Mean, range, and median values of Hg concentration in water, sediment, soil, fish, and cassava.
Although the difference was not statistically significant (p = 0.300), the ASGM area’s median Hg concentration for pond water, which is used for fish farming, was 0.113 µg/L almost twice the reference value of 0.060 µg/L. Crucially, the Hg levels in pond water from the ASGM and reference areas were significantly lower than the Indonesian standard (2 µg/L for agriculture, aquaculture, recreation, and animal husbandry [30].
Mercury concentration in tailing water, which is directly used in gold processing operations, was extremely high, with median of 18.9 µg/L and range of 2.70 to 82.0 µg/L. Accounting for 75% of the sample, these levels exceeded the wastewater quality standards of Indonesia (5 µg/L, [31]), clearly highlighting the environmental risk posed by untreated mining waste discharges.
3.1.2. Sediment
Mercury concentration in river sediments from ASGM had median of 35.3 mg/kg dw and ranged from 21.9 to 48.7 mg/kg dw, indicating significant contamination that most likely originates from ASGM activities and runoff, even though data from the reference area were not available. The ASGM area’s fish pond sediments also had high Hg levels, with a median concentration of 33.4 mg/kg dw (30.2 to 42.6 mg/kg dw), which was higher than the reference area’s median of 6.99 mg/kg dw (6.24 to 7.74 mg/kg dw). However, due to the limited number of samples (n = 2), a statistical comparison could not be performed. The highest level of contamination was found in tailing sediments, which are directly related to waste from gold processing at ASGM. With a median of 171 mg/kg, the Hg concentration ranged from 97.8 to 883 mg/kg, indicating significant and direct Hg input from amalgamation and tailings disposal procedures. All sediment samples showed that Hg concentration was concerningly high when compared to the New Zealand sediment guidelines for Hg of 0.15 mg/kg [32], which are used here because Indonesian sediment standards are lacking. The relevance of this guideline is further supported by the fact that Australia and New Zealand share diverse aquatic environments similar to those in Indonesia, particularly in tropical regions. Their flexible, tiered Hg guidelines are suitable for various sedimentary contexts, especially fine-grained, organic-rich sediments where Hg tends to accumulate. Such sediment characteristics are commonly found across riverine, estuarine, and deltaic systems, making the ANZECC guideline a practical and scientifically appropriate benchmark for this assessment. In the ASGM area, tailing sediments reached contamination levels almost 6,000 times higher than the standard, while river and fish pond sediments were roughly 200 to over 5,000 times higher. The mercury concentration in sediments in this study was higher than that in the Ciujung watershed, 0.61 ± 0.25 mg/kg dw, in Banten Province, Indonesia [33], where ASGM activities are likewise present in the upstream region. Even when compared to previous findings from the Cikaniki River in Gunung Pongkor, another ASGM-affected area in Indonesia, where Hg concentration in sediment was 10.0 mg/kg dw upstream, 6.00 mg/kg dw midstream, and 64.4 mg/kg dw downstream [11], the current study shows that Hg contamination remains a serious and spatially variable issue. This study showed extreme levels of Hg contamination in sediments impacted by ASGM and highlighted the urgent need for ongoing monitoring and remediation to prevent further environmental persistence and the transfer of Hg through aquatic food chains.
The classification of Hg contamination level in sediment based on the Geo-accumulation Index (Igeo) further underscores the impact of ASGM activities (Figure 2). In ASGM-affected areas, 37.5% of the sediment samples were categorized as “moderately to heavily contaminated,” 50% as “heavily contaminated,” and 12.5% as “heavily to extremely contaminated.” These findings indicate that the majority (62.5%) of ASGM sediments fall into high-contamination categories, reflecting substantial and ongoing anthropogenic Hg input. In contrast, all sediment samples (100%) from the reference site were classified as “moderately to heavily contaminated,” suggesting lower but still notable levels of Hg pollution. The presence of contamination in reference areas, despite the absence of direct ASGM activity, may be attributed to atmospheric transport and deposition, legacy contamination, hydrological connectivity, and undocumented ASGM activities. Notably, Hg can be transported by surface runoff, shallow groundwater flow, or soil loss during storm periods, leading to contaminants being present several kilometers away from their primary source [34]. The remobilization of legacy contamination accumulated within soil and sediments resulting from past ASGM operations can be additionally triggered with time [2]. Moreover, undocumented ASGM cannot be excluded as a possible source, especially considering the relatively short distances (~4 km) separating ASGM sites from the reference sampling site [35]. Overall, the sharp contrast in contamination severity between ASGM and reference sites highlights the significant role of ASGM in elevating Hg level in sediment.
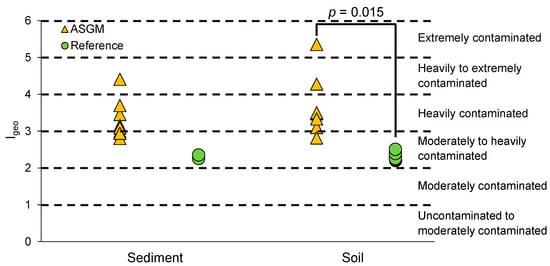
Figure 2.
Classification of Igeo in sediment and soil.
3.1.3. Soil
The median value for agricultural soil in the ASGM area was 63.1 mg/kg dw (range: 19.6–95.0 mg/kg dw (Table 1), while in reference agricultural soil was 6.69 mg/kg (range: 5.44–9.59 mg/kg). Statistical analysis revealed a significant difference between agricultural soils from ASGM and reference areas (p = 0.03), indicating elevated contamination due to mining activities. Moreover, a significant difference was also observed between reference agricultural and soil from amalgamation burning sites (p = 0.007). This elevated level is an indication that Hg is being excessively used in this area, particularly where the amalgam is burned. In fact, this study reports an exceptionally high median soil Hg concentration at the burning site (571 mg/kg dw), far exceeding those observed in Myanmar (77.4 mg/kg dw) and the Philippines (71.8 mg/kg dw) as reported by Soe et al. [36], highlighting the severity of Hg contamination in Southeast Asia’s ASGM regions. Notably, Hg concentration in all soil samples from both ASGM and reference sites was higher than the background (0.020 mg/kg dw, [22] and Indonesian guidelines (0.3 mg/kg dw [30]. Although the reference area is not directly impacted by ASGM activities, the elevated Hg level suggests contamination, likely through atmospheric deposition, surface runoff, or soil transport via wind or water from adjacent ASGM-impacted regions.
The contamination was assessed using CF through comparison with the Indonesian background, 0.020 mg/kg dw [22]. All soil samples were extremely contaminated with Hg, particularly in areas of amalgam burning. The CF ranged from 980 (agricultural soil) to 338,150 (amalgam burning area). This level of contamination may severely compromise local ecosystems and pose substantial health threats to surrounding communities.
The Igeo evaluation of Hg pollution at the ASGM and reference areas of Waluran–Sukabumi indicates an extremely alarming pattern of pollution (Figure 2). At the ASGM sites, the majority of samples (57%) were in class 4 (heavily contaminated), followed by 14% in class 3 (moderately to heavily contaminated), class 5 (heavily to extremely contaminated), and class 6 (extremely contaminated). This distribution means that 85% of the ASGM sites were heavily to extremely contaminated, reflecting the heavy environmental impact of ASGM activities.
In comparison, 100% of the reference area was in class 3, indicating moderate to high pollution. Although these sites lack on-site ASGM, the presence of Hg at this level suggests that Hg contamination has spread beyond mining sites through atmospheric deposition, water transport, soil mobilization, and historical mining activities.
3.1.4. Fish
Mercury concentration in fish samples differed markedly between the ASGM and reference sites. In the ASGM area, fish exhibited significantly (p = 0.030) higher Hg levels, with a median of 4.76 mg/kg dw (0.411 to 12.8 mg/kg dw), than the reference site, with a median of 1.14 mg/kg dw (0.413 to 2.70 mg/kg dw), indicating contamination from ASGM activities.
Considering wet weight, the median Hg concentration in fish from the ASGM area was 1.07 mg/kg ww, while the reference site had a median Hg concentration of 0.262 mg/kg ww. A total of 50% of the ASGM samples and 33% of the reference area samples exceeded the Indonesian standard of 0.03 mg/kg [37]. The elevated Hg levels at the reference site may be attributed to several factors, such as atmospheric deposition of Hg transported from nearby ASGM activities, the downstream movement of contaminated water or sediment, or historical mining impacts in the broader area. This suggests that even areas considered as reference points are not entirely free from Hg contamination, highlighting the wide-ranging environmental influence of ASGM or other Hg pollution sources.
The mercury concentration detected in tilapia (Oreochromis niloticus) from this study was notably higher than those reported in previous studies from other aquatic systems. These values exceeded the previously reported median concentrations from ASGM-impacted sites, such as Gunung Pongkor, where median values ranged from 0.082 to 0.156 mg/kg ww. Similarly elevated concentrations have been observed in other Indonesian locations affected by ASGM, including Limboto Lake Gorontalo (0.283 mg/kg ww) [38] and the Bakan River in Lolayan, Bolaang Mongondow (0.382 mg/kg ww) [39], confirming the impact of Hg released from mining processes on fish contamination. The extremely high Hg detected in tilapia from Rawa Taliwang Lake, West Sumbawa (3.44 mg/kg ww) [40] near active ASGM sites further exemplifies the severe Hg burden associated with these mining activities. When compared internationally, the tilapia Hg concentration from this study also stands out. For example, riverine tilapia from the Ghanaian Ankobra–Tano basins generally show lower muscle Hg (0.138 mg/kg ww) [41], reflecting different levels of Hg exposure and potentially lower net methylation at the sampled sites. Conversely, a Kenyan study in Migori county reported much higher tilapia muscle Hg levels (2.07 mg/kg ww) [42], aligning with stronger sediment contamination. In our study area, the fish pond from which the tilapia fish samples were taken received Hg from tailings water. Mechanistically anoxic, organic-rich fish ponds may stimulate MeHg formation by sulfate-reducing microbes, promoting MeHg bioaccumulation in tilapia. These results indicate that tilapia from the fish ponds in the study area contain substantially elevated Hg concentration compared to those in other sites, reflecting significant local contamination.
Spearman’s rank correlation in Table 2 revealed that no significant correlation was found between Hg concentration in fish and fish length (p = 0.380) or fish weight (p = 0.418). Similarly, no correlation was observed between fish Hg concentration and Hg levels in water (p = 0.223) and sediment (p = 0.230). This is in contrast with a previous study in Gunung Pongkor, a where significant correlation was observed between Hg concentration in fish and those in both water and sediment [11]. The lack of correlation may be attributed to the small sample size (ASGM n = 10, reference n = 6), and differences in Hg bioavailability and methylation dynamics across sites [43]. Methylation hotspots are areas with high organic matter, active sulfate-reducing bacteria, and favorable redox conditions, which can increase MeHg production independently of total Hg, affecting bioaccumulation in fish [43,44]. Sediment water partitioning also influences Hg availability. Hg bound to sediments is less bioavailable, while dissolved or colloidal Hg in water is more readily taken up [45,46]. Several studies have shown that fish Hg concentration is more closely linked to diet and Hg methylation hotspots than to ambient water or sediment concentrations [35,47].

Table 2.
Spearman-rank correlation of Hg concentration with various indices.
3.1.5. Cassava
In cassava leaf, the median Hg concentration in the ASGM area (15.7 mg/kg dw) was significantly higher than that in the reference area (1.12 mg/kg dw) (p = 0.002). This difference indicates elevated Hg pollution in the ASGM area, likely due to both root uptake from contaminated soil and atmospheric deposition on leaf surfaces. In the cassava root, the median Hg concentration in the contaminated ASGM location was 1.54 mg/kg dw, while cassava root from the reference site had a Hg concentration below the detection limit (<0.001 mg/kg) and a statistically significant difference was observed (p = 0.021). This indicates elevated contamination associated with ASGM activities.
Considering wet weight, Hg concentration in all cassava leaf samples from both ASGM areas and the reference area greatly exceeded the Indonesian food safety limit of 0.03 mg/kg ww. In contrast, elevated Hg levels in all cassava root samples were observed only in ASGM-impacted areas, while roots from the reference area remained below the regulatory threshold. The median Hg level in cassava leaves was 3.95 mg/kg, over 130 times higher than the standard, while the median in roots was 0.413 mg/kg ww, about 14 times higher. Even in the reference area, cassava leaves exceeded the limit (median: 0.442 mg/kg ww), though root levels remained below detection. These results highlight serious food safety concerns in ASGM-affected regions.
Evaluating the relationship between Hg concentration in cassava and soil, the Spearman-rank correlation analysis presented in Table 2 revealed significant positive correlations between Hg level in soil and cassava root (r = 0.812, p = 0.014), as well as between cassava root and leaf (r = 0.913, p = 0.002). These results indicate that cassava absorbs Hg from contaminated soils and redistributes part of it from root to leaf. However, no significant correlation was observed between Hg concentration in soil and cassava leaf (r = 0.643, p = 0.086), suggesting that soil input alone cannot explain the high Hg levels in leaves. This pattern indicates that root-to-leaf translocation occurs in combination with direct foliar uptake, likely through the atmospheric deposition from ASGM emissions, which represents the dominant pathway of Hg accumulation in cassava leaves.
Mercury concentration was significantly higher in cassava leaves compared to the root (p = 0.004), being approximately ten times greater. This further supports the important role of atmospheric inputs, as seen in studies from Ghana and Tanzania [48,49]. Regional atmospheric data from West Java ASGM villages show atmospheric Hg spanning ~6 ng m−3 to ~1800 ng m−3 [50], supporting foliar Hg uptake as a plausible contributor to the elevated leaf Hg we observed. The role of soil chemistry, including factors such as pH, organic matter, and redox conditions, also influences Hg mobility and plant uptake [51,52]. The lack of correlation between soil and leaf Hg (Table 2) suggests that additional factors, such as variable translocation efficiency or direct foliar deposition, may influence Hg accumulation in leaf [53,54]. A correlation was observed between Hg levels in the root and leaf of the plant. Overall, these results indicate that atmospheric deposition is the primary source of Hg in cassava leaves, while root uptake contributes to a lesser extent [11,48,49].
Mercury concentration in cassava root from this study was higher than that in previous studies in Gunung Pongkor–West Java (1.12 mg/kg dw) [11] and Palu–Central Sulawesi (0.33 mg/kg dw) [55]. Globally, other countries contained much lower concentrations, such as in Ghana (0.079–0.331 mg/kg dw) [56], Tanzania (0.003 mg/kg dw) [48], and Uganda (0.020 mg/kg mg/kg dw) [57]. Cassava leaves in Indonesia also showed elevated Hg; the average Hg concentration, at 9.46 mg/kg dw, obtained in this study was lower than that observed in in Bombana–Sulawesi (9.90 mg/kg dw) [58] but higher than that observed in previous studies in Gunung Pongkor (4.61 mg/kg dw) [11] and the 2 mg/kg dw found in in Mandailing Natal–North Sumatra [59]. In contrast, leaves from Tanzania (0.061–0.15 mg/kg dw) [48,60] and Uganda (0.11 mg/kg dw) [57] contained much lower concentrations. Overall, cassava roots and leaves from Indonesian ASGM sites contained substantially higher Hg than those reported in other countries, underscoring the strong impact of ASGM activities on food contamination.
3.2. Assessment of Hg Accumulation
3.2.1. Bioconcentration Factor (BCF) in Fish
In this study, BCF value of Hg in tilapia was notably high, ranging from 770 to 23,363 in the ASGM area, and 1683 to 22,464 in the reference area (Figure 3A), indicating substantial Hg accumulation even in non-mining zones. Significantly, these exceedingly elevated BCF values, particularly in the reference sites where Hg concentration in water is comparatively low, clearly demonstrate that marked bioaccumulation still occurs despite the minimal dissolved Hg levels, thereby underscoring persistent ecological and human exposure risks even outside mining areas. Table 3 shows a comparison of BCF value from this study with that obtained in previous studies, revealing that much lower BCF values were reported by Komala et al. [61] in Rasbora argyrotaenia and tilapia from West Sumatra, with values ranging from 0.668 to 4.319 across environments influenced by dense settlements, hydropower, and fish cages. In the Melawi watershed, West Kalimantan, Triswanto et al. [62] observed, in Kryptopterus species, a range from 731 to 6067, depending on proximity to anthropogenic sources. Prilia et al. [63] found similarly elevated values in Barbonymus gonionotus and Channa striata in the Ciberang River, Banten, with a BCF ranging between 1561 and 4354. In Banten Bay, a streaked spinefoot species near a power plant had a BCF of 5000 [64], suggesting industrial influence.
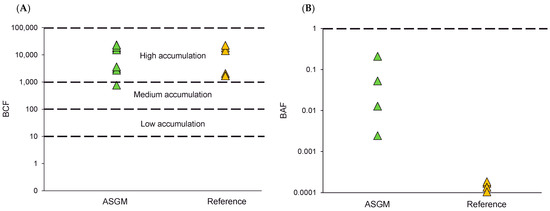
Figure 3.
BCF of Hg in fish (A) and BAF of Hg in cassava root (B).

Table 3.
Comparison of the BCF in fish with levels observed in other studies.
In freshwater fish from Lake Lanskie, Poland, Łuczyńska et al. [65] recorded a BCF value of 11,056 in fish. A study on tilapia in the River Nile, Egypt, reported a much lower BCF between 15.5 and 87.2 [66], reflecting lower Hg contamination. Lastly, Cai et al. [67] documented a BCF between 974 and 2274 for several fish species in the Wujiangdu Reservoir, China, with species like Cyprinus carpio and Culter alburnus showing moderately elevated BCF.
The very high BCF value determined in the current study, greater than those reported from other non-ASGM and ASGM areas, is at least partly the result of the uneven Hg uptake by fish relative to its concentration in water. Even if the dissolved Hg concentration in the water appears to be minimal when determined via measurements such as its binding to sediment, Hg remains highly bioavailable in its methylated form and is readily absorbed by aquatic life [68]. The high Hg input from ASGM, conducive methylation conditions, and tilapia’s benthic diet can lead to significant bioaccumulation, even in waters with low Hg concentrations, revealing hidden ecotoxicological risks [35,43,68,69]. Similarly high BCF values were also recorded in the reference site. Previous studies in Canadian oligotrophic lakes [70], Chinese subtropical reservoirs [71], and Indonesian Lake Lindu [72] have all shown extensive bioaccumulation of Hg due to in situ methylation processes, sediment–water dynamics, and efficient trophic transfer along aquatic food chains. These suggest that high BCFs may arise from both short-term Hg enrichment and natural methylation processes, highlighting the need to consider both external sources and local biogeochemical conditions when assessing mercury bioaccumulation risks.
3.2.2. Bioaccumulation Factor (BAF) in Cassava Root
The BAF of Hg in cassava roots for both ASGM and the reference areas showed low levels of bioaccumulation, with all values below 1 (Figure 3B). Specifically, in the ASGM site, the BAF ranged between 0.002 and 0.209, reflecting variability in Hg uptake; however, these values are still within the low-accumulation category. This finding suggests that even if cassava plants in ASGM-affected environments are exposed to Hg, the extent of their uptake into plant tissues remains low. Cassava from the reference site had BAF values close to zero, indicating minimal or negligible accumulation. This contrast highlights the influence of ASGM activities on environmental Hg levels, even though cassava itself exhibits a low accumulation capacity. This coincides with the findings of a previous study by Addai-Arhin [56], which also obtained a low BAF value for cassava grown in Hg-contaminated ASGM locations in Ghana. These low bioaccumulation levels indicate that limited cassava toxicity to humans occurs throughout the food chain. This indicates that while cassava plants in ASGM-affected sites are subjected to Hg, there is low bioaccumulation into plant tissues. The low BAF levels observed indicate restricted availability of Hg in the soil for plant uptake and accumulation. This suggests that soil properties, such as pH, soil organic matter (SOM), and cation exchange capacity, significantly influence the bioavailability of mercury in these environments.
3.3. Health Risk Assessment
The results revealed a health risk, particularly for children, as all HQ values were greater than one (Figure 4). Consistent with the model assumptions, children showed higher estimated risks than adults, and ingestion was the predominant exposure pathway compared to dermal and inhalation routes. Notably, site-specific results indicated that children who consumed cassava leaves were most at risk, with a mean HQ value of 44.6 and 4.88 in ASGM and the reference site, respectively. All samples for fish, cassava leaves, and roots had HQ > 1 for both age groups, and 33.3% of drinking water samples also had HQ > 1. Soil and sediment exposure pathways included ingestion, dermal contact, and inhalation. Inhalation was the least hazardous of these exposure methods, while ingestion was the most common.
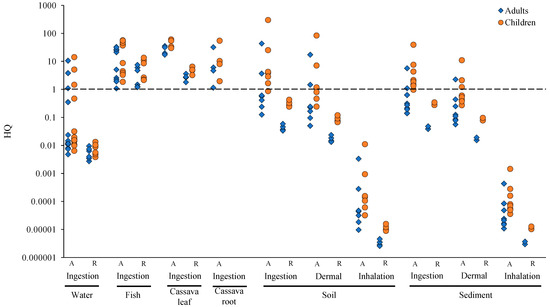
Figure 4.
HQ of Hg exposure. A; ASGM, R; reference.
Each exposure pathway makes a proportionate contribution to the overall HI, as illustrated in Figure 5. The pie chart reveals a distinct difference between the ASGM and reference areas. In the ASGM area, the primary contributor to the HI is the consumption of cassava leaves, accounting for approximately 30% of the total, closely followed by cassava root consumption, at around 29%. This indicates that both parts of the cassava plant serve as significant sources of mercury (Hg) exposure in communities near ASGM activities. In contrast, in the reference area, cassava leaves alone contribute a dominant 85% to the overall HI, highlighting a more singular exposure route. These results demonstrate that consuming contaminated cassava, particularly the leaves, is the main pathway through which the population is exposed to Hg. Therefore, reducing the health hazards to the local population requires monitoring and managing Hg contamination in food crops and agricultural soils close to ASGM areas.
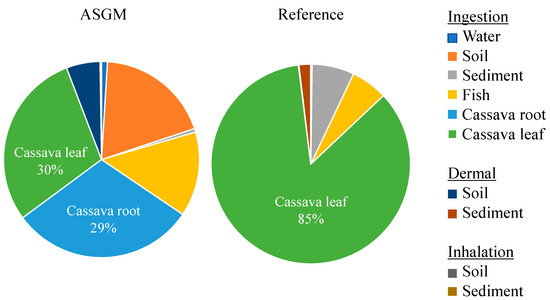
Figure 5.
Contribution of Hg exposure to HI.
Fish, sediment, soil, and water were among the other ingestion routes that contributed less to the total HI. The dermal and inhalation exposures through soil and sediment were only of minor importance.
3.4. Limitations of Study
This study has several limitations that should be acknowledged. First, the sampling design may not capture seasonal variations in Hg levels or environmental dynamics. Second, although reference areas were chosen to represent Hg-unpolluted areas, their high Hg concentration reflects the possible impact of atmospheric deposition, runoff, or previous mining; thus, they are not as useful as true baselines. Third, the study examined only total mercury (THg) in food samples, without making any distinction between the more bioavailable and toxic MeHg. This could lead to an underestimation of health risk, particularly from food exposure, as MeHg typically accounts for more than 90% of total Hg in fish muscle [35,73,74]. It has also been found in high concentrations in rice and cassava harvests near Hg-polluted areas [75,76]. Moreover, the study did not consider specific plant uptake mechanisms or soil characteristics, such as pH and organic matter, which play a key role in determining Hg mobility and availability. Finally, variability in Hg concentrations across sites and potential analytical limitations introduce further uncertainty. Future research should focus on Hg speciation, especially MeHg and implement long-term biomonitoring, to more precisely evaluate ecological and human health risks. It should also look into the properties of the soil and how plants uptake Hg to learn more about what affects the transport and availability of Hg. Also, using probabilistic methods would help account for uncertainty and improve risk estimates for human exposure.
3.5. Result-Based Recommendations
Based on our findings, we propose a local action plan in order to reduce population exposure to Hg, prioritizing contaminated food restriction. The action plan consists of applicable measures divided into three stages. First, the regular monitoring, at least once per semester, of Hg contamination of drinking water sources, crops, and freshwater fish. These data will provide a reliable pathway to delineate affected areas based on a health risk assessment of each consumable commodity. Once the contamination mappings are clear, this will provide the foundation for the local government to educate people concerning the health risks of Hg-contaminated foods. The last stage of the action plan is focused on a long-term approach to eliminate the use of Hg in ASGM through the use of alternative technology, facilitated by a research institution.
4. Conclusions
This study revealed widespread and severe Hg contamination in ASGM-affected areas in Sukabumi, Indonesia. The elevated Hg concentration in tailings, sediments, fish, soil, and cassava poses a significant ecological risk. Human health risk assessments demonstrated that the ingestion of contaminated food, especially cassava leaves, represented the primary exposure pathway in both ASGM and reference areas, with children facing the highest risk. The findings also showed that even reference areas were not entirely free from Hg contamination, highlighting the broad spatial impact of ASGM activities. Effective policy interventions, environmental remediation, and community education are urgently needed to manage Hg exposure and safeguard public and ecological health. Long-term monitoring, the inclusion of MeHg analyses, and broader health surveillance are recommended for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/earth6030110/s1, Table S1: Data of Weather in Waluran; Table S2: The classes of contamination factors (CFs); Table S3: The BCF classes of contamination; Table S4: The Igeo indexes and level of contamination; Table S5: Parameters used in the risk assessment.
Author Contributions
The manuscript was authored by T.A. (Tia Agustiani). Data collection was conducted by T.A. (Tia Agustiani), A.S., F.S., F.Y.A., E. and T.A. (Tetsuro Agusa). Data curation was conducted by T.A. (Tia Agustiani), S.S., P.A.P., M.B. and T.A. (Tetsuro Agusa). Visualization was conducted by T.A. (Tia Agustiani), F.S. and R.H. Critical revisions of the manuscript were provided by A.S., P.A.P. and T.A. (Tetsuro Agusa). Supervision was carried out by Y.I., J.K., J.S.M., Y.A. and T.A. (Tetsuro Agusa). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the International Postgraduate Scholarship for Mercury Research, of Kumamoto Prefecture Government, and Heiwa Nakajima Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the support and commitment of the Kumamoto Prefecture Government, the Prefectural University of Kumamoto, the National Research and Innovation Agency (BRIN) of the Republic of Indonesia, and Heiwa Nakajima Foundation to this study. We also thank for Dedi, Dadan, Agus, Ewok, and Waluran residents who helped with the sampling process; and Suherman and Onig for assisting in preparation for sampling, and through to handling samples after sampling.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arrighi, S.; Franceschini, F.; Petrini, R.; Fornasaro, S.; Ghezzi, L. The Legacy of Hg Contamination in a Past Mining Area (Tuscany, Italy): Hg Speciation and Health Risk Assessment. Toxics 2024, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2018; UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2019; Available online: https://www.unep.org/resources/publication/global-mercury-assessment-2018 (accessed on 8 September 2024)ISBN 978-92-807-3744-8.
- NEXUS3 Foundation. Towards Minamata COP-6: The State of Small-Scale Gold Mining (ASGM) in Indonesia; NEXUS3 Foundation: Bali, Indonesia, 2025; Available online: https://www.nexus3foundation.org/wp-content/uploads/2025/05/ENG-Indonesia-ASGM-National-Status-Report_compressed.pdf (accessed on 8 September 2024).
- GOLD-ISMIA. Annual Report 2020. 2020. Available online: https://www.goldismia.org/sites/default/files/2021-06/Annual%20Report%202020-8.pdf (accessed on 8 September 2024).
- Metaragakusuma, A.P.; Sakakibara, M.; Arifin, Y.I.; Pateda, S.M.; Jahja, M. Rural Knowledge Transformation in Terms of Mercury Used in Artisanal Small-Scale Gold Mining (ASGM)—A Case Study in Gorontalo, Indonesia. Int. J. Environ. Res. Public Health 2023, 20, 6640. [Google Scholar] [CrossRef]
- Radar Sukabumi. Penambang Illegal Rusak Lahan Perhutani. Radar Sukabumi, 2 August 2023. Available online: https://radarsukabumi.com/berita-utama/penambang-illegal-rusak-lahan-perhutani/ (accessed on 8 September 2024).
- Isa, I.G.T.; Setiawan, I.R.; Jhoansyah, D. The Potential of “Keripik Enye” Industry in Improving Local Welfare: A Case Study from Waluran Village. J. Masy. Mandiri 2019, 3, 29–40. [Google Scholar]
- Badan Pusat Statistik (BPS) Sukabumi. Luas Panen, Produktivitas dan Produksi Ubi Kayu Menurut Kecamatan di Kabupaten Sukabumi. 2023. Available online: https://sukabumikab.bps.go.id (accessed on 8 September 2024).
- Saragih, G.S.; Tapriziah, E.R.; Syofyan, Y.; Masitoh, S.; Pandiangan, Y.S.H.; Andriantoro, A. Mercury Contamination in Selected Edible Plants and Soil from Artisanal and Small-Scale Gold Mining in Sukabumi Regency, Indonesia. Makara J. Sci. 2021, 25, 222–228. [Google Scholar] [CrossRef]
- Harianja, A.H.; Saragih, G.S.; Fauzi, R.; Hidayat, M.Y.; Syofyan, Y.; Tapriziah, E.R.; Kartiningsih, S.E. Mercury Exposure in Artisanal and Small-Scale Gold Mining Communities in Sukabumi, Indonesia. J. Health Pollut. 2020, 10, 201209. [Google Scholar] [CrossRef]
- Agustiani, T.; Sulistia, S.; Sudaryanto, A.; Kurniawan, B.; Poku, P.A.; Elwaleed, A.; Kobayashi, J.; Ishibashi, Y.; Anan, Y.; Agusa, T. Mercury Contamination and Human Health Risk by Artisanal Small-Scale Gold Mining (ASGM) Activity in Gunung Pongkor, West Java, Indonesia. Earth 2025, 6, 67. [Google Scholar] [CrossRef]
- Central Bureau of Statistics of Sukabumi Regency. Waluran Subdistrict in Figures 2023; BPS Sukabumi: Sukabumi, Indonesia, 2023.
- Sukabumi Public Works and Spatial Planning Office. Topography and Landslide Risk Zoning Report for Southern Sukabumi; Sukabumi Government: Sukabumi, Indonesia, 2021.
- Meteorology, Climatology, and Geophysics Agency (BMKG). Annual Rainfall Statistics for Sukabumi Region; BMKG West Java Office: Bandung, Indonesia, 2022.
- Ministry of Home Affairs (Indonesia). Administrative Village Database of Sukabumi Regency; Directorate General of Population and Civil Registration: Jakarta, Indonesia, 2024.
- Khansa, F.R.; Hardiyono, A.; Sunarie, C.Y. Geologi Daerah Waluranmandiri dan Sekitarnya Kecamatan Waluran, Kabupaten Sukabumi, Provinsi Jawa Barat. Padjadjaran Geosci. J. 2024, 8, 1812–1820. [Google Scholar] [CrossRef]
- BMKG (Meteorology, Climatology, and Geophysics Agency of Indonesia). Online Data—Directorate of Data and Computation. Available online: https://dataonline.bmkg.go.id/dataonline-home (accessed on 20 August 2025).
- Badan Pusat Statistic (BPS), Sukabumi Regency. Sukabumi Regency in Figures 2024; BPS: Sukabumi, Indonesia, 2024. Available online: https://sukabumikab.bps.go.id/id/publication/2024/02/28/a7468802d2fddb1a70fda907/kabupaten-sukabumi-dalam-angka-2024.html (accessed on 20 August 2025).
- U.S. Environmental Protection Agency. Method 245.1: Determination of Mercury in Water by Cold Vapor Atomic Absorption Spectrometry (Revision 3.0); U.S. EPA: Cincinnati, OH, USA, 1994.
- Müller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Heumasse, H.; Omar, S.B.A.; Demmallino, E.B. Mercury (Hg) Contamination on Water, Sediment and Macrozoobenthos in Waelata River, Wamsait Village Waelata Sub-District, Buru District, Maluku Province. J. Phys. Conf. Ser. 2019, 1341, 092019. [Google Scholar] [CrossRef]
- Sudarningsih, S.; Fahruddin, F.; Lailiyanto, M.; Noer, A.A.; Husain, S.; Siregar, S.S.; Wahyono, S.C.; Ridwan, I. Assessment of Soil Contamination by Heavy Metals: A Case of Vegetable Production Center in Banjarbaru Region, Indonesia. Pol. J. Environ. Stud. 2022, 32, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control: A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- OECD. Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure; OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2012. [Google Scholar] [CrossRef]
- LaGrega, M.D.; Buckingham, P.L.; Evans, J.C. Hazardous Waste Management, 2nd ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Udiba, U.U.; Udofia, U.U.; Akpan, E.R.; Antai, E.E. Assessment of Lead (Pb) Uptake and Hazard Potentials of the Cassava Plant (Manihot esculentus cranz), Dareta Village, Zamfara, Nigeria. Int. Res. J. Public Environ. Health 2019, 6, 115–126. [Google Scholar]
- U.S. Environmental Protection Agency. Exposure Factors Handbook: 2011 Edition; National Center for Environmental Assessment: Washington, DC, USA, 2011. Available online: https://assessments.epa.gov/risk/document/&deid=236252 (accessed on 8 September 2024).
- González-Fernández, B.; Menéndez-Casares, E.; Meléndez-Asensio, M.; Fernández-Menéndez, S.; Ramos-Muñiz, F.; Cruz-Hernández, P.; González-Quirós, A. Sources of Mercury in Groundwater and Soils of West Gijón (Asturias, NW Spain). Sci. Total Environ. 2014, 481, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Aleku, D.L.; Lazareva, O.; Pichler, T. Mercury in Groundwater–Source, Transport and Remediation. Appl. Geochem. 2024, 170, 106060. [Google Scholar] [CrossRef]
- Indonesia Minister of Environment and Forestry (IMEF). Minister of Environment and Forestry Regulation No. 6 of 2021 Concerning Procedures and Requirements for Managing Hazardous and Toxic Waste; Department of Environmental Affairs of Indonesia: Jakarta, Indonesia, 2021. Available online: https://jdih.menlhk.go.id/new/uploads/files/2021pmlhk006_menlhk_06082021104752.pdf (accessed on 8 September 2024).
- Indonesia Minister of Environment and Forestry (IMEF). Minister of Environment Regulation No. 5 of 2014 Concerning Wastewater Quality Standards; Department of Environmental Affairs of Indonesia: Jakarta, Indonesia, 2014. Available online: https://jdih.menlhk.go.id/new2/home/open_indonesia_file/MLH%20P.5.pdf (accessed on 8 September 2024).
- Australian and New Zealand Environment and Conservation Council (ANZECC). Australian and New Zealand Guidelines for Fresh and Marine Water Quality; ANZECC & Agriculture and Resource Management Council of Australia and New Zealand (ARMCANZ): Canberra, Australia, 2000.
- Nugraha, W.C.; Ishibashi, Y.; Arizono, K. Assessment of Heavy Metal Distribution and Contamination in the Sediment of the Ciujung Watershed, Banten Province, Indonesia. J. Mater. Cycles Waste Manag. 2023, 25, 2619–2631. [Google Scholar] [CrossRef]
- Harvey, J.W.; Krupa, S.L.; Gefvert, C.; Mooney, R.H.; Choi, J.; King, S.A.; Giddings, J.B. Interactions Between Surface Water and Ground Water and Effects on Mercury Transport in the North-Central Everglades; Water-Resources Investigations Report 02-4050; South Florida Water Management District: Reston, VA, USA, 2002.
- Driscoll, C.T.; Han, Y.J.; Chen, C.Y.; Evers, D.C.; Lambert, K.F.; Holsen, T.M.; Kamman, N.C.; Munson, R.K. Mercury Contamination in Forest and Freshwater Ecosystems in the Northeastern United States. BioScience 2007, 57, 17–28. [Google Scholar] [CrossRef]
- Soe, P.S.; Kyaw, W.T.; Arizono, K.; Ishibashi, Y.; Agusa, T. Mercury Pollution from Artisanal and Small-Scale Gold Mining in Myanmar and Other Southeast Asian Countries. Int. J. Environ. Res. Public Health 2022, 19, 6290. [Google Scholar] [CrossRef]
- Indonesia Food and Drug Administration (IFDA). Indonesia Food and Drug Administration Regulation No. 9 of 2021 Concerning Requirements for Heavy Metal Contaminants in Processed Food; IFDA: Jakarta, Indonesia, 2022. Available online: https://standarpangan.pom.go.id/dokumen/peraturan/202x/logam_2022.pdf (accessed on 8 September 2024).
- Nakoe, M.R.; Ardian, Y.; Ruhardi, A.; Dwinugroho, F.; Yudhastuti, R.; Sulistyorini, L.; Azizah, R.; Indriani, D. Risk Assessment Exposure of Mercury (Hg) at People Who Consuming Nila Fish (Oreochromis niloticus) from Limboto Lake of Gorontalo Province. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1420–1427. [Google Scholar]
- Maddusa, S.S.; Asrifuddin, A.; Mantjoro, E.M. Public Health Risk Analysis Due to Consuming Tilapia (Oreochromis niloticus) Containing Heavy Metals in Bakan Village, Lolayan District, Bolaang Mongondow Regency. Int. J. Community Med. Public Health 2022, 10, 45. [Google Scholar] [CrossRef]
- Mulyani, I.; Yamin, M.; Khairuddin, K. Analysis of Mercury (Hg) Content in Tilapia Fish (Oreochromis mossambicus) from Rawa Taliwang Lake to Enrich the Course Materials on Ecotoxicology. J. Penelit. Pendidik. IPA 2023, 9, 4679–4684. [Google Scholar] [CrossRef]
- Boohene, M.; Poku, P.A.; Sulistia, S.; Brown, C.; Morokuma, E.; Agusa, T.; Arizono, K.; Anan, Y.; Ishibashi, Y. Human Health Risk Assessments of Mercury from the Ingestion of Edible Fishes from Birim River, Ghana. Available online: http://www.fundtoxicolsci.org/index_e.html (accessed on 8 September 2024).
- Kola, S.; Kanja, L.W.; Mbaria, J.M.; Maina, J.G.; Okumu, M.O. Levels of Mercury in Nile Tilapia (Oreochromis niloticus), Water, and Sediment in the Migori Gold Mining Belt, Kenya, and the Potential Ramifications to Human Health. F1000Research 2019, 8, 1244. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Gilmour, C.C.; Podar, M.; Bullock, A.L.; Graham, A.M.; Brown, S.D.; Somenahally, A.C.; Johs, A.; Hurt, R.A.; Bailey, K.L.; Elias, D.A. Mercury methylation by novel microorganisms from new environments. Environ. Sci. Technol. 2013, 47, 11810–11820. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.P.; Krabbenhoft, D.P.; Cleckner, B.L.; Olson, M.L.; Aiken, G.R.; Rawlik, P.S., Jr. System controls on the aqueous distribution of mercury in the northern Wisconsin lakes. Environ. Sci. Technol. 1998, 32, 1424–1432. [Google Scholar] [CrossRef]
- Wang, F.; Outridge, P.M.; Feng, X.; Meng, B.; Heimbürger-Boavida, L.E.; Mason, R.P. How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere? Sci. Total Environ. 2014, 466–467, 879–887. [Google Scholar]
- Cabana, G.; Rasmussen, J.B. Modeling Food Chain Structure and Contaminant Bioaccumulation Using Stable Nitrogen Isotopes. Nature 1994, 372, 255–257. [Google Scholar] [CrossRef]
- Nyanza, E.C.; Dewey, D.; Thomas, D.S.K.; Davey, M.; Ngallaba, S.E. Spatial Distribution of Mercury and Arsenic Levels in Water, Soil and Cassava Plants in a Community with Long History of Gold Mining in Tanzania. Bull. Environ. Contam. Toxicol. 2014, 93, 716–721. [Google Scholar] [CrossRef]
- Adjorlolo-Gasokpoh, A.; Golow, A.A.; Kambo-Dorsa, J. Mercury in the Surface Soil and Cassava, Manihot esculenta (Flesh, Leaves and Peel) Near Goldmines at Bogoso and Prestea, Ghana. Bull. Environ. Contam. Toxicol. 2012, 89, 1106–1110. [Google Scholar] [CrossRef]
- Kono, Y.; Rahajoe, J.S.; Hidayati, N.; Kodamatani, H.; Tomiyasu, T. Using native epiphytic ferns to estimate the atmospheric mercury levels in a small-scale gold mining area of West Java, Indonesia. Chemosphere 2012, 89, 241–248. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Larssen, T.; Qiu, G.; Vogt, R.D. In Inland China, Rice, Rather Than Fish, Is the Major Pathway for Methylmercury Exposure. Environ. Health Perspect. 2010, 118, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shen, Z.; Dou, C.; Dou, Z.; Li, Y.; Gao, Y.; Sun, Q. Effects of Soil Properties on Heavy Metal Bioavailability and Accumulation in Crop Grains under Different Farmland Use Patterns. Sci. Rep. 2022, 12, 9211. [Google Scholar] [CrossRef]
- Yang, Y.; Yanai, R.D.; Driscoll, C.T.; Montesdeoca, M.; Smith, K.T. Concentrations and content of mercury in bark, wood, and leaves in hardwoods and conifers in four forested sites in the northeastern USA. PLoS ONE 2018, 13, e0196293. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of Native Plant Species for Phytoremediation Potential at a Hg-Contaminated Mining Site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Ramlan; Basir-Cyio, M.; Napitupulu, M.; Inoue, T.; Anshary, A.; Mahfudz; Isrun; Rusydi, M.; Golar; Sulbadana; et al. Pollution and Contamination Level of Cu, Cd, and Hg Heavy Metals in Soil and Food Crop. Int. J. Environ. Sci. Technol. 2022, 19, 1153–1164. [Google Scholar] [CrossRef]
- Addai-Arhin, S.; Novirsa, R.; Jeong, H.H.; Phan, Q.D.; Hirota, N.; Ishibashi, Y.; Shiratsuchi, H.; Arizono, K. Potential Human Health Risk of Mercury-contaminated cassavas—Preleminary studies. Fundam. Toxicol. Sci. 2022, 9, 61–69. [Google Scholar] [CrossRef]
- Ssenku, J.E.; Naziriwo, B.; Kutesakwe, J.; Mustafa, A.S.; Kayeera, D.; Tebandeke, E. Mercury Accumulation in Food Crops and Phytoremediation Potential of Wild Plants Thriving in Artisanal and Small-Scale Gold Mining Areas in Uganda. Pollutants 2023, 3, 181–196. [Google Scholar] [CrossRef]
- Basri; Sakakibara, M.; Sera, K. Mercury in Soil and Forage Plants from Artisanal and Small-Scale Gold Mining in the Bombana Area, Indonesia. Toxics 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Arrazy, S.; Addai-Arhin, S.; Jeong, H.; Novirsa, R.; Wispriyono, B.; Agusa, T.; Ishibashi, Y.; Kobayashi, J. Spatial Distribution and Human Health Risks of Mercury in the Gold Mining Area of Mandailing Natal District, Indonesia. Environ. Monit. Contam. Res. 2023, 3, 33–42. [Google Scholar] [CrossRef]
- Sanga, T.R.; Maseka, K.K.; Ponraj, M.; Tungaraza, C.; Mng’ong’o, M.E.; Mwakalapa, E.B. Accumulation and Distribution of Mercury in Agricultural Soils, Food Crops and Associated Health Risks: A Case Study of Shenda Gold Mine-Geita Tanzania. Environ. Chall. 2023, 11, 100697. [Google Scholar] [CrossRef]
- Komala, P.S.; Azhari, R.M.; Hapsari, F.Y.; Edwin, T.; Ihsan, T.; Zulkarnaini; Harefa, M. Comparison of Bioconcentration Factor of Heavy Metals Between Endemic Fish and Aquacultured Fish in Maninjau Lake, West Sumatra, Indonesia. Biodiversitas 2022, 23, 4026–4032. [Google Scholar] [CrossRef]
- Triswanto, B.; Wibowo, M.A.; Ardiningsih, P. A Study of Mercury Pollution in Water, Sediment, and Lais (Kryptopterus) Fish in the Melawi Watershed. J. Trop. Life Sci. 2020, 10, 207–213. [Google Scholar] [CrossRef]
- Prilia, D.; Oginawati, K.; Ariesyady, H.D. Analysis of Mercury in Water and Sediment Distribution and Its Bioaccumulation Potential in Fish in the Small-Scale Gold Mining Area (Case Study: Ciberang River, Lebak, Banten). J. Water Sustain. 2013, 2, 107–116. [Google Scholar] [CrossRef]
- Tresnayaputri, C.I.A.; Lumban Batu, D.T.F.; Sulistiono. Kandungan Logam Berat (Hg, Cd, Pb, dan Cu) pada Ikan Baronang Siganus javus (Linnaeus, 1766) di Perairan Teluk Banten dan Sekitarnya. IPB University Repository. 2021. Available online: http://repository.ipb.ac.id/handle/123456789/107935 (accessed on 8 September 2024).
- Łuczyńska, J.; Paszczyk, B.; Łuczyński, M.J.; Kowalska-Góralska, M.; Nowosad, J.; Kucharczyk, D. Using Rutilus rutilus (L.) and Perca fluviatilis (L.) as Bioindicators of the Environmental Condition and Human Health: Lake Łańskie, Poland. Int. J. Environ. Res. Public Health 2020, 17, 7595. [Google Scholar] [CrossRef]
- Al-Sisi, M.; Elhawat, N.; Alshaal, T.; Eissa, F. Assessment of Trace Element Occurrence in Nile Tilapia from the Rosetta Branch of the River Nile, Egypt: Implications for Human Health Risk via Lifetime Consumption. Ecotoxicol. Environ. Saf. 2024, 285, 117079. [Google Scholar] [CrossRef]
- Cai, S.; Zeng, B.; Li, C. Potential Health Risk Assessment of Metals in the Muscle of Seven Wild Fish Species from the Wujiangdu Reservoir, China. Qual. Assur. Saf. Crops Foods 2023, 15, 73–83. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms Regulating Mercury Bioavailability for Methylating Microorganisms in the Aquatic Environment: A Critical Review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.A.; Clayden, M.G.; Jardine, T.D. Bioaccumulation and Biomagnification of Mercury Through Food Webs. In Environmental Chemistry and Toxicology of Mercury; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 455–499. [Google Scholar] [CrossRef]
- Chen, C.Y.; Stemberger, R.S.; Kamman, N.C.; Mayes, B.M.; Folt, C.L. Patterns of Hg Bioaccumulation and Transfer in Aquatic Food Webs Across Multiple Reservoirs. Environ. Pollut. 2009, 157, 2435–2444. [Google Scholar] [CrossRef]
- Kambey, J.L.; Farrell, A.P.; Bendell-Young, L.I. Influence of Illegal Gold Mining on Mercury Levels in Fish of North Sulawesi’s Minahasa Peninsula, Indonesia. Environ. Pollut. 2001, 114, 299–302. [Google Scholar] [CrossRef]
- Castilhos, Z.C.; Rodrigues-Filho, S.; Rodrigues, A.P.; Villas-Bôas, R.C.; Siegel, S.; Veiga, M.M.; Beinhoff, C. Mercury Contamination in Fish from Gold Mining Areas in Indonesia and Human Health Risk Assessment. Sci. Total Environ. 2006, 368, 320–325. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury Exposure and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Wang, S.; Feng, X.; Qiu, G. Mercury Exposure in Rice-Based Diets in SW China. Environ. Pollut. 2009, 157, 326–330. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Chen, Y.; Lu, X.; Feng, X.; Qiu, G. Bioaccumulation and Transformation of Mercury in Crops from Mining-Impacted Areas. Sci. Total Environ. 2020, 712, 135575. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).