Abstract
The reintroduction of Eurasian beaver (Castor fiber L.) results in significant changes in ecosystems. The purpose of this study is to assess the impact of the environment-forming activity of C. fiber on the riparian phytocoenoses of the Raifa forest sector of the Volga-Kama State Nature Biosphere Reserve (Middle Volga region, European Russia) after the reintroduction. Phytoindication methods of ecological–coenotic groups and indicator values were used to assess changes in environmental conditions under the influence of beaver activity. The influence of the beaver reintroduction factor on the increase in the moisture regime (by three points according to the Tsyganov indicator values) and the illumination of habitats, the richness of soils in nitrogen, and the acidity and salt regime of soils (by one point) was revealed. Under the conditions of fodder and construction activities of the beaver, an increase in the proportion of aquatic and wetland groups from 10.2% to 28.2% and boreal plant species from 15.0% to 27.6% was detected. An expansive nature of the change in the degree of landscape occupancy with wetland plants was noted. A decrease in the degree of landscape occupancy (3 to 2 points) of the distribution of ruderal species in the riparian zones of the waterbodies of the reserve due to the activity of the beaver was revealed. Based on phytoindication and ecological–coenotic analyses, it was shown that the reintroduction of C. fiber into the waterbodies of the Raifa forest sector of the reserve is responsible for maintaining the necessary microclimatic conditions for the preservation of natural southern boreal communities. The results obtained can be used for predictive assessment of the influence of the beaver on riparian (small rivers and lakes) plant communities of forest ecosystems in the Middle Volga region of European Russia and other regions of the planet with similar environmental conditions.
1. Introduction
Beavers are among the largest rodents on Earth, but ecologically they are different from all other members of the Rodentia order. There are only two species of beaver: the North American beaver (Castor canadensis Kuhl.) and the Eurasian beaver (Castor fiber L.). The beaver was once one of the most widespread mammals in North America but hunting and trapping have put an end to that. Fortunately, conservation efforts have resulted in the recovery of many populations, and C. canadensis is now present in much of its former range. The beaver is relatively common throughout most of the United States, except for very dry areas such as Arizona, Nevada, and Utah. Their geographic range extends from the northern reaches of Canada to parts of northern Mexico. The C. canadensis was introduced to parts of Russia and Finland in the 1950s, and its range is still expanding. The C. fiber disappeared from many parts of Europe and Asia due to trapping and became extinct in Great Britain in the 16th century. As a result of predatory hunting, the beaver was on the verge of extinction in Eurasia: by the beginning of the 20th century, only 6–8 isolated populations remained (from the Rhone River basin in the west to Mongolia and the upper reaches of the Yenisey River in the east), only 1200 heads. Since then, reintroduction and natural distribution have restored the species to large areas of its original range. Efforts to restore the European population have been largely successful, and this is currently happening in several locations in the UK, Spain, Central Europe, and Scandinavia. The beaver also returns to China and other parts of Asia [1]. Range expansion in 2000–2020 has been rapid, with large expansions in western and south-central Europe, southern Russia, and western and central Siberia. The beaver population is currently recovering in all countries of its former European range, except for Portugal, Italy, and the southern Balkans; they are widely distributed from Siberia to Mongolia, with scattered populations in the east. About half of the world’s beaver population lives in Russia. Populations appear to be mature in most of European Russia, Belarus, the Baltic States, and Poland [1].
The activity of C. fiber attracts the attention of researchers because of the peculiarities of its construction and food activity, resulting in the transformation of the environment [2,3]. All herbivores influence the structure and dynamics of the ecosystem [4,5,6,7], but the influence of the beaver is especially strong because this animal can cut down trees with a large trunk diameter, build dams, and flood floodplains. In addition, the biomass of trees and shrubs taken by the beaver from the ecosystem is much higher than the biomass that it consumes [8,9,10].
Thus, beavers are the key animal species that significantly affect the processes of the landscape level not only in the aquatic but also in the terrestrial environment. The impact of the beaver includes a change in the flow of energy through the water-land boundary, as well as a change in the heterogeneity of the environment. This is due to the direct impact of the beaver on the habitat: felling trees, eating grassy vegetation and woody growth, building beaver structures (dams, huts, semi-huts, burrows, cobles, and canals), and laying beaver paths. Due to this, beavers initiate long-term ecological succession processes associated with the emergence, aging, and destruction of beaver ponds, with a change in the hydrological regime of waterbodies, and a significant change in aquatic and riparian ecosystems [11].
In turn, the history of changes in the distribution of the beaver reflects the destructive ability of human economic activity and, at the same time, demonstrates its ability to compensate for the damage caused to the natural environment [12]. A typical example of such activity is the sharp decline in forest area in the central part of the Volga-Kama region of European Russia in the 19th century. This circumstance resulted in a change in the hydrological regime of small rivers and a large-scale commercial capture of the beaver, which was previously a common species in this region. The last beaver on the territory of the modern Republic of Tatarstan, one of the administrative regions of the Middle Volga region of European Russia, was captured (killed by hunters) in 1902 [13].
In the middle of the 20th century, work on the reacclimatization of C. fiber in the Middle Volga region began. By that time, due to the poor forage base and partially drying watercourses, the watersheds of which were used mainly for arable land and pastures, the habitat conditions for the beaver were unfavorable there [13]. Introduced beavers have found suitable habitats. The reintroduction of beavers is a functionally new invasion. The C. fiber is no more native species to a previously native ecosystem [14].
The modern stand of the Raifa forest sector of the Volga-Kama State Nature Biosphere Reserve (or Volga-Kama Reserve) [15], which belongs to its riparian part, was formed already in the absence of the beaver after its disappearance from the local fauna [16,17]. The fact that in the places of reacclimatization, these rodents in the riparian zone destroy plantations of some plant species should be considered as a stage in the process of restoration of the natural riparian phytocomplex [18]. The beaver acts as a natural factor. Plant species that are not adapted to the conditions of prolonged flooding and do not have the ability for rapid vegetative reproduction disappear from riparian plant communities. The purpose of this study is to assess the impact of the environment-forming activity of C. fiber on riparian phytocoenoses of the Raifa forest sector of the Volga-Kama Reserve after reintroduction.
2. Materials and Methods
2.1. Study Area
The study area is located within the Raifa forest sector of the Volga-Kama Reserve (Figure 1), on the left slope of the Volga River valley. The sector’s surface is composed of Quaternary sandy-argillaceous alluvial-lacustrine deposits. The northeastern part of this sector of the reserve lies on the upper terrace of the Volga River valley with absolute elevations of 125–130 m. The surface of the middle terrace was significantly transformed by erosion, karst, suffosion, and eolian processes in the Neopleistocene and Holocene. The surface of these terraces is dissected by the valleys of the Sumka River and its left tributary, the Ser-Bulak River. The length of the Sumka River is 37 km (within the Raifa forest sector–10 km). The bottom of the valley, hundreds of meters wide, is complicated by karst depressions and sinkholes, mainly occupied by lakes, among which the largest are karst-suffosion Lake Raifa (32 ha), karst-suffosion Lake Ilyinskoye (22 ha), and karst-suffosion Lake Beloye (6.4 ha). The length of the Ser-Bulak River is 11.5 km. A constant flow is maintained only in the upper reaches of the river. In the valley of the lower reaches of the river, 3 km upstream of the mouth, there is Lake Linevo with an area of 7 ha. Almost ubiquitous plowing of land in the basin of the upper reaches of the Sumka River in recent centuries has resulted in an increase in surface runoff, a sharp increase in erosion processes, siltation of lakes, and a decrease in their area and depth [19,20].
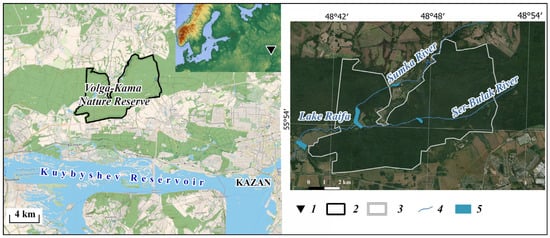
Figure 1.
Location of the Raifa forest sector of the Volga-Kama State Nature Biosphere Reserve in the Russian (East European) Plain. 1—location of the Volga-Kama Reserve; 2 and 3—the border of the Raifa forest sector of the reserve (using Open Street Map (left) and satellite imagery from Google Earth (right)); 4—main rivers; and 5—lakes.
2.2. Reintroduction of Castor fiber L.
In 1996, work on the reintroduction of C. fiber in the watercourses of the Raifa forest sector began (Figure 2). The reintroduction of the beaver on the territory of the reserve was carried out as a biotechnical measure in order to use it to reduce the level of pollution of rivers and lakes. Large areas of the watersheds of the rivers are located outside the reserve; they are mainly occupied by agricultural fields. During the spring melting of snow, the soil layer is washed away from these fields by surface runoff. A huge mass of sediment (on average, about 500 tons per year) enters the rivers flowing through the territory of the reserve, then this mass accumulates in its lakes. For example, in the largest lake of the reserve, karst-suffosion Lake Raifa, the maximum depth used to be 21 m; by the mid-1990s, it became shallow by two meters, and its area decreased by 2 ha [21]. Due to silting, there was a significant decrease in the water flow of the local rivers Sumka and Ser-Bulak. By blocking the rivers with dams, beavers reduce the mass of suspended sediment, which is carried mainly in the spring flood to Lake Raifa. The purpose of the reintroduction of the beaver was to improve the hydrological situation, as well as to increase the level of groundwater due to the flooding and underflooding of large areas by beavers, and to improve the moisture regime [21].

Figure 2.
The Eurasian beaver (C. fiber L.) (left) and the results of its construction activity (right) on the Ser-Bulak River (the photographs were kindly provided by the administration of the Volga-Kama State Nature Biosphere Reserve).
The study of the influence of beaver activity on the plant communities of the Volga-Kama State Nature Biosphere Reserve began at the very moment of the reintroduction of animals [21]. This made it possible to reveal changes in the composition and structure of riparian phytocoenoses during the reintroduction of the beaver.
2.3. Materials
In the work, fund materials of research of the scientific department of the Volga-Kama State Nature Biosphere Reserve for 1997 were used. In 2010 and 2022, we continued to study the vegetation in order to monitor the ecological succession processes caused by the reintroduction of the beaver.
2.4. Methods
In 1997, to determine the influence of the beaver on the riparian vegetation of the Raifa forest sector of the reserve and its buffer zone, 59 test plots were established according to the method proposed by [22]. At an arbitrary point located in the riparian zone (determined from a table of random numbers), the first test (trial) plot was laid. All subsequent plots were laid at a distance of 200 m from each other. The location of the first area (right or left bank) was determined by a table of random numbers, and then alternated, so that all the even numbers of the area were on the right bank, and the odd numbers were on the left, or vice versa [22]. The coordinates of the starting point were determined using GPS with an accuracy of tenths of a second. In addition, the starting point was marked with pegs and marks on the trees.
The test plot consisted of two mutually perpendicular transects (Figure 3). One of the transects, “along-bank/shore transects”, ran parallel to the riverbank/lakeshore, the second one, “inner transects”, is from the river/lake perpendicular to the first. Each of the transects included three rectangular sites (“box”) (3 m × 7 m); the distance between them was 3 m. In each of the “boxes”, the number of trees less than 10 cm in diameter (50 cm from the ground) was counted by species.
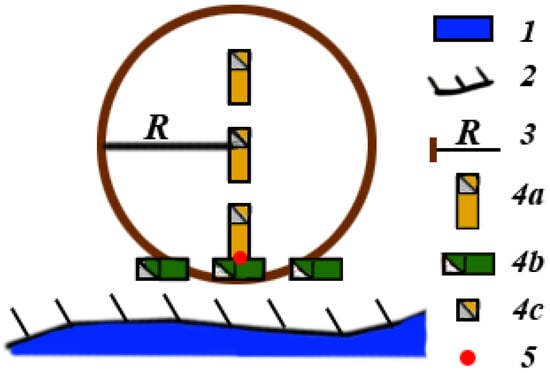
Figure 3.
Scheme of a test plot for determining the influence of beavers on riparian vegetation. 1—waterbody (river or lake); 2—riverbank/lakeshore, 3—radius R = 17.84 m; 4a—inner transects (3 m × 7 m), 4b—along-bank/shore transects (3 m × 7 m), 4c—test plots (1 m × 1 m); 5—starting point with notation sign.
All vegetation relevés in 1997, 2010, and 2022 were performed according to the above methodology. Of the 59 test plots described in 1997, only 27 were followed by subsequent vegetation relevés in 2010 and 2022 (Figure 4); data from these 27 plots formed the basis of this work.
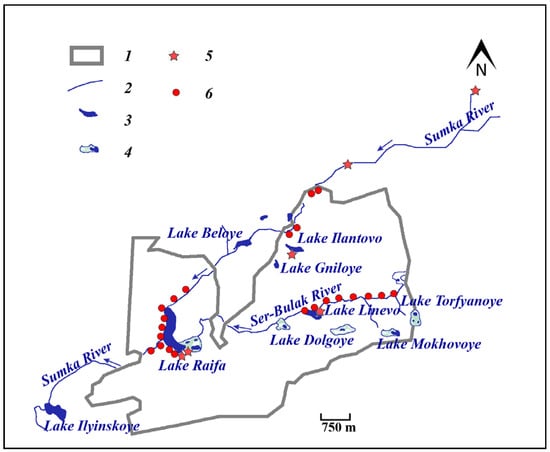
Figure 4.
Map of the location of the studied test plots. 1—the border of the Raifa forest sector of the Volga-Kama Reserve; 2—rivers; 3—lakes; 4—wetlands; 5—the places where beavers were released in 1996 on the territory of the reserve and in its buffer zone; 6—the test plots studied.
Depending on the activity of C. fiber along the rivers and lakes, the studied plots were divided into two groups: (1) 19 plots exposed to beavers (experimental group); (2) 8 plots where no beaver activity was observed (control group). The presented division was carried out based on our own observations during the collection of field material at the test plots studied. At the same time, the presence of traces of beaver activity at each plot (gnawed and fallen trees, beaver paths, burrows, huts, semi-huts, dams, canals, and cobles) was considered. We also used data on the distribution of C. fiber in the waterbodies of the Raifa forest sector of the reserve. Moreover, in this study, for a more detailed assessment of the influence of the environment-forming activity of the beaver, it was decided to divide the experimental group into two more independent subgroups. These are sites where only feeding activity of rodents was observed (8 plots), and sites where construction activity was observed (11 plots).
The herbaceous vegetation was studied in areas of 1 m × 1 m in each “box” of the transects [22]. When describing the test plots, we identified the plant species present on the site, and simultaneously visually determined their abundance for each species as a percentage of the area and on the Drude scale [23]. The Drude scale is a system for estimating the abundance of a species: soc (socialis)—plants are close to each other with full vegetable coverage (>75%); cop3 (copiosae)—very abundantly, vegetable coverage is 50–75%; cop2—abundantly, vegetable coverage is 35–50%; cop1—quite abundantly, vegetable coverage is 20–35%; sp (sparsae)—scattered, vegetable coverage is <20%; sol (solitaries)—rarely; un (unicum)—single plants [23]. The projective cover of a species was taken as an indicator of abundance [22]. All data were entered into herbaceous vegetation relevés sheets indicating the conditional name of the test plot, the name of the river or lake, and the date of relevé.
Data on trees and shrubs with a diameter of less than 10 cm were recorded on the shrub tier relevé sheet. The tree-stand relevé sheets recorded data on woody vegetation (more than 10 cm in diameter), obtained when describing a round area with a radius of 17.84 m. This includes information about the number of trees of different species, their diameter, and tree vitality, which were determined on a nine-point scale: 1—normally growing trees, 2—trees with a deteriorated state, 3—recently dead trees, 4 and 5—dead trees without bark, 6—broken trees, 7—tilted trees, 8—fallen trees, and 9—only stumps [22]. All data of vegetation relevés obtained at the stage of collecting field material were entered into the [24] for further analysis: assessment of the values of environmental factors according to the Tsyganov indicator values [25], ecological–coenotic analysis of the vegetation cover (the analysis of the flora by confinement to groups of species, which are stable regular combinations of species in plant communities) on the established test plots and calculation of the index of species activity. The indicator values developed by Tsyganov and compiled for the temperate zone of Eurasia include more than 2300 plant species and make it possible to assess a wide range of environmental factors. The Tsyganov indicator values are as follows: Tm–thermoclimatic; Kn–continentality of the climate; Om–aridity/humidity of the climate; Cr–cryoclimatic; Hd–soil moisture; Tr–soil trophicity (salt content); Nt–soil nitrogen richness; Rc–acid-alkaline conditions (pH) of soils; Lc–illumination/shading; fH–moisture variability. The number of classes in them ranges from 9 to 23 [25]. The conditional unit of ecological indicator values [26,27,28] is the score (point), which is calculated for each floristic composition.
The range of application of indicator values is wide. It includes analysis of growing conditions and position of plant communities on the axes of environmental factors; determination of ecological groups of species and ordination of plant communities; analysis of plant dynamics, including anthropogenic; prediction of habitat conditions in plant communities, etc. [29,30].
In each group of vegetation relevés, the values of environmental factors were calculated using the Tsyganov indicator values. To do this, first, for each species in each relevé, the values of the average abundance were calculated by dividing the sum of the abundance values in 1 m2 by their number (6, three on each transect, Figure 3):
where Pi is the average abundance of the i species (%), Pn is the abundance in each “box” of transects, and 6 is the number of plots with an area of 1 m2.
Pi = ∑ Pn/6,
Species data were converted to environmental factor values prior to analysis. The obtained values of the average abundance are recalculated and expressed in fractions of one. Using the data on the abundance of species and the limiting values of environmental factors for each species according to the tables of the Tsyganov indicator values, the values of each environmental factor (fi) were calculated for all test plots in the analyzed groups:
where Cav is the arithmetic mean of the environmental factor (in points), and P is the mean abundance of the species (in fractions of one).
fi = ∑(Cav × P),
Thus, information on the nature of such environmental factors as air temperature regime, climate continentality, dryness-humidity, cryoclimatism, humidity, soil salt regime, soil nitrogen content, soil acidity, illumination, and moisture variability were obtained. The entire calculation of the values of environmental factors was carried out in Microsoft Office Excel 2010. The indicator values between the control and experimental plots were compared using ANOVA. To check the normality of the distribution, the Kolmogorov-Smirnov test was used. The conformity of homoscedasticity was assessed according to the White test. The significance level (p) was chosen as 0.05. Calculations were carried out using Statistica for Windows (Version 12).
To assess the degree of success in the development of landscape species, the degree of landscape occupancy by each species (A) based on the abundance (D) and occurrence (F) indicators was calculated as follows:
A = (F × D)0.5,
The leading value is the occurrence of species in each group of plots, expressed in points (maximum 10). The occurrence of the species is automatically calculated in the FLORA database [24]. The species abundance was determined by the projective cover of the species according to the 5-point Drude scale [23].
3. Results and Discussion
The ecological–coenotic groups of the studied flora were determined. On the control test plots described in 1997, the proportion of forest species was 51.2% (Table 1), including 11.1% of boreal species (Vaccinium myrtillus L., V. vitis-idaea L., Maianthemum bifolium L., Oxalis acetosella L.), 22.4% of nemoral species (Asarum europaeum L., Viola mirabilis L., Pulmonaria obscura Dumort., Carex pilosa Scop.), and the remaining 17.7% are species that grew in forests of various type (mixed, pine and alder). The group of meadow species accounted for 37.7% of the entire described flora. Plants growing in meadows of various types (Hypericum perforatum L., Trifolium pratense L., Saponaria officinalis L., Tanacetum vulgare L.) accounted for 11.1%, in wet meadows (Filipendula ulmaria (L.) Maxim., Deschampsia cespitosa (L.) P. Beauv., Veronica longifolia L., Persicaria maculosa Gray.)—4.4%, in forest meadows (forest fringes) (Fragaria vesca L., Geranium sylvaticum L., Vicia cracca L., Lysimachia nummularia L.)—22.2%. The group of wetland species (Lysimachia thyrsiflora L., Salvinia natans (L.) All., Lemna minor L., Phragmites australis (Cav.) Trin.) was represented by 6.7%. A separate group consisted of weed (ruderal) plant species (Urtica dioica L., Arctium lappa L., Artemisia vulgaris L., Chelidonium majus L.), associated in their distribution with disturbed habitats. They accounted for 4.4%.

Table 1.
Ecological–coenotic characteristics of the studied control and experimental test plots in 1997, 2010, and 2022.
On the same test plots in 2010, there was a general decrease in the share of forest species to 47.7% (Table 1), including boreal species—9.4%, nemoral—23.8%; the remaining 14.5% are species growing in forests of various types. The proportion of wetland species increased to 12.7%. The group of meadow species reduced their share in the community to 30.6%: species growing in meadows of various types accounted for 9.5%, wet-meadow species—7.9%, and forest meadows (forest fringes)—12.7%. The appearance of steppe species was noted—1.6%. The proportion of ruderal species increased to 7.4%.
In the control plots of 2022, an increase in the share of forest species to 63.2% was noted (Table 1), including 7% of boreal species, 34.2% of nemoral species, and 22% of mixed forest species. Wetland and steppe species were not noted. The share of meadow species decreased to 21% against the background of an increase in the share of ruderal species—15.8% (10.5%—weedy species, 5.3%—cultural species).
An analysis of the ecological–coenotic groups (ECG) of the experimental plots in 1997 revealed the same regularities as in the control plots of 1997: species of the forest group also prevailed there, although in a different ratio. The share of forest species was 46% (Table 1), including boreal—15%, nemoral—10.9%, and growing in forests of various types—20.1%. The group of weed plant species accounted for 13.5%, among which the leading position belonged to ruderal species—10.1%; cultivated species accounted for 3.4%.
According to the results of the ECG analysis in the experimental plots for 2010, there was a tendency to change the dominant group. Along with the forest group, species of the wetland group accounted for the largest share. The share of forest species was 44.5% (Table 1), among them the share of boreal species increased—24%, nemoral—7.9%; the remaining 12.6% were species growing in forests of various types. 25.7% of the species were associated with wetland communities. The group of meadow species accounted for 19.4% of the total flora. Steppe plant species accounted for 1.7%. The group of weed plant species accounted for 8.7%, among which the leading position belonged to ruderal species—6.2%; cultivated species accounted for 2.5%.
The share of forest species in the experimental plots for 2022 was 46.3% (boreal species—27.6%, nemoral species—6%, 12.7%—species growing in forests of various types) (Table 1). The share of the group of wetland species decreased to 28.2%. The group of meadow species accounted for 23.1%. No steppe species were noted. The weed group accounted for 2.4% of the species.
According to the results of the ECG analysis, it is also noted that, in contrast to the control test plots, in areas where beaver activity is noted, there is an increase in the proportion of boreal phytocoenoses, conservation of meadows, and a decrease in the proportion of ruderal species. On the control plots, the proportion of boreal species decreased, and the group of wetland species completely disappeared. At the same time, a steady trend towards an increase in the role of ruderal species in communities was observed. These results correlate well with the results on changes in moisture conditions obtained from the Tsyganov indicator values and species activity.
To study the change of the flora under the influence of C. fiber, it is important to determine the nature of its changes, which makes it possible to conclude the dynamics of vegetation. The landscape occupancy scores (points) of each species were calculated in all plot groups. The calculation of species activity revealed the maximum expansion in species of the ruderal group in the control plots in 1997, which was 4 points (Urtica dioica L.), which belongs to the middle class of activity. In the plots in 2010 and 2022, the highest activity also belonged to ruderal species (U. dioica L., Chelidonium majus L.)–5 points (class–expansion). Thus, an expansive degree of landscape occupancy was observed in this group of species. The rest of the species and groups of species had rather low landscape occupancy scores (1 and 2 points) for all the years of our study. At the same time, species of the wetland and aquatic groups showed a decrease in the degree of landscape occupancy, and they were not noted at all in the relevés of 2022. This also reflects the trend of change in the flora of the Raifa forest sector of the reserve, where an increase in the degree of landscape occupancy of ruderal species was observed.
On the experimental test plots in 2010 and 2022, on the contrary, despite the general expansion of ruderals, there was a fading degree of change in the ruderal group and an expansive degree in boreal and wetland species. The maximum value of landscape occupancy of species in these areas in 1997 was for U. dioica L. (ruderal group) and amounted to 3 points (fairly expansive class), the nature of the degree of landscape occupancy was expansive. In 2010 and 2022, the maximum value was associated with Lemna minor L., belonging to the aquatic group–4 points (medium expansive class), the nature of the degree of landscape occupancy was expansive. In 2010 and 2022, there was a fading degree (from 3 to 2) for ruderals. The maximum value for this group (2–inactive class) was noted for U. dioica L. These results also provide evidence of changes in vegetation cover and environmental conditions in areas that are directly affected by C. fiber.
Based on phytoindication analysis using the Tsyganov indicator values, information on the nature of changes in such environmental factors as air temperature regime, continentality of climate, aridity-humidity, cryoclimatism, moisture content, salt regime of soils, soil nitrogen availability, soil acidity, illumination, and variability of moisture for both groups of plots studied [25] were obtained.
For control test plots in 1997, the following environmental conditions were determined:
- The air temperature regime (Tm) refers to the type of boreal-nemoral zones with an annual value of total solar radiation of 40 kcal/cm2;
- Continental climate (Kn), semi-arid, according to the ombroclimatic factor;
- The cryoclimatic factor (Cr) was characterized by moderate winters (average air temperatures of the coldest month were from −8 to −16 °C);
- Conditions of humid forest-meadow zones according to the moisture factor (Hd);
- Poor soils in terms of the salt regime (Tr);
- Nitrogen-poor soils under conditions of nitrogen richness (Nt);
- Slightly acidic soils (pH = 5.5–6.5) according to the soil acidity regime (Rc) (for light forest conditions–Lc, for a zone of relatively stable moisture–Bk [25]).
The same indicators were obtained when analyzing data from test plots for 2010 and 2022. Statistically significant differences in indicators for all years were assessed. On the control plots, the constancy of the main environmental factors was noted, except for the content of soil nitrogen, the value of which showed a steady increase (Figure 5).
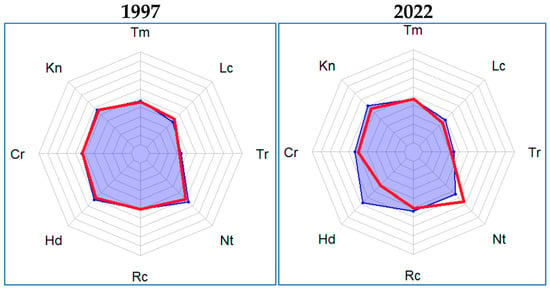
Figure 5.
Average characteristics of environmental conditions (in points, according to the Tsyganov indicator values) in 1997 and 2022.  control test plots,
control test plots,  experimental test plots.
experimental test plots.
For the experimental plots of 1997, the same indicators of environmental factors were obtained as in the control plots of 1997. However, when compared with the values of environmental conditions obtained in 2010, there was a noticeable change in the indicators of the following factors: an increase in the humidity index by 3 points, illumination, salt regime of the soil, variability of humidity (all by 1 point), a decrease in nitrogen richness and the acidity of soils (by 1 point). The results of phytoindication analysis according to the Tsyganov indicator values, obtained for geobotanical test plots in 2022, are consistent with the results of 2010. These results are confirmed by statistical calculations (p = 0.000). In contrast to the results of studies of riparian vegetation carried out in the Kostomuksha Nature Reserve (Karelia, European Russia) [31], there were no changes in environmental factors in the Raifa forest sector as a result of the reintroduction of the beaver. An increase in indicators was noted only for humidity, illumination, soil salt regime, and moisture variability. However, in contrast to [31], under the influence of the activity of the beaver, there was a decrease in nitrogen richness and soil acidity. It is possible that this difference was due to the different characteristics of the soils of these territories.
For a more detailed assessment of the impact of the activity of the beaver, the environmental conditions of the selected sites (according to the indicator values), where only feeding activity was observed, and the sites where construction work was carried out, were compared. The results obtained were completely identical to the results for the entire experimental group and differed slightly. Although the results of construction activities were most noticeable at the initial stages of colonization by beavers [32].
Currently, major transformations of the landscape, habitat conditions, and changes in riparian phytocoenoses are taking place. However, under conditions of a sufficient amount of food resources, both the stabilization of the number of beavers and their habitat and a decrease and further extinction of the construction activity of beavers occur. In the future, only the factor of food activity will remain the most significant and constant [32,33,34,35]. In our work, repeated studies of vegetation and phytoindication evaluation of control and experimental plots were carried out 13 and 25 years after the reintroduction of the beaver. Probably, during this time, the number of beavers in the Raifa forest sector stabilized, which resulted in the stabilization of new conditions [33,36,37,38].
The species composition of the flora of the control and experimental geobotanical test plots described in 1997 differed insignificantly. In 2022, 63 plant species belonging to 52 genera and 36 families were recorded on the control plots, and 118 plant species belonging to 89 genera and 53 families were recorded in the experimental plots. The control group was dominated by flowering plants (Magnoliophyta)–54 species, which is 85.7% of the total number of species. The divisions of horsetails (Equisetophyta), ferns (Polypodiophyta), and gymnosperms (Pinophyta) were represented by only 9 species (14.3%). In the experimental plots, the dominant role of angiosperms (Magnoliophyta) remains–102 species, which is 86.4% of the total number of species. The plauns (Lycopodiophyta), horsetails (Equisetophyta), ferns (Polypodiophyta), and gymnosperms (Pinophyta) were represented by only 16 species (13.6%). Thus, our study showed that in the test plots where the life activity of the beaver is noted, there is a greater taxonomic richness and diversity of riparian flora. The results reflecting an increase in the species diversity of herbaceous plants in the zone of activity of the beaver are consistent with the results of other researchers [34,37,38,39,40,41,42,43]. Against the background of an increase in the number of species, the proportion of ecological–coenotic groups characteristic of boreal phytocoenoses also increases. In the test plots of the fodder and construction activities of the beaver, the structure of riparian phytocoenoses becomes more complex. This is an indicator of increasing environmental sustainability [44] in riparian plant communities.
In [20], no statistically significant differences were found between the indicators of 1997 and 2002, collected on the control plots, while several significant changes were noted on the test plots subjected to the beaver’s environment-forming activity. According to this study, the main changes in the composition and structure of riparian phytocoenoses under the influence of the beaver occurred in the riparian zone, where the beaver feeds predominantly. In addition, the construction work of the beaver led to a rise in the level of surface and ground waters, which also directly affected the vegetation of the riparian zone [20].
According to the results of the first studies, it was revealed that the fodder (foraging) activity of the beaver limits the development of willow and aspen. These types of woody and shrubby vegetation make up the beaver’s main diet. In most plots, the willow was significantly affected by the fodder activity of the beaver; in several plots, it completely died [20].
While in several numerous works [9,31,32,36,45,46,47,48], the influence of beavers on phytocoenoses was studied, such studies have not been previously carried out in the Volga-Kama Reserve. Our data document for the first time the role of beaver reintroduction in the riparian vegetation of the Raifa forest sector. Previous studies did not assess the activity of plant species under the influence of beaver activity. Our results indicate a decrease in the activity and participation of ruderal plant species in riparian phytocoenoses. Ruderal species are mainly adventitious elements and indicators of increased anthropogenic impact. There is a change of ruderals by forest and moisture-loving species, as well as a restoration of more characteristic groups of herbaceous vegetation for the boreal forest.
Overall, the results obtained correlate well with the results of the studies of other researchers who investigated the environment-forming activity of C. fiber in European Russia: in Novgorod Oblast (Valdai National Park, Priilmenskaya Lowland) [36], Bryansk Oblast (Bryansk Forest Nature Reserve) [45], Vologda Oblast (Darwin Nature Reserve) [46], Moscow Oblast (Prioksko-Terrasnyi Nature Biosphere Reserve) [47], Leningrad Oblast (Nizhnesvirsky Nature Reserve) [48], and Orenburg Oblast (Burtinskaya Steppe) [49]. The activity of C. fiber in its natural habitats is one of the main components in maintaining a higher species diversity of the flora and in preserving the microclimatic factors characteristic of these territories.
In the riparian zones of the studied waterbodies, where beaver activity was noted, the composition and structure of plant communities had features identified for the riparian zones of similar waterbodies in the taiga, forest, forest-steppe, and steppe zones of the Russian (East European) Plain. In particular, this concerns an increase in species diversity and diversity of ecological–coenotic groups of plants. Therefore, we can conclude that the results obtained can be used in practice for predictive assessment of the impact of the beaver on plant communities in the conditions of riparian zones of small rivers and lakes.
4. Limitations
A significant limitation of this study is that for the applied phytoindication analysis, data on the characteristics of the herbaceous vegetation were used. This is because it is in the structure of the herbaceous vegetation that the first ecological succession processes are noted, which reflect the directions and degree of changes in environmental factors, and the direct death of a part of the plantation (due to eating and flooding) is accepted by us as manifestations of the environment-forming activity of the beaver. In addition, the grass cover is characterized by greater heterogeneity, which allows a more detailed assessment of the influence of environmental factors. Another important limitation is the lack of data on several plots due to their inaccessibility, as well as the loss of primary information about the location of some of them.
The limitations of the study also include the fact that relevés in test plots were carried out not annually, but with an interval of 12–13 years, which did not allow for assessing the interannual dynamics of riparian phytocoenoses and changes in environmental conditions in the compared sites. The work also did not consider the peculiarities of the seasonal dynamics of flora and vegetation for various phenological phases during the growing season. The initial relevés of vegetation in 1997 were carried out in August, which falls on the phases of flowering and ripening of seeds and fruits. In this regard, in order to eliminate seasonal fluctuations based on the results of research, further study of vegetation in subsequent years (2010 and 2022) was also carried out only in August.
5. Conclusions
- The activity of C. fiber does not affect the functioning of riparian phytocoenoses associated with such more global factors as climate continentality, air temperature regime, ombroclimatism, and cryoclimatism. Statistically significant changes in microclimatic factors of the environment under the influence of the activity of C. fiber in the Raifa forest sector of the Volga-Kama State Nature Biosphere Reserve were revealed. The influence of the beaver reintroduction factor on the change in the regime of moistening and illumination of habitats, the richness of soils with nitrogen, and the acidity and salt regime of soils was also revealed.
- Under the conditions of fodder and construction activities of the beaver, an increase in the proportion of groups of aquatic, wetland, and boreal plant species, as well as an expansive nature of the change in their activity, were noted. A decrease in the activity of distribution of ruderal species under the conditions of beaver activity was noted.
- Changes in factors such as humidity and illumination of the habitat are most likely directly related to the activity and lifestyle of the beaver. This is due to the direct removal of trees from the forest stand, which increases the illumination of riparian zones.
- Beaver-induced flooding and underflooding result in an increase in moisture of riparian soils and grounds in quantities sufficient to cause the replacement of some ecological–coenotic groups by others that are more adapted to changing conditions.
- From the obtained results, it follows that in the areas affected by C. fiber, the effect of adverse climatic phenomena, such as drought, is smoothed out, and the most optimal conditions for the conservation and development of boreal plant species are provided.
In this paper, for the first time, the influence of the reintroduction of the beaver on the vegetation of the riparian forests of the Volga-Kama Nature Reserve, as well as the in the Middle Volga region, is assessed. The results obtained can be considered representative (key) at least for similar forest ecosystems in the south of the forest belt (southern taiga subzone) of the east of the Russian Plain.
Author Contributions
Conceptualization, N.G.N. and A.V.G.; data curation, N.G.N. and V.E.P.; formal analysis, N.G.N. and V.E.P.; funding acquisition, A.G.S.; investigation, N.G.N., V.E.P. and A.G.S.; methodology, N.G.N. and V.E.P.; project administration, A.G.S. and A.V.G.; software, V.E.P., A.G.S. and A.V.G.; supervision, A.V.G.; validation, N.G.N. and V.E.P.; visualization, A.G.S. and V.E.P.; writing—original draft, N.G.N.; writing—review and editing, A.V.G. and F.N.L. All authors have read and agreed to the published version of the manuscript.
Funding
The investigation was carried out at the expense of the grant of the Russian Science Foundation No. 22-77-10087, https://rscf.ru/project/22-77-10087/, accessed on 25 April 2023, (field research; comparative analysis of the ecological–coenotic characteristics of the studied control and experimental plots). The work was also carried out in accordance with the Strategic Academic Leadership Program “Priority 2030” of the Kazan Federal University of the Government of the Russian Federation (assessment of the role of environmental factors).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors thank the administration of the Volga-Kama State Nature Biosphere Reserve and Cristopher Mac Quiroa Andre, Kazan Federal University, for informational and technical assistance in the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Halley, D.J.; Saveljev, A.P.; Rosell, F. Population and distribution of beavers Castor fiber and Castor canadensis in Eurasia. Mammal Rev. 2021, 51, 1–24. [Google Scholar] [CrossRef]
- Dezhkin, V.V.; D’yakov, Y.V.; Safonov, V.G. Beaver; Agroprom Publisher: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Balodis, M.M. Biological Bases of Beaver Farming (on the Example of Latvia). Ph.D. Thesis, A.N. Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia, 1989. (In Russian). [Google Scholar]
- Abaturov, B.D. Mammals as a Component of the Ecosystems; Nauka Publisher: Moscow, Russia, 1984. (In Russian) [Google Scholar]
- Bratton, S.P. The effect of the European wild boar, Sus scrofa, on gray beech forest in the Great Smokey Mountains. Ecology 1975, 56, 1356–1366. [Google Scholar] [CrossRef]
- McNaughton, S.J. Ecology of a grazing ecosystem: The Serengheti. Ecol. Monogr. 1985, 55, 259–294. [Google Scholar] [CrossRef]
- Huntley, N.J. Influence of refuging consumers (pikas: Ochotona princeps) on subalpine meadow vegetation. Ecology 1987, 68, 274–283. [Google Scholar] [CrossRef]
- Johnston, C.A.; Naiman, R.J. Boundary dynamics at the aquatic-terrestrial interface: The influence of beaver and geomorphology. Landsc. Ecol. 1987, 1, 47–57. [Google Scholar] [CrossRef]
- Gorshkov, D.Y.; Gorshkov, Y.A.; Kochetkov, N.V. The features of the feeding behavior of the beavers in Volga-Kama National Nature Zapovednik. In Proceedings of the First Euro-American Beaver Congress, 24–28 August 1999; Busher, P.E., Gorshkov, Y., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 14–16. [Google Scholar]
- Zavyalov, H.A.; Krylov, A.V.; Bobrov, A.A.; Ivanov, V.K.; Dgebuadze, Y.Y. Effects of River Beaver on the Ecosystems of Small Rivers; Nauka Publisher: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Dezhkin, V. Beaver in the modern world: Necessity of the population management on the national and the international level. In Proceedings of the First Euro-American Beaver Congress, 24–28 August 1999; Busher, P.E., Gorshkov, Y., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 20–26. [Google Scholar]
- Popov, V.A. Mammals of the Volga-Kama Region; Kazan Branch of the Academy of Sciences of the USSR Publisher: Kazan, Russia, 1960. (In Russian) [Google Scholar]
- Grigoriev, N.D. The current state of beaver colonies in the Volga-Kama region, growth prospects and using. Proc. Voronezhsky State Reserve 1969, 16, 86–99. (In Russian) [Google Scholar]
- Dgebuadze, Y.Y. Ecology of invasions and population contacts of animals: General approaches. In Invasive Species in the European Seas of Russia; Matishev, G.G., Ed.; Kola Scientific Center of the Russian Academy of Sciences Publisher: Apatity, Russia, 2000; pp. 35–50. (In Russian) [Google Scholar]
- Great Volzhsko-Kamsky Biosphere Reserve, According to UNESCO. Available online: https://en.unesco.org/biosphere/eu-na/great-volzhsko-kamsky (accessed on 31 March 2023).
- Porfiriev, V.S. Vegetation of Raifa. Proc. Volga-Kama Nat. Reserve 1968, 1, 17–23. (In Russian) [Google Scholar]
- Taisin, A.S. Raifa Forest in the Boreal Forests of Eurasia; Kazan University Publisher: Kazan, Russia, 2008. (In Russian) [Google Scholar]
- Legeyda, I.S. Environment-Forming Activity of Beavers and Protection of Coastal Biogeocenoses of Ukraine. Ph.D. Thesis, Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia, 1992. (In Russian). [Google Scholar]
- Lisetskii, F.N.; Poletaev, A.O.; Buryak, Z.A. Geoinformation support for studies of the boundaries of flood zones in urban areas. J. Phys. Conf. Ser. 2022, 2388, 012134. [Google Scholar] [CrossRef]
- Taisin, A.S.; Dedkov, A.P. Pliocene valleys and Quaternary terraces of the Raifa. Proc. Volga-Kama Nat. Reserve 2005, 6, 115–127. (In Russian) [Google Scholar]
- Gorshkov, Y.A.; Gorshkov, D.Y.; Easter-Pilcher, A.L.; Pilcher, B.K. First results of beaver (Castor fiber) reintroduction in Volga-Kama National Nature Zapovednik (Russia). Folia Zool. 2002, 51, 67–74. [Google Scholar]
- Easter-Pilcher, A.L. Forage Utilization, Habitat Selection and Population Indices of Beaver in Northwest Montana. Master’s Thesis, University of Montana, Missoula, MT, USA, 1987. [Google Scholar]
- Drude, O. Handbuch der Pflanzengeographie; J. Engelhorn: Stuttgart, Germany, 1890; 582p. (In German) [Google Scholar]
- Prokhorov, V.E.; Rogova, T.V.; Kozhevnikova, M.V. Vegetation Database of Tatarstan. Pytocoenologia 2017, 47, 309–313. [Google Scholar] [CrossRef]
- Tsyganov, D.N. Phytoindication of Ecological Regimes in the Subzone of Coniferous-Deciduous Forests; Nauka Publisher: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Ellenberg, H. Zeigerwerte der Gefässpflanzen Mitteleuropas. Scr. Geobot. 1974, 9, 1–166. (In German) [Google Scholar]
- Landolt, E. Okologische Zeigerwerts zur Sweizer Flora. In Veröffentlichungen des Geobotanischen Institutes der ETH; Stiftung Rübel: Zürich, Switzerland, 1977; Volume 64, 208p. (In German) [Google Scholar]
- Ramensky, L.G.; Tsatsenkin, I.A.; Chizhikov, O.N.; Antipin, N.A. Ecological Assessment of Fodder Lands by Vegetation Cover; Sel’khozhiz: Moscow, Russia, 1956; 472p. (In Russian) [Google Scholar]
- Egorova, N.Y. Influence of Ecological Factors on the Population-Ontogenetic Parameters of Vaccinium vitis-idaea L. in Forest Ecosystems of the European Northeast of Russia. Contemp. Probl. Ecol. 2020, 13, 656–662. [Google Scholar] [CrossRef]
- Hellegers, M.; Ozinga, W.A.; Hinsberg, A.; Huijbregts, M.A.J.; Hennekens, S.M.; Schaminée, J.H.J.; Dengler, J.; Schipper, A.M. Evaluating the ecological realism of plant species distribution models with ecological indicator values. Ecography 2020, 43, 161–170. [Google Scholar] [CrossRef]
- Fyodorov, F.; Yakimova, A. Changes in Ecosystems of the Middle Taiga due to the Impact of Beaver Activities, Karelia, Russia. Balt. For. 2012, 18, 278–287. [Google Scholar]
- Danilov, P.I.; Fyodorov, F.V. The history and legacy of reintroduction of beaver in the European North of Russia. Russ. J. Theriol. 2016, 15, 43–48. [Google Scholar] [CrossRef]
- Law, A.; Jones, K.C.; Willby, N.J. Medium vs. short- term effects of herbivory by Eurasian beaver on aquatic vegetation. Aquat. Bot. 2014, 116, 27–34. [Google Scholar] [CrossRef]
- Elmeros, M.; Madsen, A.B.; Berthelsen, J.P. Monitoring of reintroduced beavers (Castor fiber) in Denmark. Lutra 2003, 46, 153–162. [Google Scholar]
- Donkor, N.T.; Fryxell, J.M. Lowland boreal forests characterization in Algonquin Provincial Park relative to beaver (Castor canadensis) foraging and edaphic factors. Plant Ecol. 2000, 148, 1–12. [Google Scholar] [CrossRef]
- Porokhov, A.A. Reacclimatization and Biocenotic Role of River Beavers Castor fiber L. in the Territory of the Priilmenskaya Lowland. Ph.D. Thesis, Russian Research Institute of Game Management and Fur Farming, Kirov, Russia, 1998. (In Russian). [Google Scholar]
- Pollock, M.M.; Beechie, T.; Wheaton, J.; Jordan, C.; Bouwes, N.; Weber, N.; Volk, C. Using beaver dams to restore incised stream ecosystems. BioScience 2014, 64, 279–290. [Google Scholar] [CrossRef]
- Sjoberg, G.; Ball, J.P. (Eds.) Restoring the European Beaver: 50 Years of Experience; Pensoft Publisher: Sofia, Bulgaria, 2011. [Google Scholar]
- McMaster, R.T.; McMaster, N.D. Composition, structure, and dynamics of vegetation in fifteen beaver-impacted wetlands in Western Massachusetts. Rhodora 2001, 103, 293–320. [Google Scholar]
- Müller-Schwarze, D.; Sun, L. The Beaver. Natural History of a Wetlands Engineer; Cornell University Press: New York, NY, USA, 2003. [Google Scholar]
- Rosell, F.; Bozser, O.; Collen, P.; Parker, H. Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems. Mammal Rev. 2005, 35, 248–276. [Google Scholar] [CrossRef]
- Simonavičiūtė, L.; Ulevičius, A. Structure of phytocenoses in beaver meadows in Lithuania. Ekologija 2007, 53, 34–44. [Google Scholar]
- Bonner, J.; Anderson, J.; Rentch, J.; Grafton, W. Vegetative composition and community structure associated with beaver ponds in Canaan valley, West Virginia, USA. Wetl. Ecol. Manag. 2009, 17, 543–554. [Google Scholar] [CrossRef]
- Odum, E.P. Fundamentals of Ecology, 3rd ed.; W.B. Saunders Publisher: Philadelphia, PA, USA, 1971. [Google Scholar]
- Aleinikov, A.A. Castor fiber as an ecosystem engineer in «Bryanskiy Les» reserve and its protective zone. Bull. Tver State Univ. Ser. Biol. Ecol. 2010, 18, 60–68. (In Russian) [Google Scholar]
- Zavyalov, N.A. Dynamics of the Number and Environment-Forming Activity of the Beaver in the Darwin Reserve. Ph.D. Thesis, Institute of Forestry of Russian Academy of Sciences, Moscow, Russia, 1999. (In Russian). [Google Scholar]
- Albov, S.A.; Andreeva, M.V.; Bashinskiy, I.V.; Golubkov, V.V.; Katsman, E.A.; Goriaynova, Z.I.; Krylov, A.V.; Onipchenko, V.G.; Prokin, A.A.; Khlyap, L.A. European Beaver (Castor fiber L.) as a Key Species of a Small River Ecosystem (Prioksko-Terrasnyi Nature Biosphere Reserve); Association of Scientific Publications Publisher: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Oliger, T.I. Environment-forming role of the beaver in the Nizhnesvirsky Reserve. In Proceedings of the Preservation and Study of Geo- and Biodiversity in the Protected Areas of the European North of Russia, Izhevsk, Russia, 2–5 September 2014; pp. 166–170. (In Russian). [Google Scholar]
- Ustabaeva, E.V. Regional Ecological Features of the River Beaver Population with an Assessment of Its Impact on Steppe Bio-Coenoses of Orenburg Oblast. Ph.D. Thesis, Russian State Agrarian University named after Timiryazev, Moscow, Russia, 2013. (In Russian). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).