Earthworm Abundance Increased by Mob-Grazing Zero-Tilled Arable Land in South-East England
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Field Treatments
- 1.
- zero tillage + grass-clover ley (ZT)
- 2.
- zero tillage + grass-clover ley + mob-grazing (ZTMG)
- 3.
- permanent grassland (PG)
2.3. Earthworm Sampling
2.4. Soil Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Cultivation Legacy
4.2. Crop Diversification: Grass-Clover Ley
4.3. Livestock and Mob-Grazing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. Conservation Agriculture Factsheet. In Three Principles of Conservation Agriculture; CB8350EN/1/03.22—Revised Version; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- Brown, G. Dirt to Soil: One Family’s Journey into Regenerative Agriculture; Chelsea Green Publishing: Hartford, VT, USA, 2018. [Google Scholar]
- Montgomery, D.R. Growing a Revolution: Bringing Our Soil Back to Life; W. W. Norton & Company Inc.: London, UK, 2017. [Google Scholar]
- Teague, W.R.; Apfelbaum, R.; Lal, R.; Kreuter, J.; Rowntree, J.; Davies, C.A.; Conser, R.; Rasmussen, M.; Hatfield, J.; Wang, T.; et al. The role of ruminants in reducing agriculture’s carbon footprint in North America. J. Soil Water Cons. 2016, 71, 156–164. [Google Scholar] [CrossRef]
- Earl, J.M.; Jones, C.E. The need for a new approach to grazing management-is cell grazing the answer? Rangel. J. 1996, 18, 327–350. [Google Scholar] [CrossRef]
- Humerickhouse, N. Productivity and Quality of Smooth Brome Pastures under Continuous, Rotational, and Mob Grazing by Sheep. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2014. [Google Scholar]
- Tracy, B.F.; Bauer, R.B. Evaluating mob stocking for beef cattle in a temperate grassland. PLoS ONE 2019, 14, e0226360. [Google Scholar] [CrossRef] [PubMed]
- Battini, F.; Pecetti, L.; Romani, M.; Annicchiarico, P.; Ligabue, M.; Piano, E. Persistence of lucerne cultivars under grazing in organic farms of northern and central Italy. In Breeding and Seed Production for Conventional and Organic Agriculture, Proceedings of the XXVI Meeting of the EUCARPIA Fodder Crops and Amenity Grasses Section, XVI Meeting of the European Association for Research on Plant Breeding (EUCARPIA) Medicago spp . group, Perugia, Italy, 2–7 September 2006; European Association for Research on Plant Breeding (EUCARPIA): Wageningen, The Netherlands, 2007; pp. 145–149. [Google Scholar]
- AHDB—Agriculture and Horticulture Development Board. Planning Grazing Strategies for Better Returns; Stoneleigh Park: Warwickshire, UK, 2018. [Google Scholar]
- Soil Association. What Is Mob Grazing? Available online: https://www.soilassociation.org/our-work-in-scotland/scotland-farming-programmes/mob-grazing/what-is-mob-grazing/ (accessed on 20 January 2021).
- Gordon, K. Mastering Mob Grazing. ANGUS J. August 2010, 80–81. [Google Scholar]
- Gompert, T. The power of stock density. In Proceedings of the Grazing Lands Conservation Initiative’s 4th National Conference on Grazing Lands, Sparks, NV, USA, 13–16 December 2009. [Google Scholar]
- Defra—Department for Environment, Food and Rural Affairs. Agriculture in the United Kingdom 2021. Annual Statistics about Agriculture in the United Kingdom. Available online: https://www.gov.uk/government/collections/agriculture-in-the-united-kingdom#agriculture-in-the-united-kingdom-2021 (accessed on 29 June 2022).
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, V.; Roger, P.; Ineson, P.O.W.H.; Dhillion, S. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.J.; Brown, G.G.; De Deyn, G.B.; van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G.; Doube, B.M.; Edwards, C.A. Functional interactions between earthworms, microorganisms, organic matter, and plants. Earthworm Ecol. 2004, 2, 213–239. [Google Scholar]
- Briones, M.J.; Schmidt, O. Conventional tillage decreases the abundance and biomass of earthworms and alters the community structure in a global meta-analysis. Glob. Change Biol. 2017, 23, 4396–4419. [Google Scholar] [CrossRef]
- Chan, K.Y. An overview of some tillage impacts on earthworm population abundance and diversity—Implications for functioning in soils. Soil Tillage Res. 2001, 57, 179–191. [Google Scholar] [CrossRef]
- Edwards, C.A.; Lofty, J.R. The influence of invertebrates on root growth of crops with minimal or zero cultivation. Ecol. Bull. 1977, 25, 348–356. [Google Scholar]
- Stroud, J.; Irons, D.E.; Carter, J.E.; Watts, C.W.; Murray, P.J.; Norris, S.L.; Whitmore, A.P. Lumbricus terrestris middens are biological and chemical hotspots in a minimum tillage arable ecosystem. App. Soil Ecol. 2016, 105, 31–35. [Google Scholar] [CrossRef]
- Palm, J.; van Schaik, N.L.M.; Schröder, B. Modelling distribution patterns of anecic, epigeic and endogeic earthworms at catchment-scale in agro-ecosystems. Pedobiologia 2013, 56, 23–31. [Google Scholar] [CrossRef]
- UK Meteorological Office. UK Climate Averages 2022. Available online: https://www.metoce.gov.uk/research/climate/maps-and-data/uk-climate-averages/gcq89t680 (accessed on 25 March 2022).
- British Cattle Movement Service. Official Cattle Breeds and Codes. 2022. Available online: https://www.gov.uk/guidance/official-cattle-breeds-and-codes (accessed on 10 June 2022).
- Stroud, J. Soil health pilot study in England: Outcomes from an on-farm earthworm survey. PLoS ONE 2019, 14, e0203909. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). World Reference Base for Soil Resources. International soil classification system for naming soils and creating legends for soil maps. In World Soils Resources Reports; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2014; Volume 106. [Google Scholar]
- Santisteban, J.I.; Mediavilla, R.; Lopez-Pamo, E.; Dabrio, C.J.; Blanca Ruiz Zapata, M.; Jose Gil Garcia, M.; Castano, S.; Martinez-Alfaro, P.E. Loss on ignition: A qualitative or quantitative method for organic matter and carbonate mineral content in sediments? J. Paleolimnol. 2004, 32, 287–299. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeon. Electron. 2001, 4, 9. [Google Scholar]
- Lee, K.E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Academic Press Inc.: London, UK, 1985. [Google Scholar]
- van Capelle, C.; Schrader, S.; Brunotte, J. Tillage-induced changes in the functional diversity of soil biota—A review with a focus on German data. Eur. J. Soil Biol. 2012, 50, 165–181. [Google Scholar] [CrossRef]
- Capowiez, Y.; Cadoux, S.; Bouchant, P.; Ruy, S.; Roger-Estrade, J.; Richard, G.; Boizard, H. The effect of tillage type and cropping system on earthworm communities, macroporosity and water infiltration. Soil Tillage Res. 2009, 105, 209–216. [Google Scholar] [CrossRef]
- Ernst, G.; Müller, A.; Göhler, H.; Emmerling, C. C and N turnover of fermented residues from biogas plants in soil in the presence of three different earthworm species (Lumbricus terrestris, Aporrectodea longa, Aporrectodea caliginosa). Soil Biol. Biochem. 2008, 40, 1413–1420. [Google Scholar] [CrossRef]
- Pelosi, C.; Bertrand, M.; Roger-Estrade, J. Earthworm community in conventional, organic and direct seeding with living mulch cropping systems. Agron. Sustain. Dev. 2009, 29, 287–295. [Google Scholar] [CrossRef]
- Simonsen, J.; Posner, J.; Rosemeyer, M.; Baldock, J. Endogeic and anecic earthworm abundance in six Midwestern cropping systems. App. Soil Ecol. 2010, 44, 147–155. [Google Scholar] [CrossRef]
- Kuntz, M.; Berner, A.; Gattinger, A.; Scholberg, J.M.; Mäder, P.; Pfiffner, L. Influence of reduced tillage on earthworm and microbial communities under organic arable farming. Pedobiologia 2013, 56, 251–260. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Wyss, E.; Glasstetter, M. Tillage treatments and earthworm distribution in a Swiss experimental corn field. Soil Biol. Biochem. 1992, 24, 1635–1639. [Google Scholar] [CrossRef]
- Wilkes, T.I.; Warner, D.J.; Davies, K.G.; Edmonds-Brown, V. Tillage, glyphosate and beneficial arbuscular mycorrhizal fungi: Optimising crop management for plant–fungal symbiosis. Agriculture 2020, 10, 520. [Google Scholar] [CrossRef]
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. Zero tillage systems conserve arbuscular mycorrhizal fungi, enhancing soil glomalin and water stable aggregates with implications for soil stability. Soil Syst. 2021, 5, 4. [Google Scholar] [CrossRef]
- Marinissen, J.C.Y.; Van den Bosch, F. Colonization of new habitats by earthworms. Oecologia 1992, 91, 371–376. [Google Scholar] [CrossRef]
- Schmidt, O.; Clements, R.O.; Donaldson, G. Why do cereal–legume intercrops support large earthworm populations? App. Soil Ecol. 2003, 22, 181–190. [Google Scholar] [CrossRef]
- Edwards, C.A.; Lofty, J.R. The effect of direct drilling and minimal cultivation on earthworm populations. J. App. Ecol. 1982, 19, 723–734. [Google Scholar] [CrossRef]
- Riley, H.; Pommeresche, R.; Eltun, R.; Hansen, S.; Korsaeth, A. Soil structure, organic matter and earthworm activity in a comparison of cropping systems with contrasting tillage, rotations, fertilizer levels and manure use. Agric. Ecosyst. Environ. 2008, 124, 275–284. [Google Scholar] [CrossRef]
- Torppa, K.A.; Taylor, A.R. Alternative combinations of tillage practices and crop rotations can foster earthworm density and bioturbation. App. Soil Ecol. 2022, 175, 104460. [Google Scholar] [CrossRef]
- Crush, J.R.; Nichols, S.N.; Ouyang, L. Adventitious root mass distribution in progeny of four perennial ryegrass (Lolium perenne L.) groups selected for root shape. N. Zeal. J. Agric. Res. 2010, 53, 193–200. [Google Scholar] [CrossRef]
- Curry, J.P.; Doherty, P.; Purvis, G.; Schmidt, O. Relationships between earthworm populations and management intensity in cattle-grazed pastures in Ireland. App. Soil Ecol. 2008, 39, 58–64. [Google Scholar] [CrossRef]
- Tian, G.; Brussaard, L. Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions: Effects on soil fauna. Soil Biol. Biochem. 1993, 25, 731–737. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J. Plant litter quality influences the contribution of soil fauna to litter decomposition in humid tropical forests, southwestern China. Soil Biol. Biochem. 2009, 41, 910–918. [Google Scholar] [CrossRef]
- Bertrand, M.; Barot, S.; Blouin, M.; Whalen, J.; de Oliveira, T.; Roger-Estrade, J. Earthworm services for cropping systems. A review. Agron. Sustain. Dev. 2015, 35, 553–567. [Google Scholar] [CrossRef]
- Zaller, J.G.; Saxler, N. Selective vertical seed transport by earthworms: Implications for the diversity of grassland ecosystems. Eur. J. Soil Biol. 2007, 43, S86–S91. [Google Scholar] [CrossRef]
- Piotrowska, K.; Connolly, J.; Finn, J.; Black, A.; Bolger, T. Evenness and plant species identity affect earthworm diversity and community structure in grassland soils. Soil Biol. Biochem. 2013, 57, 713–719. [Google Scholar] [CrossRef]
- Schon, N.L.; Mackay, A.D.; Gray, R.A.; Dodd, M.B.; Van Koten, C. Quantifying dung carbon incorporation by earthworms in pasture soils. Eur. J. Soil Sci. 2015, 66, 348–358. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Hosseinibai, S. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Change Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef]
- Edwards, C.A. Earthworm ecology in cultivated soils. In Earthworm Ecology; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar]
- Satchell, J.E. Earthworm microbiology. In Earthworm Ecology; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar]
- Sharpley, A.; McDowell, R.; Moyer, B.; Littlejohn, R. Land application of manure can influence earthworm activity and soil phosphorus distribution. Commun. Soil Sci. Plant Anal. 2011, 42, 194–207. [Google Scholar] [CrossRef]
- Gougoulias, N.; Leontopoulos, S.; Makridis, C. Influence of food allowance in heavy metal’s concentration in raw milk production of several feed animals. Emir. J. Food. Agric. 2014, 26, 828–834. [Google Scholar]
- Mosier, S.; Apfelbaum, S.; Byck, P.; Calderon, F.; Teague, R.; Thompson, R.; Cotrufo, M.F. Adaptive multi-paddock grazing enhances soil carbon and nitrogen stocks and stabilization through mineral association in southeastern US grazing lands. J. Environ. Manag. 2021, 288, 112409. [Google Scholar] [CrossRef]
- Russelle, M.; Entz, M.; Franzluebbers, A. Reconsidering integrated crop-livestock systems in North America. Agron. J. 2007, 99, 325–334. [Google Scholar] [CrossRef]
- Soussana, J.F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ineson, P.; Huish, S.A. Nitrogen and cation mobilization by soil fauna feeding on leaf litter and soil organic matter from deciduous woodlands. Soil Biol. Biochem. 1983, 15, 463–467. [Google Scholar] [CrossRef]
- Amador, J.A. Soil Ecology. Soil Sci. 2003, 168, 218–219. [Google Scholar] [CrossRef]
- Garg, V.K.; Kaushik, P.; Yadav, Y.K. Effect of stocking density and food quality on the growth and fecundity of an epigeic earthworm (Eisenia fetida) during vermicomposting. Environmentalist 2008, 28, 483–488. [Google Scholar] [CrossRef]
- Scown, J.; Baker, G. The influence of livestock dung on the abundance of exotic and native earthworms in a grassland in south-eastern Australia. Eur. J. Soil Biol. 2006, 42, S310–S315. [Google Scholar] [CrossRef]
- Knight, D.; Elliott, P.W.; Anderson, J.M.; Scholefield, D. The role of earthworms in managed, permanent pastures in Devon, England. Soil Biol. Biochem. 1992, 24, 1511–1517. [Google Scholar] [CrossRef]
- Brown, G.G.; Barois, I.; Lavelle, P. Regulation of soil organic matter dynamics and microbial activityin the drilosphere and the role of interactions with other edaphic functional domains. Eur. J. Soil Biol. 2000, 36, 177–198. [Google Scholar] [CrossRef]
- McInerney, M.; Bolger, T. Decomposition of Quercus petraea litter: Influence of burial, comminution and earthworms. Soil Biol. Biochem. 2000, 32, 1989–2000. [Google Scholar] [CrossRef]
- Sheehan, C.; Kirwan, L.; Connolly, J.; Bolger, T. The effects of earthworm functional diversity on microbial biomass and the microbial community level physiological profile of soils. Eur. J. Soil Biol. 2008, 44, 65–70. [Google Scholar] [CrossRef]
- Gunadi, B.; Edwards, C.A. The effect of multiple applications of different organic wastes on the growth, fecundity and survival of Eisenia foetida (Savigny) (Lumbricidae). Pedobiologia 2003, 47, 321–330. [Google Scholar] [CrossRef]
- O’hea, N.M.; Kirwan, L.; Finn, J.A. Experimental mixtures of dung fauna affect dung decomposition through complex effects of species interactions. Oikos 2010, 119, 1081–1088. [Google Scholar] [CrossRef]
- Teague, R.; Provenza, F.; Kreuter, U.; Steffens, T.; Barnes, M. Multi-paddock grazing on rangelands: Why the perpetual dichotomy between research results and rancher experience. J. Environ. Manag. 2013, 128, 699–717. [Google Scholar] [CrossRef]
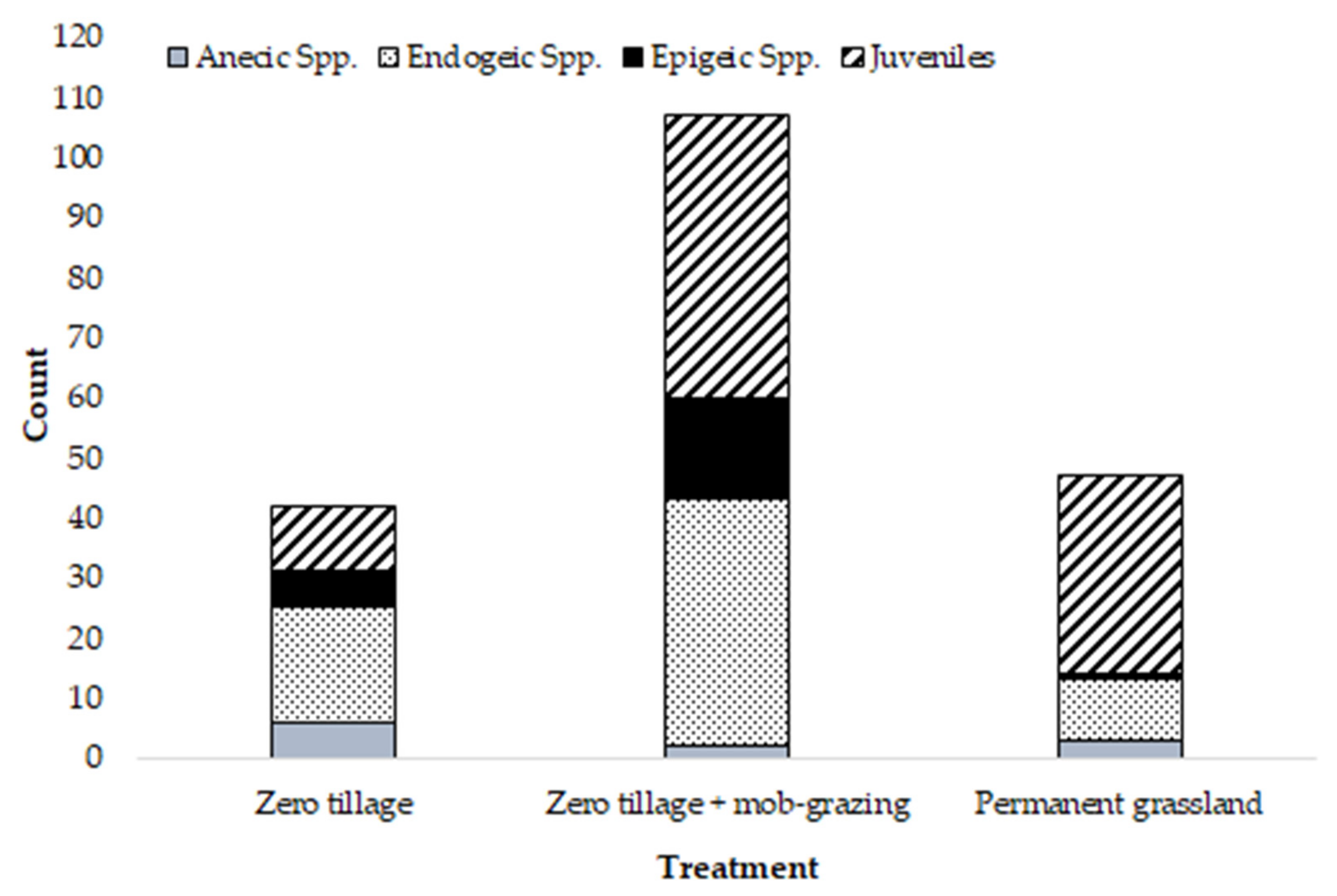
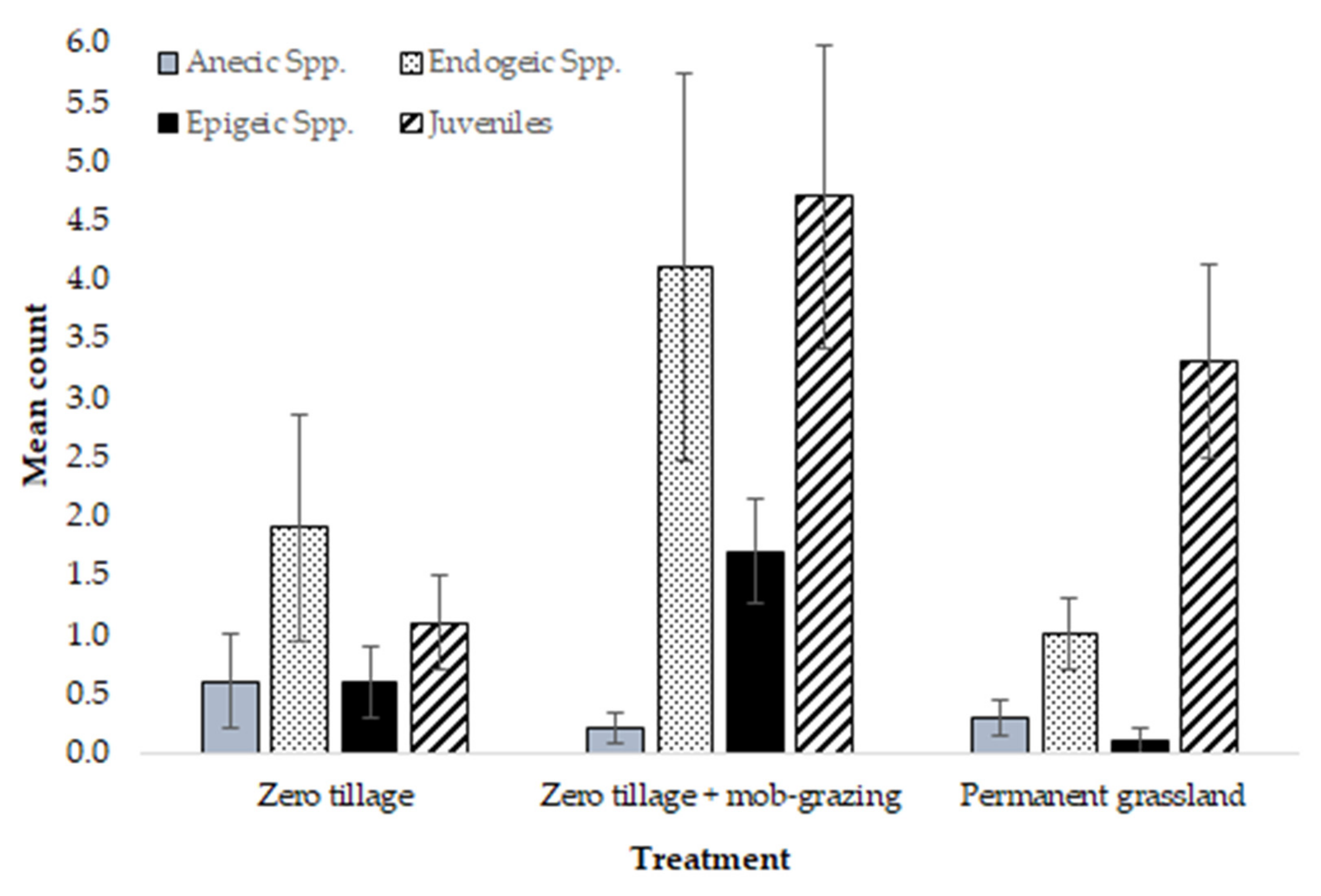
 : zero tillage;
: zero tillage;  : zero tillage + mob-grazing;
: zero tillage + mob-grazing;  : permanent grassland), n = 10 per treatment. Axis 1: Eigenvalue 0.0075, 96.6% variation; Axis 2: Eigenvalue 0.0003, 3.4% variation.
: permanent grassland), n = 10 per treatment. Axis 1: Eigenvalue 0.0075, 96.6% variation; Axis 2: Eigenvalue 0.0003, 3.4% variation.
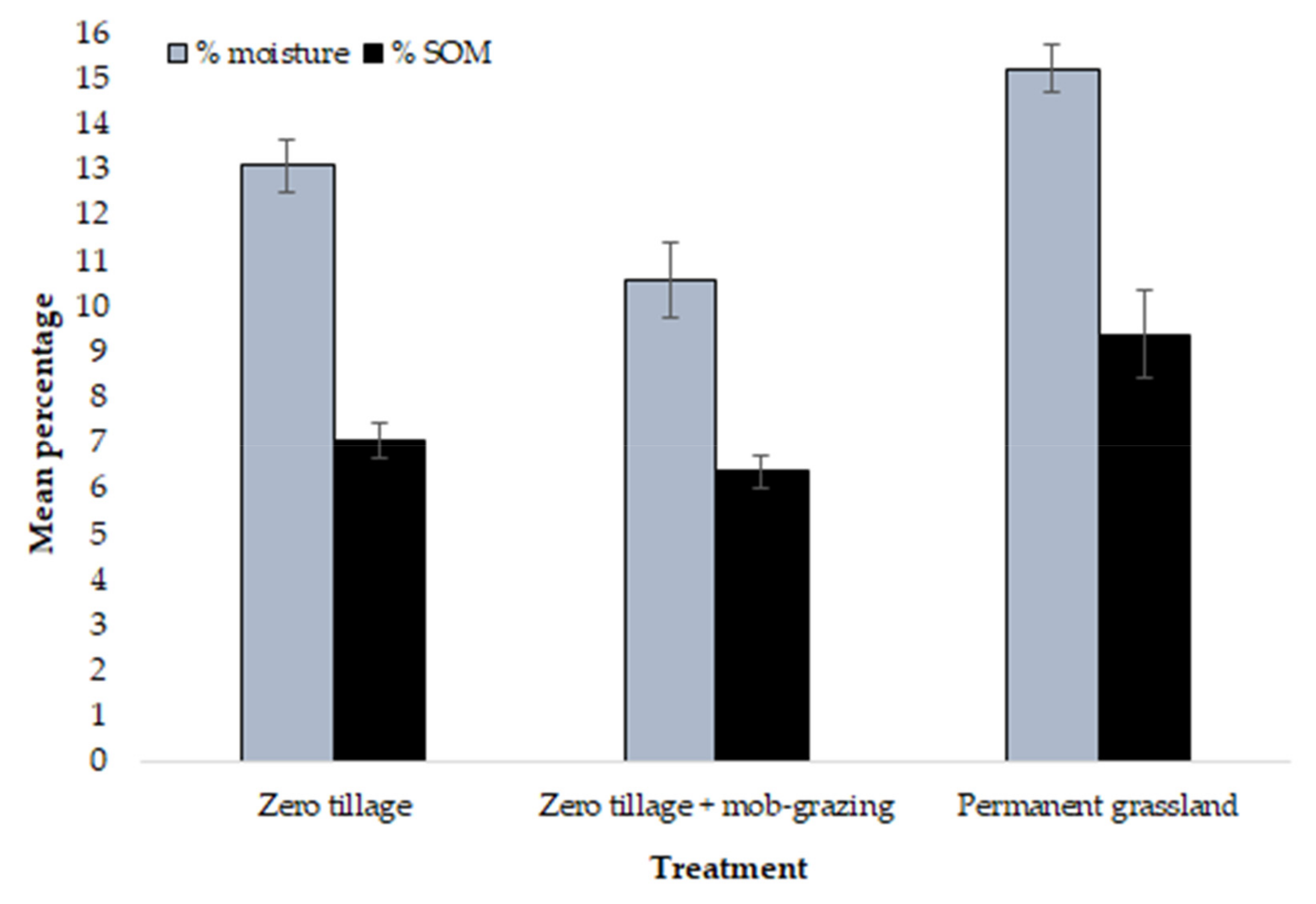
 : zero tillage;
: zero tillage;  : zero tillage + mob-grazing;
: zero tillage + mob-grazing;  : permanent grassland), n = 10 per treatment. Axis 1: Eigenvalue 0.0075, 96.6% variation; Axis 2: Eigenvalue 0.0003, 3.4% variation.
: permanent grassland), n = 10 per treatment. Axis 1: Eigenvalue 0.0075, 96.6% variation; Axis 2: Eigenvalue 0.0003, 3.4% variation.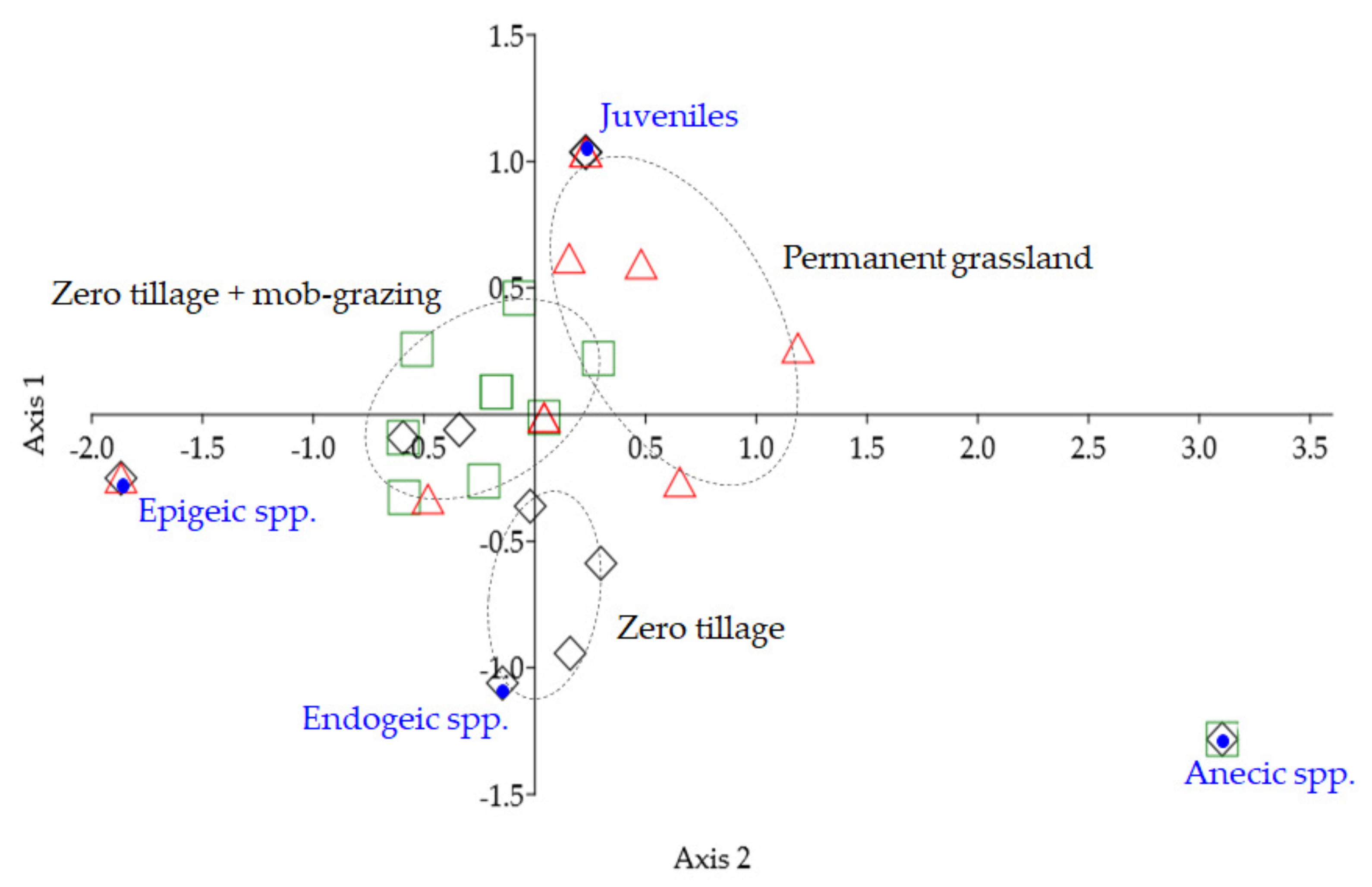
| Treatment | |||
|---|---|---|---|
| Management | ZT | ZTMG | PG |
| Permanent grass | - | - | ✓ |
| Previous soil inversion | 11 years | 11 years | - |
| Annual cropping | ✓ | ✓ | - |
| 3-year grass-clover ley | ✓ | ✓ | - |
| Mob-grazing ley with cattle | - | ✓ | - |
| Total Count | Anecic spp. | Endogeic spp. | Epigeic spp. | Juveniles | % Moisture | % SOM | |
|---|---|---|---|---|---|---|---|
| Kruskal-Wallis H | 9.89 | 0.412 | 5.774 | 11.026 | 8.902 | 13.025 | 7.884 |
| df | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Asymp. Sig. | 0.007 ** | 0.814 | 0.056 | 0.004 ** | 0.012 * | 0.001 ** | 0.019 * |
| Total Count | Epigeic spp. | Juveniles | % Moisture | % SOM | |
|---|---|---|---|---|---|
| zero tillage + mob grazing compared to zero tillage | |||||
| Mann–Whitney U | 15.5 | 24.5 | 14.5 | 17 | 33 |
| Wilcoxon W | 70.5 | 79.5 | 69.5 | 72 | 88 |
| Z | −2.625 | −2.024 | −2.727 | −2.495 | −1.287 |
| Asymp. Sig. (2-tailed) | 0.009 ** | 0.043 * | 0.006 ** | 0.013 * | 0.198 |
| zero tillage compared to permanent grassland | |||||
| Mann–Whitney U | 29.5 | 34.5 | 21 | 19 | 24.5 |
| Wilcoxon W | 84.5 | 89.5 | 76 | 74 | 79.5 |
| Z | −1.564 | −1.55 | −2.25 | −2.346 | −1.928 |
| Asymp. Sig. (2-tailed) | 0.118 | 0.121 | 0.024 * | 0.019 * | 0.054 |
| zero tillage + mob grazing compared to permanent grassland | |||||
| Mann–Whitney U | 18 | 12.5 | 38 | 11 | 16.5 |
| Wilcoxon W | 73 | 67.5 | 93 | 66 | 71.5 |
| Z | −2.443 | −3.124 | −0.92 | −2.949 | −2.534 |
| Asymp. Sig. (2-tailed) | 0.015 * | 0.002 ** | 0.358 | 0.003 ** | 0.011 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trickett, T.; Warner, D.J. Earthworm Abundance Increased by Mob-Grazing Zero-Tilled Arable Land in South-East England. Earth 2022, 3, 895-906. https://doi.org/10.3390/earth3030052
Trickett T, Warner DJ. Earthworm Abundance Increased by Mob-Grazing Zero-Tilled Arable Land in South-East England. Earth. 2022; 3(3):895-906. https://doi.org/10.3390/earth3030052
Chicago/Turabian StyleTrickett, Toni, and Douglas James Warner. 2022. "Earthworm Abundance Increased by Mob-Grazing Zero-Tilled Arable Land in South-East England" Earth 3, no. 3: 895-906. https://doi.org/10.3390/earth3030052
APA StyleTrickett, T., & Warner, D. J. (2022). Earthworm Abundance Increased by Mob-Grazing Zero-Tilled Arable Land in South-East England. Earth, 3(3), 895-906. https://doi.org/10.3390/earth3030052







