Spatial and Temporal Dynamics of Birch Populations in Residential Areas of St. Petersburg, Russia, from 2002 to 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. The City
2.2. Target Species
2.3. Study Sites
2.4. Ground Surveys
2.5. Remote Sensing
2.6. Data Analysis
3. Results
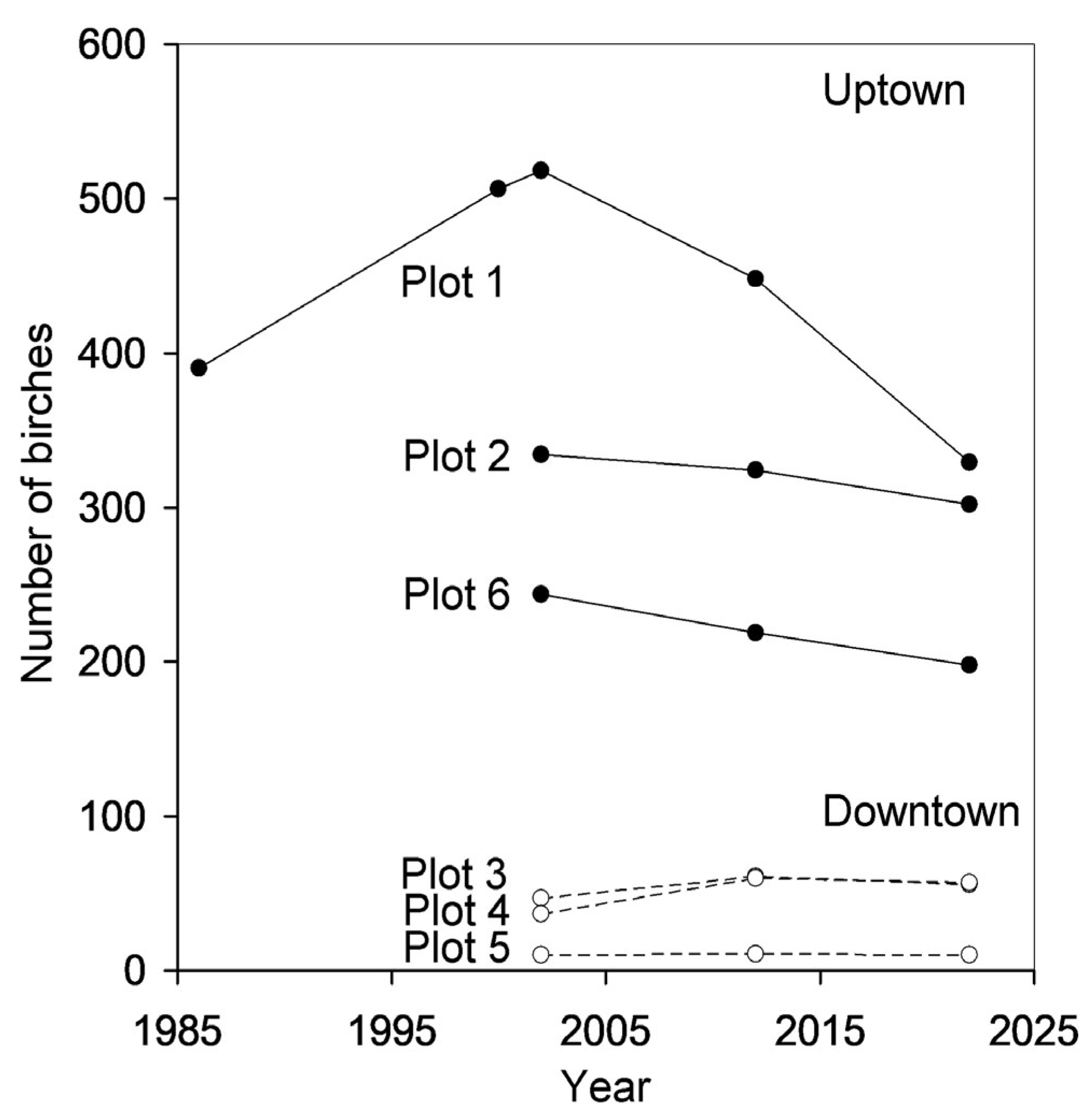
3.1. Outcomes of Ground Surveys
3.2. Outcomes of Remote Sensing
3.3. Patterns in Birch Population Density
4. Discussion
4.1. Spatial Changes in Birch Population Density
4.2. Temporal Changes in Birch Population Density
4.3. Limitations of Space-for-Time Substitution in Urban Ecology
4.4. Future of the Birch Population in St. Petersburg
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPD | birch population density |
| E.V.-C. | Elena Valdés-Correcher |
| M.V.K. | Mikhail V. Kozlov |
| V.Z. | Vitali Zverev |
References
- Liu, Z.; He, C.; Zhou, Y.; Wu, J. How much of the world’s land has been urbanized, really? A hierarchical framework for avoiding confusion. Landsc. Ecol. 2014, 29, 763–771. [Google Scholar] [CrossRef]
- United Nations. World Urbanization Prospects: The 2018 Revision; ST/ESA/SER.A/420; United Nations: New York, NY, USA, 2018. [Google Scholar]
- OECD. Cities in the World: A New Perspective on Urbanisation; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, J. Ecology of urban green spaces: The way forward in answering major research questions. Landsc. Urban Plan. 2014, 125, 298–303. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- McDonald, R.I.; Colbert, M.L.; Hamann, M.; Simkin, R.; Walsh, B. Nature in the Urban Century: A Global Assessment of Where and How to Conserve Nature for Biodiversity and Human Wellbeing; The Nature Conservancy: Arlington, VA, USA, 2018. [Google Scholar]
- Luna, A.; Romero-Vidal, P.; Hiraldo, F.; Tella, J.L. Cities may save some threatened species but not their ecological functions. PeerJ 2018, 6, e4908. [Google Scholar] [CrossRef]
- Planchuelo, G.; Kowarik, I.; von der Lippe, M. Plant traits, biotopes and urbanization dynamics explain the survival of endangered urban plant populations. J. Appl. Ecol. 2020, 57, 1581–1592. [Google Scholar] [CrossRef]
- Kozlov, M.V. Changes in distribution of an archaic moth, Micropterix calthella in St. Petersburg, Russia, between 1989 and 2005. J. Biogeogr. 2007, 34, 231–236. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Song, X.P.; Tan, P.Y.; Edwards, P.; Richards, D. The economic benefits and costs of trees in urban forest stewardship: A systematic review. Urban Forest. Urban Green. 2018, 29, 162–170. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Power, S.A.; Tjoelker, M.G.; Rymer, P.D. Future climate risk and urban tree inventories in Australian cities: Pitfalls, possibilities and practical considerations. Urban Forest. Urban Green. 2022, 78, 127769. [Google Scholar] [CrossRef]
- Von Döhren, P.; Haase, D. Geospatial assessment of urban ecosystem disservices: An example of poisonous urban trees in Berlin, Germany. Urban Forest. Urban Green. 2022, 67, 127440. [Google Scholar] [CrossRef]
- Sjöman, H.; Östberg, J.; Bühler, O. Diversity and distribution of the urban tree population in ten major Nordic cities. Urban Forest. Urban Green. 2012, 11, 31–39. [Google Scholar] [CrossRef]
- Vogt, J.M.; Watkins, S.L.; Mincey, S.K.; Patterson, M.S.; Fischer, B.C. Explaining planted-tree survival and growth in urban neighborhoods: A social–ecological approach to studying recently-planted trees in Indianapolis. Landsc. Urban Plan. 2015, 136, 130–143. [Google Scholar] [CrossRef]
- Smart, N.; Eisenman, T.S.; Karvonen, A. Street tree density and distribution: An international analysis of five capital cities. Front. Ecol. Evol. 2020, 8, 562646. [Google Scholar] [CrossRef]
- Ren, X.C.; Torquato, P.R.; Arndt, S.K. Urban density does not impact tree growth and canopy cover in native species in Melbourne, Australia. Urban Forest. Urban Green. 2023, 81, 127860. [Google Scholar] [CrossRef]
- Martinuzzi, S.; Locke, D.H.; Ramos-González, O.; Sanchez, M.; Grove, J.M.; Muñoz-Erickson, T.A.; Arendt, W.J.; Bauer, G. Exploring the relationships between tree canopy cover and socioeconomic characteristics in tropical urban systems: The case of Santo Domingo, Dominican. Urban Forest. Urban Green. 2021, 62, 127125. [Google Scholar] [CrossRef]
- Mix, C.; Hunt, N.; Stuart, W.; Hossain, A.; Bishop, B.W. A spatial analysis of urban tree canopy using high-resolution land cover data for Chattanooga, Tennessee. Appl. Sci. 2024, 14, 4861. [Google Scholar] [CrossRef]
- Ock, Y.; Shandas, V.; Ribeiro, F.; Young, N. Drivers of tree canopy loss in a mid-sized growing city: Case study in Portland, OR (USA). Sustainability 2024, 16, 1803. [Google Scholar] [CrossRef]
- Locke, D.H.; Ossola, A.; Schmit, J.P.; Grove, J.M. Sub-parcel scale analysis is needed to capture socially-driven canopy cover change in Baltimore, MD. Landsc. Urban Plan. 2025, 253, 1505187. [Google Scholar] [CrossRef]
- Berland, A. Long-term urbanization effects on tree canopy cover along an urban–rural gradient. Urban Ecosyst. 2012, 15, 721–738. [Google Scholar] [CrossRef]
- Bonney, M.T.; He, Y. Attributing drivers to spatio-temporal changes in tree density across a suburbanizing landscape since 1944. Landsc. Urban Plan. 2019, 192, 103652. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Tripler, C.E. Forest remnants along urban–rural gradients: Examining their potential for global change research. Ecosystems 2005, 8, 568–582. [Google Scholar] [CrossRef]
- Nagy, R.C.; Lockaby, B.G. Urbanization in the Southeastern United States: Socioeconomic forces and ecological responses along an urban-rural gradient. Urban Ecosyst. 2011, 14, 71–86. [Google Scholar] [CrossRef]
- Jiao, M.; Xue, H.; Yan, J.; Zheng, Z.; Wang, J.; Zhao, C.; Zhang, L.; Zhou, W. Tree abundance, diversity and their driving and indicative factors in Beijing’s residential areas. Ecol. Indic. 2021, 125, 107462. [Google Scholar] [CrossRef]
- Kozlov, M.V. Changes in distribution of birches and birch-feeding Eriocrania moths in St. Petersburg, Russia, between 1986 and 2001. J. Biogeogr. 2002, 29, 913–918. [Google Scholar] [CrossRef]
- Firsov, G.A.; Egorov, A.A.; Fadeyeva, I.V.; Byalt, V.V. To the question of the assortment of arboreal plants of the Saint-Petersburg’s city parks. Hortus Bot. 2010, 5, 1–14. (In Russian) [Google Scholar]
- Atkinson, M.D. Betula pendula Roth. (B. verrucosa Ehrh.) and B. pubescens Ehrh. J. Ecol. 1992, 80, 837–870. [Google Scholar] [CrossRef]
- Fedotova, N.B. Green spaces of St. Petersburg and monitoring of their conditions. For. Bull. 2009, 13, 202–206. (In Russian) [Google Scholar]
- Kovyazin, V.F.; Lipetskaya, A.A.; Nguyen, T.L. Diversity and ecological condition of plants in Saint Petersburg. In Actual Problems of the Forest Complex; Pamphilov, E.A., Ed.; Bryansk State Engineering and Technology Academy: Bryansk, Russia, 2015; Volume 41, pp. 190–193. (In Russian) [Google Scholar]
- Eränen, J.K.; Nielsen, J.; Zverev, V.E.; Kozlov, M.V. Mountain birch under multiple stressors—Heavy metal resistant populations co-resistant to biotic stress but maladapted to abiotic stress. J. Evol. Biol. 2009, 22, 840–851. [Google Scholar] [CrossRef]
- Goryshina, T.K. Greenery of the Old Petersburg; Iskusstvo-SPB: St. Petersburg, Russia, 2003. (In Russian) [Google Scholar]
- Nwilo, P.C.; Okolie, C.J.; Onyegbula, J.C.; Arungwa, I.D.; Ayoade, O.Q.; Daramola, O.E.; Orji, M.J.; Maduako, I.D.; Uyo, I.I. Positional accuracy assessment of historical Google Earth imagery in Lagos State, Nigeria. Appl. Geomat. 2022, 14, 545–568. [Google Scholar] [CrossRef]
- SAS Institute. SAS/Stat. User’s Guide, Version 9.2; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Chatelain, M.; Da Silva, A.; Celej, M.; Kurek, E.; Bulska, E.; Corsini, M.; Szulkin, M. Replicated, urban-driven exposure to metallic trace elements in two passerines. Sci. Rep. 2021, 11, 19662. [Google Scholar] [CrossRef] [PubMed]
- Nilon, C.H.; Aronson, M.F.J.; Cilliers, S.S.; Dobbs, C.; Frazee, L.J.; Goddard, M.A.; O’Neill, K.M.; Roberts, D.; Stander, E.K.; Werner, P.; et al. Planning for the future of urban biodiversity: A global review of city-scale initiatives. BioScience 2017, 67, 332–342. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J. The increase of impervious cover and decrease of tree cover within urban areas globally (2012–2017). Urban Forest. Urban Green. 2020, 49, 126638. [Google Scholar] [CrossRef]
- Kovyazin, V.F.; Makarov, A.I.; Minkevich, I.I.; Polovtsev, I.N. The State of Greenery in Vasileodtrovskij District of Saint Petersburg; Saint Petersburg Union of Gardeners: St. Petersburg, Russia, 2006. (In Russian) [Google Scholar]
- Gaston, K.J. Urban Ecology; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Lewis, J.S.; Logan, K.A.; Alldredge, M.W.; Bailey, L.L.; VandeWoude, S.; Crooks, K.R. The effects of urbanization on population density, occupancy, and detection probability of wild felids. Ecol. Appl. 2015, 25, 1880–1895. [Google Scholar] [CrossRef]
- Calfapietra, C.; Peñuelas, J.; Niinemets, Ü. Urban plant physiology: Adaptation-mitigation strategies under permanent stress. Trends Plant Sci. 2015, 20, 72–75. [Google Scholar] [CrossRef]
- Czaja, M.A.; Kołton, A.; Muras, P. The complex issue of urban trees—Stress factor accumulation and ecological service possibilities. Forests 2020, 11, 932. [Google Scholar] [CrossRef]
- Zverev, V.E. Mortality and recruitment of mountain birch (Betula pubescens ssp. czerepanovii) in the impact zone of a copper-nickel smelter in the period of significant reduction of emissions: The results of 15-year monitoring. Russ. J. Ecol. 2009, 40, 254–260. [Google Scholar] [CrossRef]
- Stavrova, N.I.; Gorshkov, V.V.; Katjutin, P.N. Dynamics of tree distribution in Siberian spruce and white birch populations according to trunk diameter in the course of postfire succession in northern taiga spruce forests. Lesovedenie Russ. J. For. Sci. 2010, 2010(3), 21–31. (In Russian) [Google Scholar]
- Hansen, R.; Olafsson, A.S.; van der Jagt, A.P.N.; Rall, E.; Pauleit, S. Planning multifunctional green infrastructure for compact cities: What is the state of practice? Ecol. Indic. 2019, 96, 99–110. [Google Scholar] [CrossRef]



| City Area | Plot | Distance a, km | Year of Building Erection | Area (ha) | ||
|---|---|---|---|---|---|---|
| First b | Current c | Total | Vegetated d | |||
| Uptown | 1 | 8.1 | 1940 | 1970 | 30.36 | 13.74 |
| Uptown | 2 | 6.0 | 1930 | 1964 | 24.67 | 13.50 |
| Downtown | 3 | 2.4 | 1730 | 1907 | 22.88 | 2.96 |
| Downtown | 4 | 2.7 | 1730 | 1894 | 24.18 | 3.04 |
| Downtown | 5 | 2.0 | 1730 | 1882 | 26.07 | 0.77 |
| Uptown | 6 | 7.6 | 1930 | 1952 | 30.54 | 10.86 |
| Characteristics of Birch Populations | Study Area (Uptown vs. Downtown) | Study Year | Study Area × Study Year | |||
|---|---|---|---|---|---|---|
| Statistics | p | Statistics | p | Statistics | p | |
| Proportion of area covered by vegetation | F1,4 = 31.7 | 0.0049 | F2,8 = 17.1 | 0.0013 | F2,8 = 2.83 | 0.12 |
| Proportion of area covered by trees | F1,4 = 18.2 | 0.0130 | F2,8 = 17.8 | 0.0011 | F2,8 = 2.81 | 0.12 |
| Proportion of small birches | F1,4 = 1.98 | 0.23 | F2,8 = 5.58 | 0.0304 | F2,8 = 3.17 | 0.10 |
| Absolute birch population density | F1,4 = 19.2 | 0.0119 | F2,8 = 2.66 | 0.13 | F2,8 = 4.25 | 0.0554 |
| Relative birch population density | F1,4 = 6.07 | 0.07 | F2,8 = 3.98 | 0.06 | F2,8 = 2.45 | 0.15 |
| Absolute density of planted birches | F1,4 = 2.26 | 0.21 | F1,4 = 0.23 | 0.66 | F1,4 = 1.75 | 0.26 |
| Absolute density of self-established birches | F1,4 = 0.96 | 0.38 | F1,4 = 1.26 | 0.33 | F1,4 = 0.18 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, M.V.; Valdés-Correcher, E.; Zverev, V. Spatial and Temporal Dynamics of Birch Populations in Residential Areas of St. Petersburg, Russia, from 2002 to 2022. Earth 2025, 6, 41. https://doi.org/10.3390/earth6020041
Kozlov MV, Valdés-Correcher E, Zverev V. Spatial and Temporal Dynamics of Birch Populations in Residential Areas of St. Petersburg, Russia, from 2002 to 2022. Earth. 2025; 6(2):41. https://doi.org/10.3390/earth6020041
Chicago/Turabian StyleKozlov, Mikhail V., Elena Valdés-Correcher, and Vitali Zverev. 2025. "Spatial and Temporal Dynamics of Birch Populations in Residential Areas of St. Petersburg, Russia, from 2002 to 2022" Earth 6, no. 2: 41. https://doi.org/10.3390/earth6020041
APA StyleKozlov, M. V., Valdés-Correcher, E., & Zverev, V. (2025). Spatial and Temporal Dynamics of Birch Populations in Residential Areas of St. Petersburg, Russia, from 2002 to 2022. Earth, 6(2), 41. https://doi.org/10.3390/earth6020041







