Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon
Abstract
:1. Introduction
2. Geology and Background Review of Study Area
3. Materials and Methods
3.1. Sample Collection and Preparation
3.2. Data Analysis
3.2.1. Sample Analysis
3.2.2. Estimation of Contamination Indices
Index of Geo-Accumulation of Heavy Metals
Contamination Factor (CF)
Pollution Load Index (PLI)
Ecological Risk Factor (Eri)
- Eri < 40, low potential ecological risk;
- 40 ≤ Eri < 80, moderate potential ecological risk;
- 80 ≤ Eri < 160, considerable potential ecological risk;
- 160 ≤ Eri < 320, high potential ecological risk;
- Eri ≥ 320, very high ecological risk.
Potential Ecological Risk (RI)
Enrichment Factor of Heavy Metals
Single Pollution Index (Pi)
4. Results
4.1. Soil Chemical Characterization
4.2. Estimation of Contamination Indices
4.2.1. Geo-Accumulation Index (Igeo)
4.2.2. Pollution Load Index (PLI) and Ecological Risk Index (RI)
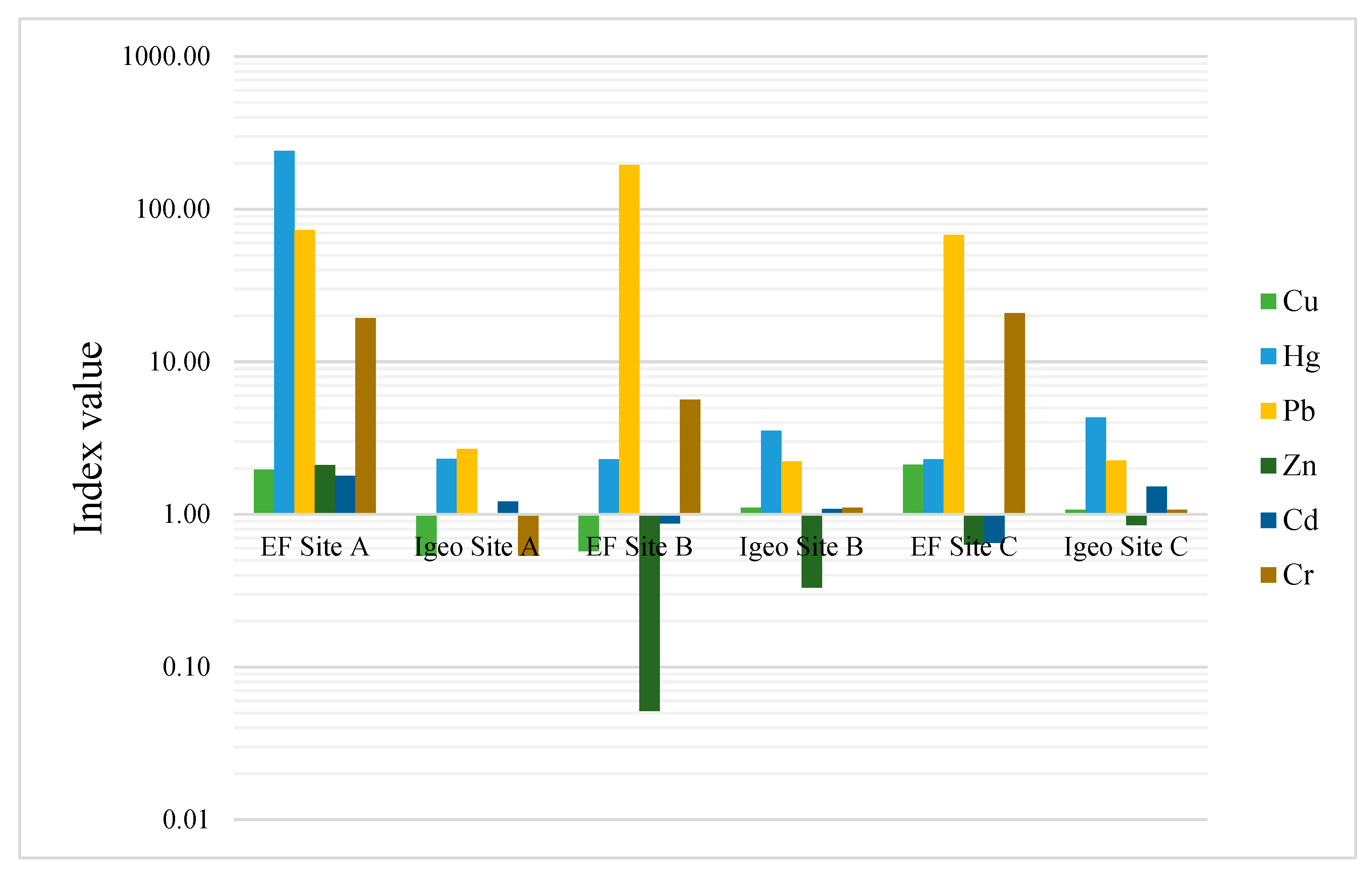
4.2.3. Enrichment Factor (EF) and Single Pollution Index (PI)
5. Discussion
5.1. Heavy Metal Concentration in the Pawara Soils
5.2. Petrogenetic Significance
| Abandoned Mines in the World (U.V: Undetermined Value) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pb (mg kg−1) | Cd (mg kg−1) | Cu (mg kg−1) | Zn (mg kg−1) | Cr (mg kg−1) | Site | Target | Reference | |
| Cameroon | 12,274 | 12.2 | 33.03 | 10.39 | 171 | Pawara | Au | This Work |
| Morocco | 9527.6 | 24.1 | 29.1 | 147.56 | 121.94 | Tourtit and Ichoumellal Mine | Sb | [71] |
| Tunisia | 10,599 | 43 | 90 | 21,353 | 1147 | Oued Mellegue | Pb-Zn-Ba | [64] |
| Zambia | 11 | 0.09 | 5254 | 35 | 23 | Kafue River | Pb-Zn-Co | [72] |
| Italy | 2541.5 | 8.75 | 465.8 | 1792.8 | u.v | Boccheggiano | Cu | [73] |
| China | 429 | 6.42 | 1486 | 2516 | u.v | Guangdong Province | Polymeta-llic sulfides | [66] |
| Nigeria | 0.22 | 0.13 | 0.63 | 0.70 | 0.15 | Southwestern zone | Au | [74,75] |
| Morocco background | 9.81 | 1 | 6.78 | 9.43 | 45.25 | - | [71] | |
| Upper continental crust | 17 | 0.1 | 14 | 79 | 140 | - | [67] | |
| Sampling Sites | Hg Levels (mg kg−1) | Reference |
|---|---|---|
| Southwestern Amazon Basin (Brazil) | 0.122 | [76] |
| Talawan Watershed (Indonesia) | 6.820 | [77] |
| Mindanao Island (Philippines) | 21.03 | [78] |
| Sansu (Japan) | 2.600 | [79] |
| Canadian reference value | 6.6 | [72] |
| Pawara Site B (Cameroon) | 15.21 | This work |
| Pawara Site A (Cameroon) | 1590 | This work |
| Pawara Site C (Cameroon) | 261.9 | This work |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.G.; Xu, Y.N.; Zhang, J.H.; Hu, S.H. Evaluation of ecological risk and primary empirical research on heavy metals in polluted soil over Xiaoqinling gold mining region, Shaanxi, China. Trans. Nonferrous Met. Soc. China 2010, 20, 688–694. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Cui, L.J.; Sheng, L.X.; Wang, L.F. Distribution and enrichment of heavy metals among sediments, water body and plants in Hengshuihu Wetland of Northern China. Ecol. Eng. 2009, 35, 563–569. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Van der Kuijp, T.J.; Yuan, Z.; Lei, H. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Sharma, R.K.; Agrawal, M.; Marshall, F.M. Heavy metals contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Pollut. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Wang, M.W.; Zhu, H.W.; Guo, Z.H.; Han, X.Q.; Zeng, P. Response of soil microbial activities and microbial community structure to vanadium stress. Ecotoxicol. Environ. Saf. 2017, 142, 200–206. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.H.; Yang, J.C.; Liu, H.; Dai, J.L. Health risk to residents and stimulation to inherent bacteria of various heavy metals in soil. Sci. Total Environ. 2015, 508, 29–36. [Google Scholar] [CrossRef]
- Lei, P.; Zhang, H.; Boaqing, S.; Lv, S.; Tang, W. Heavy metals in estuarine surface sediments of the Hai River Basin, variation characteristics, chemical speciation and ecological risk. Environ. Sci. Pollut. Res. 2016, 23, 7869–7879. [Google Scholar] [CrossRef]
- Feng, H.; Han, X.; Zhang, W.; Yu, L. A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar. Pollut. Bull. 2004, 49, 910–915. [Google Scholar] [CrossRef]
- Banza, C.L.N.; Nawrot, T.S.; Haufroid, V.; Decrée, S.; De Putter, T.; Smolders, E.; Kabyla, B.I.; Luboya, O.N.; Ilunga, A.N.; Mutombo, A.M.; et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009, 109, 745–752. [Google Scholar] [CrossRef]
- Velasquez-Lopez, P.C.; Veiga, M.M.; Hall, K. Mercury balance in amalgamation in artisanal and small scale gold mining: Identifying strategies for reducing environmental pollution in Portovelo-Zaruma, Ecuador. J. Clean. Prod. 2010, 18, 226–232. [Google Scholar] [CrossRef]
- Tarras-Wahlberg, N. Environmental management of small-scale and artisanal mining: The Portovelo-Zaruma gold mining area, southern Ecuador. J. Environ. Manag. 2002, 65, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.Y.; Wang, W.X. Metal accumulation in the green macroalgae Ulva fasciata: Effect of nitrate, ammonium and phosphate. Sci. Total Environ. 2001, 213, 273–277. [Google Scholar]
- Navarro, M.; Pérez-Sirvent, C.; Martínez-Sánchez, M.; Vidal, J.; Tovar, P.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils-Trace Metals and Metalloids in Soils and Their Bioavailability; Dordrecht. Environmental Pollution; Springer Science Business Media: Berlin, Germany, 2013; Volume 22. [Google Scholar] [CrossRef]
- Toteu, S.F.; Penaye, J.; Deloule, E.; Van Schmus, W.R.; Tchameni, R. Diachronous evolution of volcano-sedimentary basins North of the Congo craton: Insights from U-Pb ion microprobe dating of zircons from the Poli, Lom and Yaounde’ Groups (Cameroon). J. Afr. Earth Sci. 2006, 44, 428–442. [Google Scholar] [CrossRef]
- Toteu, S.F.; Penaye, J.; Poudjom Djomani, Y. Geodynamic evolution of the Pan-African belt in central Africa with special reference to Cameroon. Can. J. Earth Sci. 2004, 41, 73–85. [Google Scholar] [CrossRef]
- Toteu, S.F.; Van Schmus, W.R.; Penaye, J.; Michard, A. New U-Pb and Sm-Nd data north-central Cameroon and its bearing on the pre-Pan African history of central Africa. Precambrian Res. 2001, 108, 45–73. [Google Scholar] [CrossRef]
- Van Schmus, W.R.; Oliveira, E.P.; Da Silva Filho, A.F.; Toteu, S.F.; Penaye, J.; Guimaraes, I.P. The Central African Fold Belt Proterozoic Links between the Borborema Province, NE Brazil, and the Central African Fold Belt. Special Publications. Geol. Soc. Lond. 2008, 294, 69–99. [Google Scholar] [CrossRef]
- Tchameni, R.; Pouclet, A.; Penaye, J.; Ganwa, A.A.; Toteu, S.F. Petrography and geochemistry of the Ngaoundere Pan-African granitoids in Central North Cameroon: Implications for their sources and geological setting. J. Afr. Earth Sci. 2006, 44, 511–529. [Google Scholar] [CrossRef] [Green Version]
- Tchakounté, J.; Eglinger, A.; Toteu, S.F.; Zeh, A.; Nkoumbou, C.; Mvondo-Ondoa, J.; Penaye, J.; De Wit, M.; Barbe, M. The Adamawa-Yadé domain, a piece of Archaean crust in the Neoproterozoic central african orogenic belt (Bafia area, Cameroon). Precambrian Res. 2017, 299, 210–229. [Google Scholar] [CrossRef]
- Milési, J.P.; Toteu, S.F.; Deschamps, Y.; Feybesse, J.L.; Lerouge, C.; Cocherie, A.; Penaye, J.; Tchameni, R.; Moloto-A-Kenguemba, G.; Kampunzu, H.A.B.; et al. An overview of the geology and major ore deposits of Central Africa: Explanatory note for the 1:4,000,000 map “Geology and major ore deposits of Central Africa”. J. Afr. Earth Sci. 2006, 44, 571–595. [Google Scholar] [CrossRef]
- Soba, D. La série du Lom: Étude géologique et géochronologique d’un bassin volcano-sédimentaire de la chaïne panafricaine à l’Est du Cameroun. Thése De Dr. D’etat Univ. Pierre Et Marie Curie Paris 1989, 6, 198. [Google Scholar]
- Ngako, V.; Affaton, P.; Nnange, J.M.; Njanko, T. Pan-African tectonic evolution in central and southern Cameroon: Transpression and transtension during sinistral shear movements. J. Afr. Earth Sci. 2003, 36, 207–214. [Google Scholar] [CrossRef]
- Ngangom, E. Etude tectonique du fossé crétacé de la Mbéré et du Djérem, Sud-Adamaoua, Cameroun. Bull. Du Cent. Des Rech. Des Explor. Et Prod. Elf Aquitaine 1983, 7, 339–347. [Google Scholar]
- Ndonfack, K.I.A.; Xie, Y.; Goldfar, R.; Zhong, R.; Qu, Y. Genesis and mineralization style of gold occurrences of the Lower Lom Belt, B’etar’e Oya district, eastern Cameroon. Ore Geol. Rev. 2021, 139, 104586. [Google Scholar] [CrossRef]
- Vishit, A.; Suh, C.E.; Lehmann, B.; Shemang, E.M.; Ngome, N.L.J.; Nshanji, N.J.; Chinjo, F.E.; Mongwe, O.Y.; Egbe, A.J.; Petersen, S.; et al. Mineral chemistry, bulk rock geochemistry, and S-isotope signature of lode-gold mineralization in the B’etar’e Oya gold district, south-east Cameroon. Geol. J. 2017, 53, 2579–2596. [Google Scholar] [CrossRef]
- Suh, C.E.; Lehmann, B. Morphology and Electron-probe microanalysis of residual gold-grain at Dimako, south east Cameroon. Neues Jabrb. Mineral. Mon. 2003, 6, 225–275. [Google Scholar]
- Maurya, A.; Kesharwani, L.; Mishra, M.K. Analysis of Heavy Metal in Soil through Atomic Absorption Spectroscopy for Forensic Consideration. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 1188–1192. Available online: www.ijraset.com (accessed on 23 March 2022). [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. Geol. J. 1979, 2, 108–118. [Google Scholar]
- Loska, K.; Wiechulab, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Nowrouzi, M.; Pourkhabbaz, A. Application of Geoaccumulation Index and Enrichment Factor for Assessing Metal Contamination in the Sediments of Hara Biosphere Reserve, Iran. Chem. Special. Bioavailable 2014, 26, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Ogundele, L.T.; Adejoro, I.A.; Ayeku, P.O. Health risk assessment of heavy metals in soil samples from an abandoned Industrial waste dumpsite in Ibadan, Nigeria. Environ. Monit. Assess 2019, 191, 290. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Biasioli, M.; Barberis, R.; Ajmone-Marsan, F. The influence of a large city on some soil properties and metals content. Sci. Total Environ. 2006, 356, 154–164. [Google Scholar] [CrossRef]
- Biasioli, M.; Ajmone-Marsan, F. Organic and inorganic diffuse contamination in urban soils: The case of Torino (Italy). J. Environ. Monit. 2007, 9, 862–868. [Google Scholar] [CrossRef]
- Ajmone-Marsan, F.; Biasioli, M. Trace elements in soils of urban areas. Water Air Soil Pollut. 2010, 213, 121–143. [Google Scholar] [CrossRef]
- Rahman, M.S.; Saha, N.; Molla, A.H. Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ. Earth Sci. 2014, 71, 2293–2308. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, J.S.; Chin, H.T.; Sager, M. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au-Ag mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- Adlane, B.; Xu, Z.; Xu, X.; Liang, L.; Han, J.; Qiu, G. Evaluation of the potential risks of heavy metal contamination in rice paddy soils around an abandoned Hg mine area in Southwest China. Acta Geochim. 2020, 39, 85–95. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; pp. 1–312. [Google Scholar]
- Karbassi, A.R.; Nabi-Bidhendi, G.R.; Biati, A. Environmental geochemistry of heavy metals in a sediment core off Bushehr, Persian Gulf. Iran. J. Environ. Health Sci. Eng. 2005, 2, 255–260. [Google Scholar]
- Tasdemir, Y.; Kural, C. Atmospheric dry deposition fluxes of trace elements measured in Bursa, Turkey. Environ. Pollut. 2005, 138, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Szolnoki, Z.; Farsang, A.; Puskas, I. Cumulative impacts of human activities on urban garden soils: Origin and accumulation of metals. Environ. Pollut. 2003, 177, 106–115. [Google Scholar] [CrossRef]
- Liang, J.; Chen, C.; Song, X.; Han, Y.; Liand, Z. Assessment of Heavy Metal Pollution in Soil and Plant from Dunhua Sewage Irrigation Area. Int. J. Electrochem. Sci. 2011, 6, 5314–5324. [Google Scholar]
- Gong, Q.; Jun, G.; Yunchuan, X.; Qingfei, W.; Liqian, Y. Calculating Pollution Indices by Heavy Metals in Ecological Geochemistry Assessment and a Case Study in Parks of Beijing. J. China Univ. Geosci. 2008, 19, 230–241. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Canadian Council of Ministers of the Environment. Canadian Council of Ministers of the Environment. Canadian soil quality guidelines for the protection of environmental and human health: Summary tables. Updated. In Canadian Environmental Quality Guidelines, 1999; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2001. [Google Scholar]
- Bhuiyan, M.A.H.; Parvez, L.; Islam, M.A.; Dampare Samuel, B.; Suzuki, S. Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J. Hazard. Mater. 2010, 173, 384–392. [Google Scholar] [CrossRef]
- Félix, O.I.; Csavina, J.; Field, J.; Rine, K.P.; Sáez, A.E.; Betterton, E.A. Use of lead isotopes to identify sources of metal and metalloid contaminants in atmospheric aerosol from mining operations. Chemosphere 2015, 122, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Ona, L.; Alberto, A.; Prudente, J.A. Levels of lead in urban soils from selected cities in a central region of the Philippines. Environ. Sci. Pollut. Res. Int. 2006, 1, 177–183. [Google Scholar] [CrossRef]
- Mico, C.; Recatala, L.; Peris, M.; Sánchez, J. Assessing heavy metalsources in agricultural soils of a European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef]
- Singh, H.; Pandey, R.; Singh, S.K. Assessment of heavy metal contamination in the sediment of the River Ghaghara, a major tributary of the River Ganga in Northern India. Appl. Water Sci. 2017, 7, 4133–4149. [Google Scholar] [CrossRef]
- Wen, Q.; Zhao, Y.; Cao, W. Distribution characteristics, sources and potential ecological risks of heavy metal pollution in the middle reaches of Chaobai River. Res. Environ. Sci. 2020, 33, 599–607. [Google Scholar]
- Yua, H.; Zhang, Z.; Zhang, Y.; Fan, P.; Xia, B.; Tan, W. Metal type and aggregate microenvironment govern the response sequence of speciation transformation of different heavy metals to microplastics in soil. Sci. Total Environ. 2021, 752, 141956. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.X. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- Martinez, C.E.; Motto, H.L.X. Solubility of lead, zinc and copper added to mineral soils. Environ. Pollut. 2005, 170, 153–158. [Google Scholar] [CrossRef]
- Campbell, P.G.; Cadmium, A. priority pollutant. Environ. Chem. 2006, 3, 387–388. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 20, 402647. [Google Scholar] [CrossRef] [Green Version]
- Ahmedat, C.; Dabi, S.; Zahraoui, M.; Iz-Eddine, E.A.E.H. Spatial distribution of stream sediment pollution by toxic trace elements at Tourtit and Ichoumellal abandoned mining areas (central Morocco). Arab. J. Geosci. 2018, 11, 55. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Islam, S.; Rahman, M.S.; Haque, M.R.; Islam, M.M. Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int. J. Environ. Resour. 2010, 4, 321–332. [Google Scholar]
- Mlayah, A.; Da Silva, E.F.; Rocha, F. The OuedMellègue: Mining activity, stream sediments and dispersion of base metals in natural environments, Northwestern Tunisia. J. Geochem. Explor. 2009, 102, 27–36. [Google Scholar] [CrossRef]
- Benvenuti, M.; Mascaro, I.; Corsini, F.; Lattani, P.; Parrini, P.; Tanelli, G. Mine waste dumps and heavy metal pollution in abandoned mining district of Boccheggiano (Southern Tuscany, Italy). Environ. Geol. 1997, 30, 238–243. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhi, D.; Cai, M.F. Soil heavy metal pollution around the Dabaoshan mine, Guangdong province, China. Pedosphere 2007, 17, 588–594. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. The Composition of the Continental Crust. In Treatise Geochem; Holl, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, the Netherlands, 2003; Volume 3, pp. 1–64. [Google Scholar]
- Smith, L.A.; Means, J.L.; Chen, A. Remedial Options for Metals-Contaminated Sites; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Yannah, M.; Suh, C.E.; Mboudou, M.G.M. Quartz veins characteristics and Au Mineralization within the Batouri Au District, East Cameroon. Sci. Res. 2015, 3, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Ngako, V.; Affaton, P.; Njonfang, E. Pan-African Tectonics in North western Cameroon: Implication for History of Western Gondwana. Gondwana Res. 2008, 14, 509–522. [Google Scholar] [CrossRef]
- Abbaslou, H.; Abtahi, A.; Baghernejad, M. Effect of weathering and mineralogy on the distribution of major and trace elements (Hormozgan province, Southern Iran). Int. J. For. Soil Eros. 2013, 3, 15–25. [Google Scholar]
- Ikenaka, Y.; Nakayama, S.M.M.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Heavy metal contamination of soil and sediment in Zambia. Afr. J. Environ. Sci. Technol. 2010, 4, 729–739. [Google Scholar]
- Mungai, T.M.; Wang, J. Heavy metal pollution in suburban topsoil of Nyeri, Kapsabet, Voi, Ngong and Juja towns, in Kenya. SN Appl. Sci. 2019, 1, 960. [Google Scholar] [CrossRef] [Green Version]
- Adewumi, A.J.; Laniyan, T.A. Contamination, sources and risk assessments of metals in media from Anka artisanal gold mining area, Northwest Nigeria. Sci. Total Environ. 2020, 718, 137235. [Google Scholar] [CrossRef]
- Abiya, S.E.; Odiyi, B.O.; Ologundudu, F.A.; Akinnifesi, O.J.; Akadiri, S. Assessment of Heavy Metal Pollution in a Gold Mining Site in Southwestern Nigeria. J. Genet. Cell Biol. JGCB 2018, 1, 30–35. [Google Scholar]
- Limbong, D.; Kumampung, J.; Ayhuan, D.; Arai, T.; Miyazaki, N. Mercury Pollution Related to Artisanal Gold Mining in North Sulawesi Island, Indonesia. Bull. Environmental Contam. Toxicol. 2003, 75, 989–996. [Google Scholar] [CrossRef]
- Aspinall, C. Small-Scale Mining in Indonesia; International Institute for Environment and Development: London, UK, 2001; Available online: http://pubs.iied.org/pdfs/G00725.pdf (accessed on 3 February 2022).
- Drasch, G.; Boese, S.; Beinhoff, C.; Roider, G.; Maydl, S. Assessing mercury intoxication of the population by small scale gold mining. Sci. Total Environ. 1999, 267, 151–168. [Google Scholar] [CrossRef]
- Macklin, M.K.; Rumsby, B.T.; Heap, T. Flood alluviation and entrenchment: Holocene valley-floor development and transformation in the British uplands. GSA Bull. 1992, 104, 631–643. [Google Scholar] [CrossRef]
- Ngako, V. Les déformations Continentales Panafricaines en Afrique Centrale: Résultat d’un Poinconnement de Type Himalayen. Ph.D. Thesis., Université de Yaoundé I, Yaoundé, Cameroon, 1999; 301p. [Google Scholar]
- Ganwa, A.A.; Wolfgang, S.; Wolfgang, F.; Shang, C.K. Geochemistry of magmatic rocks and time constraints on deformational phases and shear zone slip in the Méiganga area, central Cameroon. Int. Geol. Rev. 2011, 53, 759–784. [Google Scholar] [CrossRef]
- Tchaptchet Tchato, D.P.; Tchakounte, J.; Kamwa, A.N.; Tchouankoue, J.P.; Mukherjee, S. Geometry and kinematics of brittle deformation in the Central Cameroon Shear Zone (Kékem area): Implication for gold exploration within the Central Africa Fold Belt in Cameroon. China Geol. 2021, 4, 245–255. [Google Scholar] [CrossRef]
- Ngancha, B.; Suh, C.E.; Kah, F.; Shemang, E.; Mpelane, S. Base metals-enriched gold-quartz veins in the eastern Cameroon goldfield, West-Central Africa. Episodes 2021. [Google Scholar] [CrossRef]
- Kankeu, B.; Greiling, R.O.; Nzenti, J.P. Pan-African strike–Slip tectonics in eastern Cameroon-Magnetic fabrics (AMS) and structure in the Lom basin and its gneissic basement. Precambrian Res. 2009, 174, 258–272. [Google Scholar] [CrossRef]
- Asaah, A.V.; Zoheir, B.; Lehmann, B.; Frei, D.; Burgess, R.; Suh, C.E. Geochemistry and geochronology of the ~620 Ma gold-associated Batouri granitoids, Cameroon. Int. Geol. Rev. 2015, 57, 1485–1509. [Google Scholar] [CrossRef]
- Tata, E.; Suh, C.E.; Vishiti, A.; Shemang, E.M.; Fon, A.N.; Ateh, K.I.; Chombong, N.N. Wallrock alteration categories and their geochemical signatures in gold-bearing Neoproterozoic granitoids, Batouri gold district, southeastern Cameroon. Geochem. Explor. Environ. Anal. 2018, 19, 269–288. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Weijden, C.H.V.; Woittiez, J.R.W. Chemical processes affecting the mobility of major, minor and trace elements during weathering of granitic rocks. Chem. Geol. 1988, 68, 253–273. [Google Scholar] [CrossRef]
- Colmel-Daage, E.; Lagache, P. Caractéristiques de quelques groupes de sols dérivés de roches volcaniques aux Antilles françaises. Cah. ORSTOM. Série Pédologie 1965, 3, 91–121. [Google Scholar]
- Sarkar, B.; Naidu, R.; Megharaj, M. Simultaneous adsorption of tri- and hexavalent 685 chromium by organoclay mixtures. Water Air Soil Pollut. 2013, 224, 1704. [Google Scholar] [CrossRef]
- Sababa, E.; Owona, L.G.E.; Temga, J.P.; Ndjigui, P.D. Petrology of weathering materials developed on granites in Biou area, North-Cameroon: Implication for rare-earth elements (REE) exploration in semi-arid regions. Heliyon 2021, 7, e08581. [Google Scholar] [CrossRef] [PubMed]
- Penaye, J.; Toteu, S.F.; Van Schmus, W.R.; Tchakounté, J.; Ganwa, A.; Minyem, D.; Nsifa, E.N. The 2.1-Ga West Central African Belt in Cameroon: Extension and evolution. J. Afr. Earth Sci. 2004, 39, 159–164. [Google Scholar] [CrossRef]
- Ganwa, A.A.; Klotzli, U.S.; Hauzenberger, C. Evidence for Archean inheritance in the pre-Panafrican crust of Central Cameroon: Insight from zircon internal structure and LA-MC-ICP-MS U-Pb ages. J. Afr. Earth Scences 2016, 120, 12–22. [Google Scholar] [CrossRef]
- Bouyo, H.M.; Penaye, P.; Barbey, P.; Toteu, S.F.; Wandji, P. Petrology of high-pressure granulite facies metapelites and metabasites from Tcholliré and Banyo regions: Geodynamic implication for the Central African Fold Belt (CAFB) of northcentral Cameroon. Precambrian Res. 2013, 224, 412–433. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Theologou, E.; Bompoti, N.; Dermatas, D.; Panagiotakis, I. Occurrence, Origin and Transformation Processes of Geogenic Chromium in Soils and Sediments. Curr. Pollut. Rep. 2016, 2, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.P.; Ye, X.; Feng, H.; Jing, Y.; Ouyang, T.; Yu, X.; Liang, R.; Gao, C.; Chen, W. Heavy metal contamination in Western Xiamen bay sediments and Its Vicinity, China. Mar. Pollut. Bull. 2007, 54, 974–982. [Google Scholar] [CrossRef]
- Inengite, A.K.; Abasi, C.Y.; Walter, C. Application of pollution indices for the assessment of heavy metal pollution in flood impacted soil. Int. Res. J. Pure Appl. Chem. 2015, 8, 175–189. [Google Scholar] [CrossRef]
- Yeboah, I.B.; Tuffour, H.P.; Abubakari, A.; Melenya, C.; Bonsu, M.; Quansah, C.; Adjei-Gyapong, T. Mobility and transport behavior of lead in agricultural soils. Sci. Afr. 2019, 5, e00117. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gasiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination–A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, M.; Nigro, A.; Sappa, G. Soil contamination evaluation by Enrichment Factor (EF) and Geoaccumulation Index (Igeo). Senses Sci. 2015, 2, 94–97. [Google Scholar]
- Adebola, B.A.K.; Kayode, S.J.; Akeem, O.A. Integrated assessment of the heavy metal pollution status and potential ecological risk in the Lagos Lagoon, South West, Nigeria. Hum. Ecol. Risk Assess. 2017, 24, 377–397. [Google Scholar] [CrossRef]
- Hu, B.; Jia, X.; Hu, J.; Xu, D.; Xia, F.; Li, Y. Assessment of Heavy Metal Pollution and Health Risks in the Soil-Plant-Human System in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health 2017, 14, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimoh, A.; Agbaji, E.B.; Ajibola, V.O.; Funtua, M.A. Application of Pollution Load Indices, Enrichment Factors, Contamination Factor and Health Risk Assessment of Heavy Metals Pollution of Soils of Welding Workshops at Old Panteka Market, Kaduna-Nigeria. Open J. Anal. Bioanal. Chem. 2020, 4, 11–19. [Google Scholar] [CrossRef]

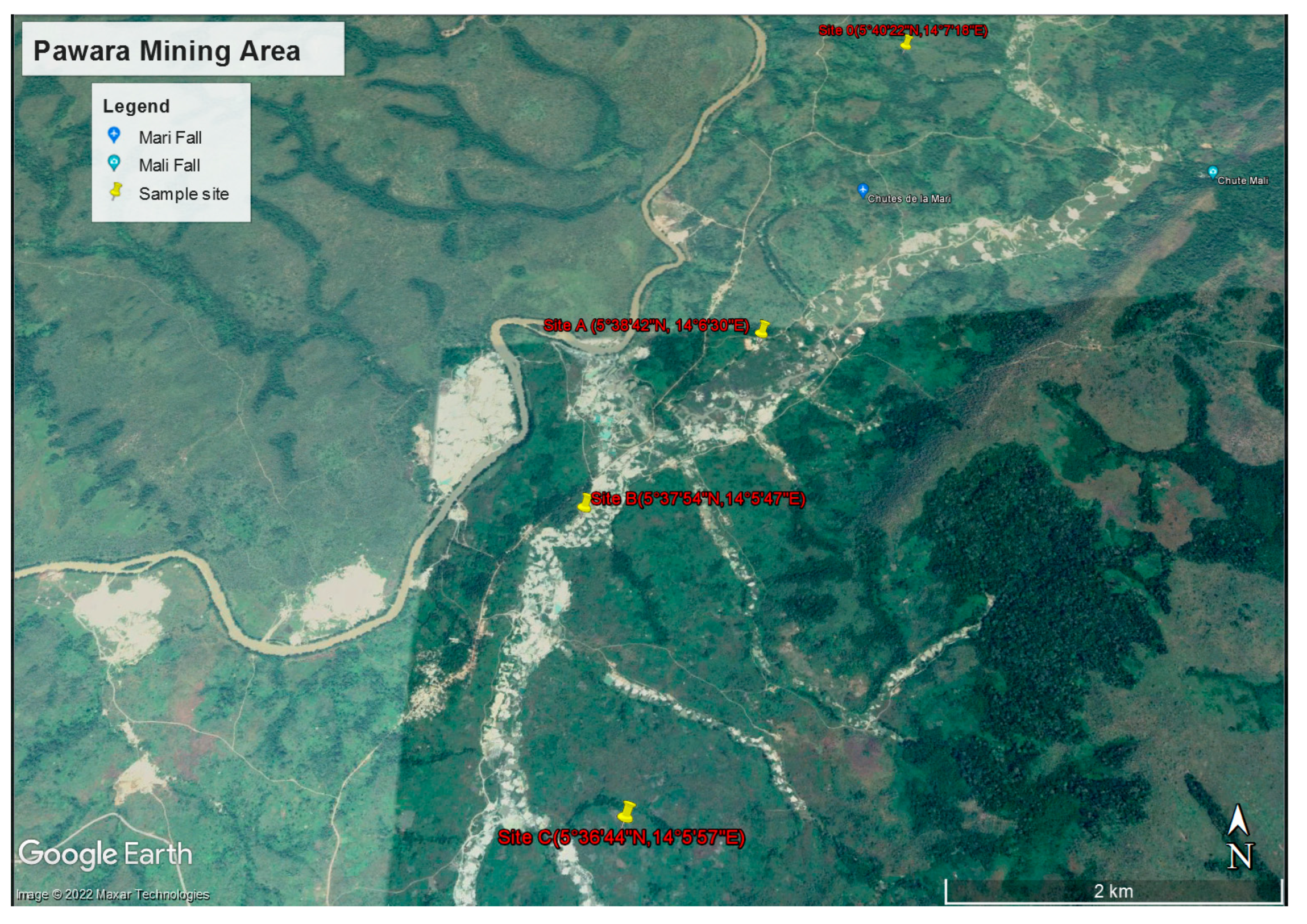
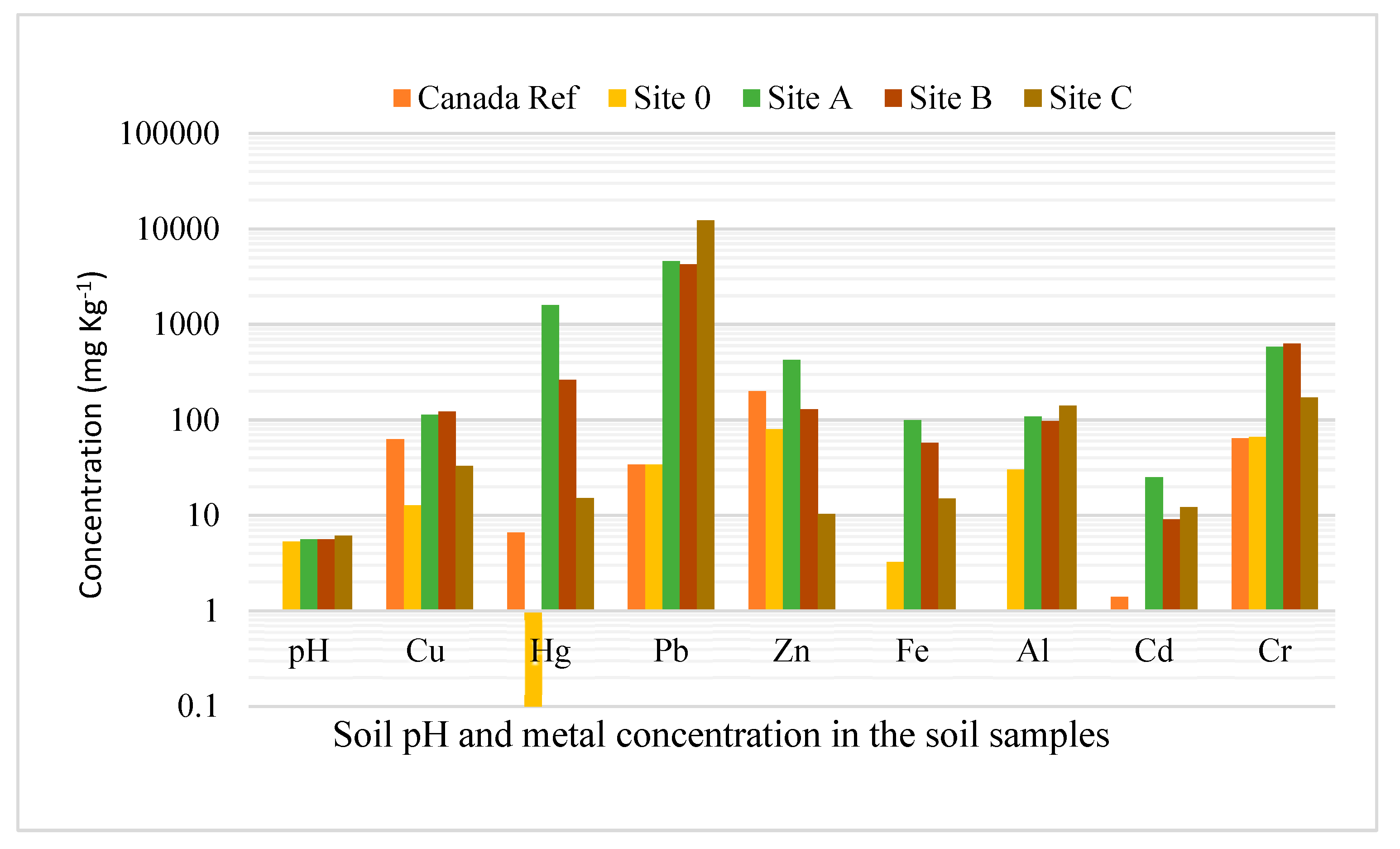
| World Reference Values | Canadian Soil Value References [50] | Control Site | Site B | Site C | Site A | |
|---|---|---|---|---|---|---|
| pH | - | 6–8 | 5.3 | 6.1 | 5.6 | 5.6 |
| Cu (mg kg−1) | 14 | 63 | 12.8 | 33.03 | 122.27 | 113.2 |
| Hg (mg kg−1) | 0.1 | 6.6 | 0.1 | 15.21 | 261.9 | 1590 |
| Pb (mg kg−1) | 25 | 34.1 | 12274 | 4265 | 4607 | |
| Zn (mg kg−1) | 62 | 200 | 80 | 10.39 | 128.7 | 427.8 |
| Fe (mg kg−1) | - | - | 3.24 | 15.03 | 57.55 | 99.66 |
| Al (mg kg−1) | - | - | 30.15 | 140.4 | 97.2 | 108 |
| Cd (mg kg−1) | 1.1 | 1.4 | 1 | 12.2 | 9.09 | 25 |
| Cr (mg kg−1) | 42 | 64 | 66.3 | 171 | 633.6 | 585.4 |
| Metal | Control Site 0 | Igeo Site A | Igeo Site B | Igeo Site C |
|---|---|---|---|---|
| Cu | 12.8 | 0.54 | 1.072 | 1.11 |
| Hg | 0.1 | 2.31 | 4.33 | 3.54 |
| Pb | 34.1 | 2.68 | 2.26 | 2.22 |
| Zn | 80 | −0.76 | 0.85 | 0.33 |
| Fe | 3.24 | 0.79 | 1.61 | 1.37 |
| Al | 30.15 | 0.79 | 0.68 | 0.63 |
| Cd | 1 | 1.21 | 1.52 | 1.08 |
| Cr | 66.3 | 0.54 | 1.07 | 1.11 |
| Metal | CF Site A | CF Site B | CF Site C | Ti | Eri Site A | Eri Site B | Eri Site C |
|---|---|---|---|---|---|---|---|
| Cu | 8.8 | 2.6 | 9.6 | 5 | 64 | 12.9 | 47.8 |
| Pb | 15,900 | 152.1 | 152.1 | 5 | 170.5 | 1799.7 | 625.4 |
| Zn | 146.7 | 390.9 | 135.8 | 1 | 80 | 0.1 | 1.6 |
| Hg | 5.4 | 0.13 | 1.6 | 40 | 4 | 6084 | 104,760 |
| Cd | 25 | 12.2 | 9.1 | 10 | 10 | 122 | 90.9 |
| Metal | EF Site A | EF Site B | EF Site C | Pi Site A | Pi Site B | Pi Site C |
|---|---|---|---|---|---|---|
| Cu (mg kg−1) | 1.8 | 0.6 | 2.1 | 8.84 | 2.58 | 9.55 |
| Hg (mg kg−1) | 240.9 | 2.3 | 2.3 | 15,900 | 152.1 | 2619 |
| Pb (mg kg−1) | 73.1 | 194.9 | 67.7 | 135.10 | 359.94 | 125.07 |
| Zn (mg kg−1) | 2.1 | 0.1 | 0.6 | 5.35 | 0.13 | 1.61 |
| Fe (mg kg−1) | - | - | - | 30.76 | 4.64 | 17.76 |
| Al (mg kg−1) | - | - | - | 3.58 | 4.66 | 3.22 |
| Cd (mg kg−1) | 1.8 | 0.9 | 0.6 | 25 | 12.2 | 9.09 |
| Cr (mg kg−1) | 19.3 | 5.6 | 20.9 | 8.83 | 2.58 | 9.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodoué, Y.; Ismaila, A.; Yannah, M.; Wirmvem, M.J.; Mana, C.B. Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon. Earth 2022, 3, 907-924. https://doi.org/10.3390/earth3030053
Fodoué Y, Ismaila A, Yannah M, Wirmvem MJ, Mana CB. Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon. Earth. 2022; 3(3):907-924. https://doi.org/10.3390/earth3030053
Chicago/Turabian StyleFodoué, Yaya, Ahmadou Ismaila, Mero Yannah, Mengnjo Jude Wirmvem, and Christian Bouba Mana. 2022. "Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon" Earth 3, no. 3: 907-924. https://doi.org/10.3390/earth3030053
APA StyleFodoué, Y., Ismaila, A., Yannah, M., Wirmvem, M. J., & Mana, C. B. (2022). Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon. Earth, 3(3), 907-924. https://doi.org/10.3390/earth3030053






