The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework
Abstract
1. Introduction
2. Methods
- Country.
- Time frame: 1960 to 2020.
- Production source: capture (harvest) or aquaculture production.
- Species name: Gelidium seaweeds (Gelidium spp.), Giant Gelidium (Gelidium corneum), Gracilaria seaweeds (Gracilaria spp.), Warty Gracilaria (Gracilaria gracilis). The data on “Red Seaweeds” were added, for all the countries where Gelidium harvesting was not reported for all the period, and for which there were references of Gelidium harvesting; these include, e.g., Canada, France, India, Indonesia, Ireland, Japan, Mexico, Morocco, Portugal, and New Zealand. “Red seaweeds” from Spain, South Korea, and South Africa were also incorporated, whenever “red seaweeds” were counted in a one-time frame and "Gelidium seaweeds" in another. Other red seaweed species besides Gelidium spp. may have been included and, therefore, the data reported may be overestimated.
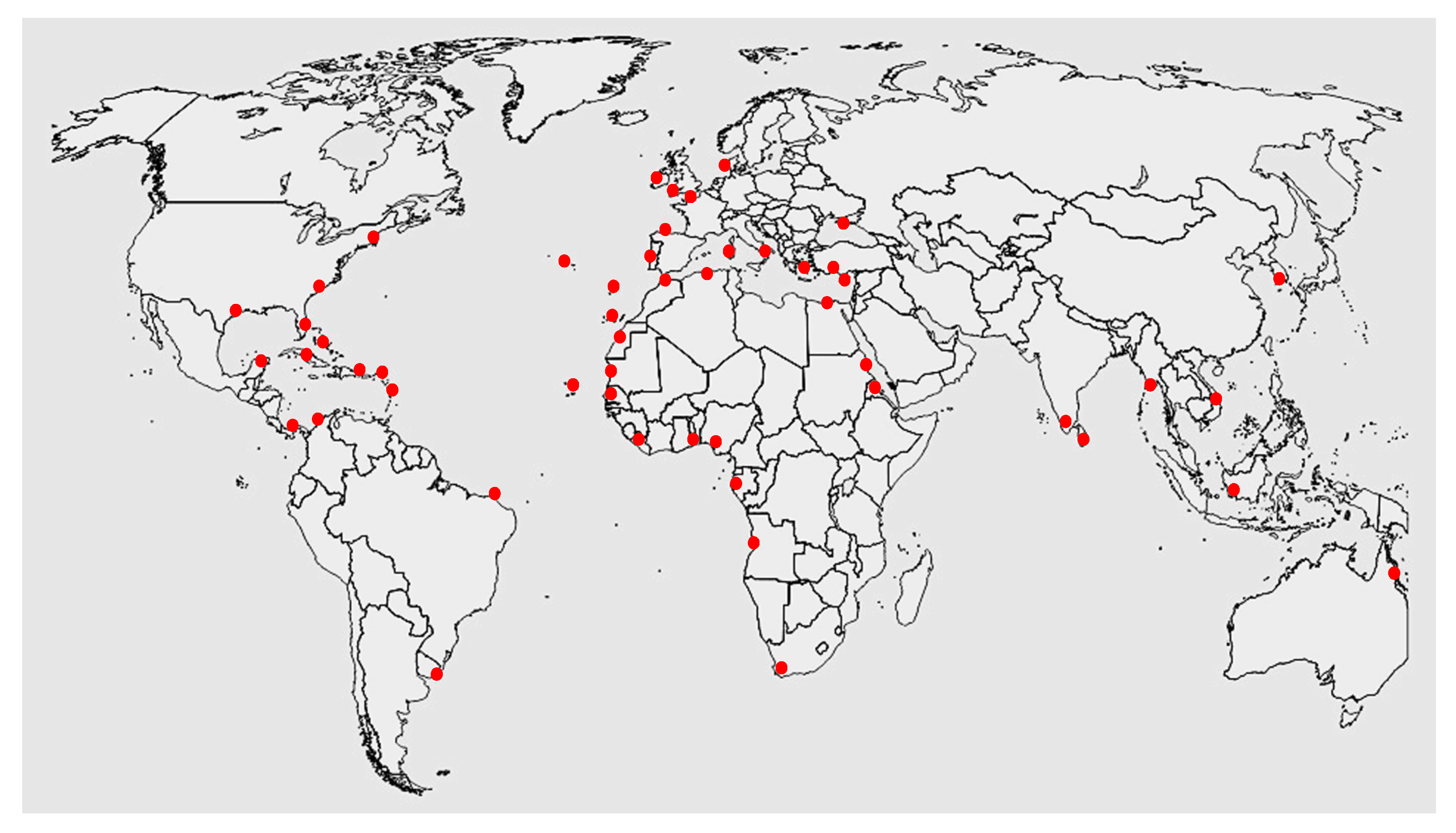
3. Gelidium corneum Biology, Distribution, and Ecology
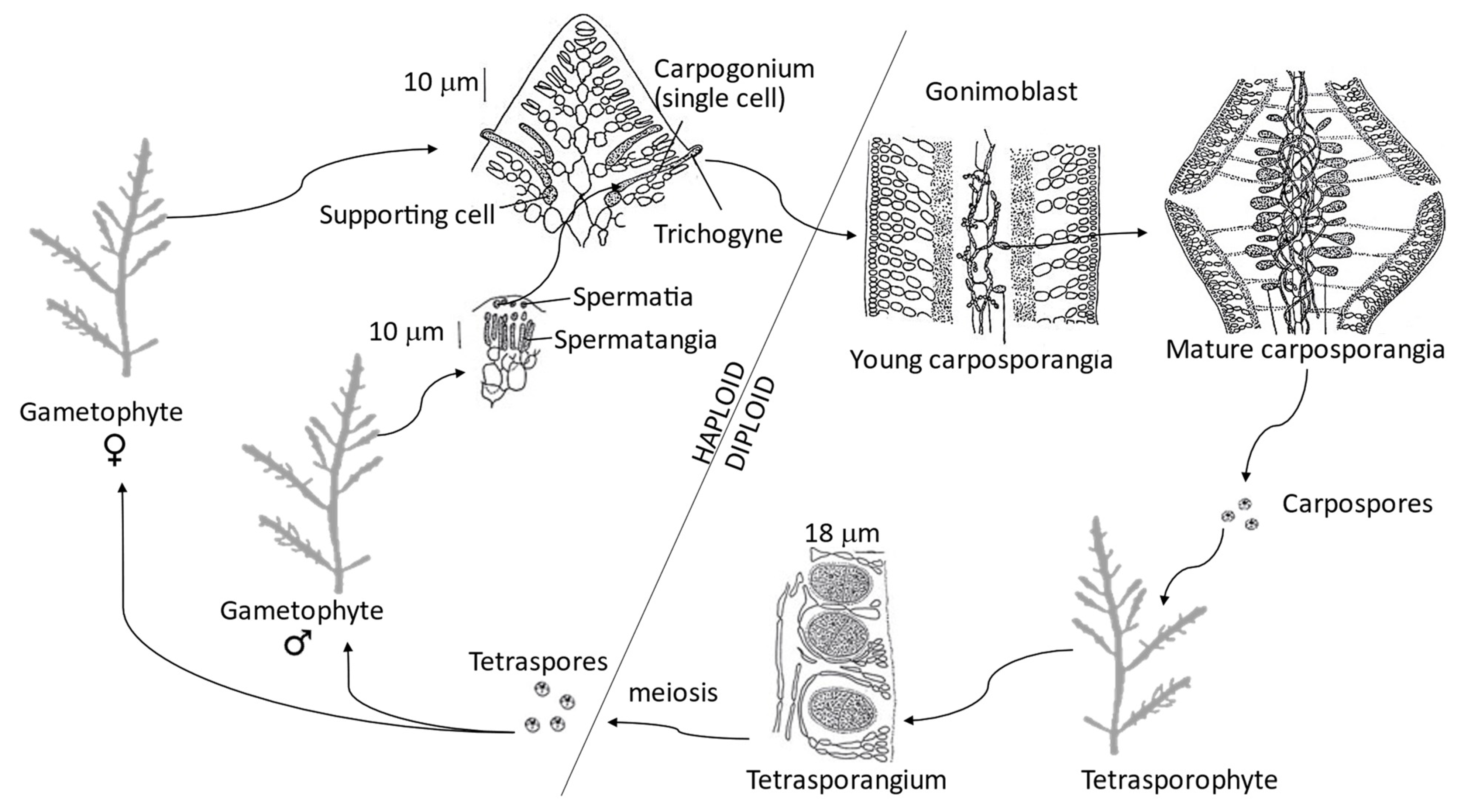
4. Gelidium corneum Life Cycle
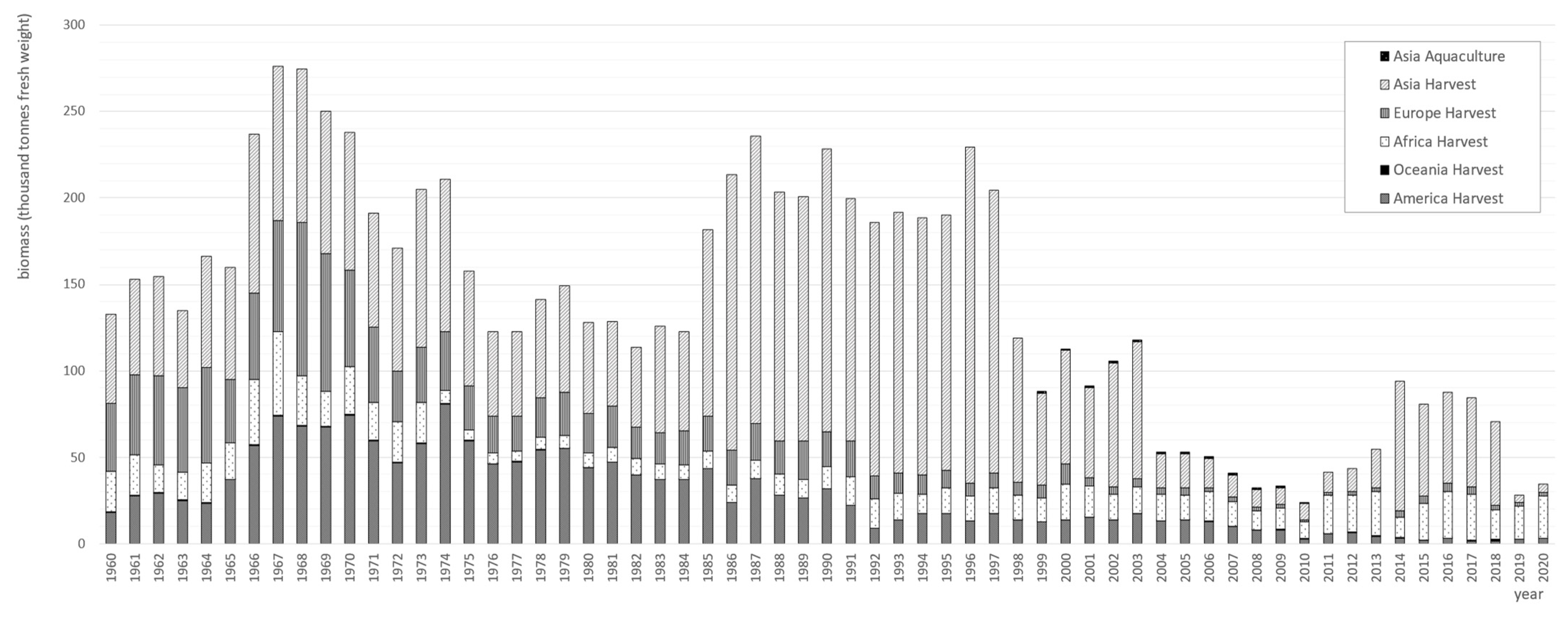
5. Gelidium Harvesting
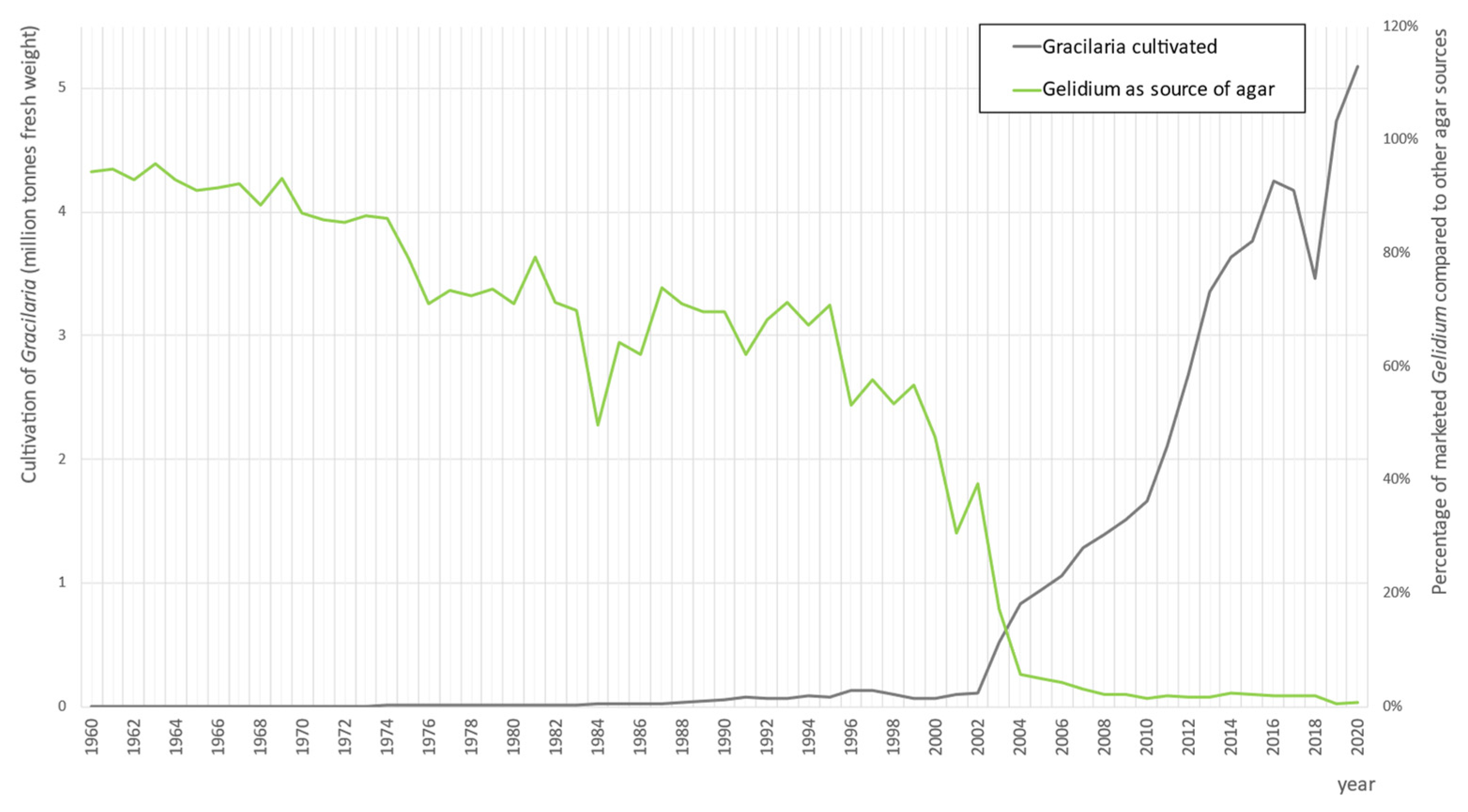
6. Gelidium Cultivation
7. Gelidium Market
8. Innovative Uses for Gelidium corneum
8.1. Primary Metabolites
8.2. UV Protection
8.3. Biofertilizer and Biostimulant
8.4. Production of Biochar
8.5. Biosorption Capacity
8.6. Biomaterials for Packaging and Coatings
8.7. Seaweeds for Biofuels
9. Integrated Approaches and Future Perspectives
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baweja, P.; Kumar, S.; Sahoo, D.; Lewiston, M. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK; Elsevier: London, UK, 2016; ISBN 9780128027721. [Google Scholar]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-0521145954. [Google Scholar]
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Coimbra, Portugal, 2011; Volume 4, pp. 15–47. ISBN 978-1-61470-878-0. [Google Scholar]
- Macartain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef]
- Cavaco, M.; Duarte, A.; Freitas, M.V.; Afonso, C.; Bernardino, S.; Pereira, L.; Martins, M.; Mouga, T. Seasonal Nutritional Profile of Gelidium Corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal. Foods 2021, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal Changes in the Nutritional Composition of Agarophyton Vermiculophyllum (Rhodophyta, Gracilariales) from the Center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Burtin, P. Nutricional Value of Seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.; de Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive Compounds from Brown Seaweeds: Phloroglucinol, Fucoxanthin and Fucoidan as Promising Therapeutic Agents against Breast Cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological Activities and Potential Industrial Applications of Fucose Rich Sulfated Polysaccharides and Fucoidans Isolated from Brown Seaweeds: A Review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and Challenges for Industrial Production of Seaweed Bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef]
- Matias, M.; Pinteus, S.; Martins, A.; Silva, J.; Alves, C.; Mouga, T.; Gaspar, H.; Pedrosa, R. Gelidiales Are Not Just Agar—Revealing the Antimicrobial Potential of Gelidium Corneum for Skin Disorders. Antibiotics 2022, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, S.; Pathak, J.; Ahmed, H.; Singh, V.; Singh, S.P.; Sinha, R.P. Mycosporine-Like Amino Acids (MAAs) Profile of Two Marine Red Macroalgae, Gelidium sp. and Ceramium sp. Int. J. Appl. Sci. Biotechnol. 2017, 5, 12–21. [Google Scholar] [CrossRef]
- Pimentel, F.; Alves, R.; Rodrigues, F.; PP Oliveira, M. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef]
- Hernández Carmona, G. Seaweed as Potential Plant Growth Stimulants for Agriculture in Mexico. Hidrobiológica 2018, 28, 129–140. [Google Scholar] [CrossRef]
- Villares, R.; Fernández-Lema, E.; López-Mosquera, M.E. Evaluation of Beach Wrack for Use as an Organic Fertilizer: Temporal Survey in Different Areas. Thalassas 2016, 32, 19–36. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Critchley, A.T.; Hurtado, A.Q. The Impacts of AMPEP K+ (Ascophyllum Marine Plant Extract, Enhanced with Potassium) on the Growth Rate, Carrageenan Quality, and Percentage Incidence of the Damaging Epiphyte Neosiphonia Apiculata on Four Strains of the Commercially Importan. J. Appl. Phycol. 2020, 32, 1907–1916. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Neish, I.C.; Majahar Ali, M.K.; Norrie, J.; Pereira, L.; Michalak, I.; Shukla, P.S.; Critchley, A.T. Extracts of Seaweeds Used as Biostimulants on Land and Sea Crops—An Efficacious, Phyconomic, Circular Blue Economy: With Special Reference to Ascophyllum (Brown) and Kappaphycus (Red) Seaweeds. In Biostimulants for Crops from Seed Germination to Plant Development; Shubhpriya, G., van Staden, J., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 263–288. ISBN 9781119130536. [Google Scholar]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Bird, M.I.; de Nys, R. Biochar from Commercially Cultivated Seaweed for Soil Amelioration. Sci. Rep. 2015, 5, 9665. [Google Scholar] [CrossRef]
- Grebe, G.S.; Byron, C.J.; Gelais, A.S.; Kotowicz, D.M.; Olson, T.K. An Ecosystem Approach to Kelp Aquaculture in the Americas and Europe. Aquac. Rep. 2019, 15, 100215. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Macroalgae Products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Trigueros, E.; Sanz, M.T.; Beltran, S.; García-Tojal, J. Pressurized Hot Water-Assisted Recovery of Crude Residual Agar from a Never-Dried Algae Industry Waste Stream: A Box-Behnken Design Approach. Food Hydrocoll. 2022, 129, 107664. [Google Scholar] [CrossRef]
- Fasahati, P.; Dickson, R.; Saffron, C.M.; Woo, H.C.; Liu, J.J. Seaweeds as a Sustainable Source of Bioenergy: Techno-Economic and Life Cycle Analyses of Its Biochemical Conversion Pathways. Renew. Sustain. Energy Rev. 2022, 157, 112011. [Google Scholar] [CrossRef]
- Thakur, N.; Salama, E.S.; Sharma, M.; Sharma, P.; Sharma, D.; Li, X. Efficient Utilization and Management of Seaweed Biomass for Biogas Production. Mater. Today Sustain. 2022, 18, 100120. [Google Scholar] [CrossRef]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed Bioethanol Production: A Process Selection Review on Hydrolysis and Fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Schiener, P.; Atack, T.; Wareing, R.; Kelly, M.S.; Hughes, A.D. The By-Products from Marine Biofuels as a Feed Source for the Aquaculture Industry: A Novel Example of the Biorefinery Approach. Biomass Convers. Biorefinery 2016, 6, 281–287. [Google Scholar] [CrossRef]
- Guillot, J.D. Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 25 May 2022).
- Martínez-Sanz, M.; Gomez-Barrio, L.P.; Zhao, M.; Tiwari, B.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Nilsson, A.E.; Krewer, C.; Östergren, K.; et al. Alternative Protocols for the Production of More Sustainable Agar-Based Extracts from Gelidium Sesquipedale. Algal Res. 2021, 55, 102254. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of Unpurified Agar-Based Extracts from Red Seaweed Gelidium Sesquipedale by Means of Simplified Extraction Protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Santos, R.; Cristo, C.; Jesus, D. Stock Assessment of the Agarophyte Gelidium Sesquipedale Using Harvest Effort Statistics. In Proceedings of the 17th International Seaweeds Symposium; Chapman, A., Anderson, R., Vreeland, V., Davison, I., Eds.; Oxford University Press: Cape Town, South Africa, 2001; pp. 145–150. [Google Scholar]
- Nil, S.; Ali-Mehidi, S.; Zellal, A.; Abi-Ayad, S.M.E.A. Effects of Season on the Yield and Quality of Agar from Gelidium Sesquipedale (Rhodophyta) from Mostaganem, Algeria. Afr. J. Biotechnol. 2016, 15, 350–355. [Google Scholar] [CrossRef]
- Bellatmania, Z.; Bentiss, F.; Jama, C.; Nadri, A.; Reani, A.; Sabour, B.; Belattmania, Z. Spectroscopic Characterization and Gel Properties of Agar from Two Gelidium Species from the Atlantic Coast of Morocco. Biointerface Res. Appl. Chem. 2021, 2021, 12642–12652. [Google Scholar] [CrossRef]
- Cardoso, S.S.M.; Carvalho, L.L.G.L.; Silva, P.P.J.; Rodrigues, M.S.M.; Pereira, O.; Pereira, L. Bioproducts From Seaweeds: A Review With Special Focus On The Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Williams, P. Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; ISBN 9780824759223. [Google Scholar]
- Kim, S.-K. Handbook of Marine Macroalgae—Biotechnology and Applied Phycology, 1st ed.; Kim, S.-K., Ed.; John Wiley & Sons: West Sussex, UK, 2012; ISBN 9780470979181. [Google Scholar]
- Bixler, H.J.; Porse, H. A Decade of Change in the Seaweed Hydrocolloids Industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Gallardo, T. Marine Algae: General Aspects (Biology, Systematics, Field and Laboratory Techniques). In Marine Algae Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2015; ISBN 9781466581814. [Google Scholar]
- FAO Fishery and Aquaculture Statistics. Global Capture Production 1950–2020 (FishStatJ). Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj/en (accessed on 19 May 2022).
- Borja, Á.; Fontán, A.; Muxika, I. Interactions between Climatic Variables and Human Pressures upon a Macroalgae Population: Implications for Management. Ocean. Coast. Manag. 2013, 76, 85–95. [Google Scholar] [CrossRef]
- Santos, R.; Melo, R.A. Global Shortage of Technical Agars: Back to Basics (Resource Management). J. Appl. Phycol. 2018, 30, 2463–2473. [Google Scholar] [CrossRef]
- Friedlander, M. Advances in Cultivation of Gelidiales. J. Appl. Phycol. 2008, 20, 451–456. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Filipigh, A.; Beltrán, S.; Riaño, P. Enzymatic Hydrolysis of the Industrial Solid Residue of Red Seaweed after Agar Extraction: Extracts Characterization and Modelling. Food Bioprod. Processing 2021, 126, 356–366. [Google Scholar] [CrossRef]
- Grina, F.; Ullah, Z.; Kaplaner, E.; Moujahid, A.; Eddoha, R.; Nasser, B.; Terzioğlu, P.; Yilmaz, M.A.; Ertaş, A.; Öztürk, M.; et al. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Fingerprints of Five Moroccan Seaweeds. South Afr. J. Bot. 2020, 128, 152–160. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. The Examination of Polysaccharides as Potential Antioxidative Compounds for Topical Administration Using a Lipid Model System. Int. J. Pharm. 2005, 298, 153–163. [Google Scholar] [CrossRef]
- Lakhdar, F.; Sidi, O.A.; Ben, M. Antimicrobial Effect of Two Marine Algae Gelidium Sesquipedale and Laminaria Ochroleuca Collected from the Coast of El Jadida-Morocco. J. Bio. Innov. 2016, 5, 16–23. [Google Scholar]
- Öztürk, B.Y.; Gürsu, B.; Dağ, İ. Antibiofilm and Antimicrobial Activities of Green Synthesized Silver Nanoparticles Using Marine Red Algae Gelidium Corneum. Process Biochem. 2020, 89, 208–219. [Google Scholar] [CrossRef]
- Matos, J.; Gomes, A.; Cardoso, C.; Afonso, C.; Campos, A.M.; Gomes, R.; Falé, P.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Commercial Red Seaweed in Portugal (Gelidium Sesquipedale and Pterocladiella Capillacea, Florideophyceae): Going beyond a Single-Purpose Product Approach by Valorizing Bioactivity. Thalassas 2020, 36, 213–224. [Google Scholar] [CrossRef]
- Metidji, H.; Dob, T.; Toumi, M.; Krimat, S.; Ksouri, A.; Nouasri, A. In Vitro Screening of Secondary Metabolites and Evaluationof Antioxidant, Antimicrobial and Cytotoxic Properties of Gelidium Sesquipedale Thuret et Bornet Red Seaweed from Algeria. J. Mater. Environ. Sci. 2015, 6, 3184–3196. [Google Scholar]
- Grozdanic, N.; Stanojkovic, T.P.P.; Kljajic, Z.; Etahiri, S.; Assobhei, O.; Konic-Ristic, A.; Srdic-Rajic, T.; Kardum, N.; Backovic, S.; Osmak, M.; et al. In Vitro Evaluation of Antioxidant and Antitumoral Activities of Marine Algae Gelidium Sesquipedale and Fucus Spiralis. Eur. J. Cancer 2012, 48, S26. [Google Scholar] [CrossRef]
- Vilar, V.; Botelho, C.; Boaventura, R. Biosorption Performance of a Binary Metal Mixture by Algal Biomass: Column Experiments. In Combined and Hybrid Adsorbents; Loureiro, J.M., Kartel, M.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 281–286. [Google Scholar]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Methylene Blue Adsorption by Algal Biomass Based Materials: Biosorbents Characterization and Process Behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Equilibrium and Kinetic Modelling of Cd(II) Biosorption by Algae Gelidium and Agar Extraction Algal Waste. Water Res. 2006, 40, 291–302. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Copper Desorption from Gelidium Algal Biomass. Water Res. 2007, 41, 1569–1579. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Metal Biosorption by Algae Gelidium Derived Materials from Binary Solutions in a Continuous Stirred Adsorber. Chem. Eng. J. 2008, 141, 42–50. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Lead Uptake by Algae Gelidium and Composite Material Particles in a Packed Bed Column. Chem. Eng. J. 2008, 144, 420–430. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Copper Removal by Algae Gelidium, Agar Extraction Algal Waste and Granulated Algal Waste: Kinetics and Equilibrium. Bioresour. Technol. 2008, 99, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Effect of Cu(II), Cd(II) and Zn(II) on Pb(II) Biosorption by Algae Gelidium-Derived Materials. J. Hazard. Mater. 2008, 154, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Lebbar, S. Valorisation Biologique de Co-Produits de l’extraction de l’agar Issu Du Gelidium Sesquipedale. Ph.D. Thesis, Cadre de École doctorale Chimie, Ecologie, Géosciences et AgroSciences Théodore Monod (Poitiers), Limoges, France, 2018. [Google Scholar]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade Approach of Red Macroalgae Gracilaria Gracilis Sustainable Valorization by Extraction of Phycobiliproteins and Pyrolysis of Residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of Marine Macroalgal Biomass for Production of Biofuel and Commodity Chemicals. Green Chem. 2015, 17, 2436–2443. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol Production from Gracilaria Verrucosa, a Red Alga, in a Biorefinery Approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-Based Cellulose: Applications, and Future Perspectives. Carbohydr. Polym. 2021, 267, 118241. [Google Scholar] [CrossRef]
- Cavaco, M.; Duarte, A.; Bernardino, S.; Afonso, C.; Mouga, T. Sustainable Use of Seaweeds from S. Martinho Do Porto, Portugal—Past, Present, and Future Perspective. In Proceedings of the 1st International Conference on Water Energy Food and Sustainability (ICoWEFS 2021); Galvão, J., Brito, P., Neves, F., Craveiro, F., Almeida, H., Vasco, J., Neves, L., Gomes, R., Mourato, S., Ribeiro, V., Eds.; Springer Nature: Leiria, Portugal, 2021; pp. 1–11. [Google Scholar]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498730501. [Google Scholar]
- FAO Fisheries and Aquaculture—List of Species for Fishery Statistics Purposes—Gelidium Corneum. Available online: https://www.fao.org/fishery/en/species/18380 (accessed on 19 May 2022).
- Guiry, M.D.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland. Available online: www.Algaebase.Org (accessed on 26 April 2022).
- Bunker, F.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Seaweeds of Britain and Ireland, 2nd ed.; Wild Nature Press: Plymouth, MA, USA, 2017; ISBN 978-0-9955673-3-7. [Google Scholar]
- Santelices, B. Synopsis of Biological Data on the Seaweed Genera Gelidium and Pterocladia; FAO: Rome, Italy, 1988; ISBN 925102717X. [Google Scholar]
- Silva, J.; Santos, R. Comparative Ecophysiology of Gelidium Sesquipedale (Rhodophyta) Erect Fronds and Prostrate System. In Proceedings of the 17th International Seaweed Symposium; Chapman, A., Anderson, R., Vreeland, V., Davison, I., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 1–8. [Google Scholar]
- Santelices, B. Production Ecology of Gelidium. Hydrobiologia 1991, 221, 31–44. [Google Scholar] [CrossRef]
- Gorostiaga, J.M.; Santolaria, A.; Secilla, A.; Casares, C. Check-List of the Basque Coast Benthic Algae (North of Spain). An. Del Jardín Botánico De Madr. 2004, 61, 155–180. [Google Scholar] [CrossRef]
- Díez, I.; Santolaria, A.; Gorostiaga, J.M. The Relationship of Environmental Factors to the Structure and Distribution of Subtidal Seaweed Vegetation of the Western Basque Coast (N Spain). Estuar. Coast. Shelf Sci. 2003, 56, 1041–1054. [Google Scholar] [CrossRef]
- Díez, I.; Muguerza, N.; Santolaria, A.; Ganzedo, U.; Gorostiaga, J.M. Seaweed Assemblage Changes in the Eastern Cantabrian Sea and Their Potential Relationship to Climate Change. Estuar. Coast. Shelf Sci. 2012, 99, 108–120. [Google Scholar] [CrossRef]
- Burel, T.; Le Duff, M.; Gall, E.A. Updated Check-List of the Seaweeds of the French Coasts, Channel and Atlantic Ocean. Les Cah. Nat. De L’observatoire Mar. 2019, 7, 1–38. [Google Scholar]
- Araújo, R.; Bárbara, I.; Tibaldo, M.; Berecibar, E.; Tapia, P.D.; Pereira, R.; Santos, R.; Pinto, I.S. Checklist of Benthic Marine Algae and Cyanobacteria of Northern Portugal. Bot. Mar. 2009, 52, 24–46. [Google Scholar] [CrossRef]
- Dizerbo, A.H. La Répartition Géographique Du Gelidium Sesquipedale (Clem.) Thur. (Gélidiacées, Rhodophycées) Sur Les Côtes Du Massif Armoricain. Vegetatio 1967, 19, 8–10. [Google Scholar] [CrossRef]
- Tsiamis, K.; Taşkin, E.; Orfanidis, S.; Stavrou, P.; Argyrou, M.; Panayotidis, P.; Tsioli, T.; Cicek, B.A.; Marcou, M.; Küpper, F.C. Checklist of Seaweeds of Cyprus (Mediterranean Sea). Bot. Mar. 2014, 57, 153–166. [Google Scholar] [CrossRef]
- Azevedo Neto, A.I.; Parente, M.I.; Botelho, A.Z.; Prestes, A.C.L.; Resendes, R.; Afonso, P.; Alvaro, N.V.; Milla-Figueras, D.; Raul, R.M.; Tittley, I.; et al. Marine Algal Flora of Graciosa Island, Azores. Biodivers. Data J. 2020, 8, 1–29. [Google Scholar] [CrossRef]
- Neto, A.I.; Cravo, D.C.; Haroun, R.T. Checklist of the Benthic Marine Plants of the Madeira Archipelago. Bot. Mar. 2001, 44, 391–414. [Google Scholar] [CrossRef]
- Gabriel, D.; Fredericq, S. The Marine Macroalgae of Cabo Verde Archipelago: An Updated Checklist. Arquipélago Life Mar. Sci. 2019, 36, 39–60. [Google Scholar]
- John, D.M.; van Reine, W.P.; Lawson, G.W.; Kostermans, T.B.; Price, J.H. A Taxonomic and Geographical Catalogue of the Seaweeds of the Western Coast of Africa and Adjacent Islands. Beih. Zur Nova Hedwig. 2004, 127, 1–139. [Google Scholar]
- Adama, D.; Mohammed, A.; Maroua, H.; Mohammed, E.; Essalmani, H.; Mouna, D. Distribution and Biomass Assessment of Macroalgae from Moroccan Strait of Gibraltar. Acta Ecol. Sin. 2021, 41, 442–450. [Google Scholar] [CrossRef]
- Bahammou, N.; Cherifi, O.; Bouamama, H.; Rezzoum, N.; Sabri, H.; Boundir, Y. Checklist of Rhodophyceae and the First Report of Aglaothamnion Tripinnatum and Gaillona Gallica in the Moroccan Coastline. Egypt. J. Aquat. Res. 2021, 47, 101–107. [Google Scholar] [CrossRef]
- Akrong, M.O.; Anning, A.K.; Addico, G.N.D.; deGraft-Johnson, K.A.A.; Adu-Gyamfi, A.; Ale, M.; Meyer, A.S. Spatio-Temporal Variations in Seaweed Diversity and Abundance of Selected Coastal Areas in Ghana. Reg. Stud. Mar. Sci. 2021, 44, 101719. [Google Scholar] [CrossRef]
- Mendoza-González, A.C.; Mateo-Cid, L.E.; García-López, D.Y. Inventory of Benthic Marine and Estuarine Algae and Cyanobacteria for Tabasco, México. Biota Neotrop. 2017, 17, e20170379. [Google Scholar] [CrossRef]
- de Casamajor, M.N.; Lalanne, Y.; Derrien-Courtel, S.; Maria Gorostiaga, J.; le Gal, A.; Huguenin, L.; Quintano, E.; Lissardy, M. Cystoseira Baccata Meadows along the French Basque Coast (Bay of Biscay) as a Reference for the Implementation of the Water Framework and Marine Strategy EU Directives. Cont. Shelf Res. 2019, 182, 12–21. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; Technical Paper n. 441; Food & Agriculture Organization: Rome, Italy, 2003; ISBN 92-5-104958-0. [Google Scholar]
- Santelices, B. Patterns of Reproduction, Dispersal and Recruitment in Seaweeds. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 177–276. [Google Scholar]
- mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable Harvesting of Wild Seaweed Resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefalì, M.E.; Cebrian, E. Restoration of a Canopy-Forming Alga Based on Recruitment Enhancement: Methods and Long-Term Success Assessment. Front. Plant Sci. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Carmona, R.; Vergara, J.J.; Lahaye, M.; Niell, F.X. Light Quality Affects Morphology and Polysaccharide Yield and Composition of Gelidium Sesquipedale. J. Appl. Phycol. 1998, 10, 323–331. [Google Scholar] [CrossRef]
- Quintano, E.; Díez, I.; Muguerza, N.; Figueroa, F.L.; Gorostiaga, J.M. Bed Structure (Frond Bleaching, Density and Biomass) of the Red Alga Gelidium Corneum under Different Irradiance Levels. J. Sea Res. 2017, 130, 180–188. [Google Scholar] [CrossRef]
- Alfonso, B.; Sansón, M.; Sangil, C.; Expósito, F.J.; Díaz, J.P.; Hernández, J.C. Herbarium Macroalgae Specimens Reveal a Rapid Reduction of Thallus Size and Reproductive Effort Related with Climate Change. Mar. Environ. Res. 2022, 174, 105546. [Google Scholar] [CrossRef]
- Borja, Á. Cartografía y Evaluación de La Biomasa Del Alga Gelidium Sesquipedale En La Costa Guipuzcoana. Inv. Pesq. 1987, 51, 199–224. [Google Scholar]
- Borja, Á. Impacto de La Cosecha y Recuperación de La Biomasa Del Alga Gelidium Sesquipedale Sometida a Dos Formas de Explotación En El País Vasco (España). Aquat. Living Resour. 1994, 7, 59–66. [Google Scholar] [CrossRef]
- Borja, A.; Chust, G.; Fontán, A.; Garmendia, J.M.; Uyarra, M.C. Long-Term Decline of the Canopy-Forming Algae Gelidium Corneum, Associated to Extreme Wave Events and Reduced Sunlight Hours, in the Southeastern Bay of Biscay. Estuar. Coast. Shelf Sci. 2018, 205, 152–160. [Google Scholar] [CrossRef]
- Pascual, M.; Borja, A.; Eede, S.V.; Deneudt, K.; Vincx, M.; Galparsoro, I.; Legorburu, I. Marine Biological Valuation Mapping of the Basque Continental Shelf (Bay of Biscay), within the Context of Marine Spatial Planning. Estuar. Coast. Shelf Sci. 2011, 95, 186–198. [Google Scholar] [CrossRef]
- Quintano, E.; Ganzedo, U.; Díez, I.; Figueroa, F.L.; Gorostiaga, J.M. Solar Radiation (PAR and UVA) and Water Temperature in Relation to Biochemical Performance of Gelidium Corneum (Gelidiales, Rhodophyta) in Subtidal Bottoms off the Basque Coast. J. Sea Res. 2013, 83, 47–55. [Google Scholar] [CrossRef]
- Gorostiaga, J.M.; Santolaria, A.; Secilla, A.; Díez, I. Sublittoral Benthic Vegetation of the Eastern Basque Coast (N. Spain): Structure and Environmental Factors. Bot. Mar. 1998, 41, 455–465. [Google Scholar] [CrossRef]
- Lee, R.E. Phycology, 5th ed.; Cambridge University Press: Cambridge, UK, 2018; ISBN 9781107555655. [Google Scholar]
- Seoane-Camba, J. Gelidium Sesquipedale (Clem) Thuret Cultivation in Galicia (Spain). Lagascalia 1997, 19, 179–186. [Google Scholar]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The Seaweed Resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Melo, R. Gelidium Commercial Exploitation: Natural Resources and Cultivation. J. Appl. Phycol. 1998, 10, 303–314. [Google Scholar] [CrossRef]
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-Based Management of Seaweed Harvesting. Bot. Mar. 2019, 62, 395–409. [Google Scholar] [CrossRef]
- Hossein Hoseinifar, S.; Chew, K.W.; Araújo, R.; Calderón, F.V.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Veeragurunathan, V.; Vadodariya, N.; Chaudhary, J.P.; Gogda, A.; Saminathan, K.R.; Meena, R. Experimental Cultivation of Gelidium Pusillum in Open Sea along the South East Indian Coast. Indian J. Geo Mar. Sci. 2018, 47, 336–345. [Google Scholar]
- Melo, R.A.; Harger, B.W.W.; Neushul, M. Gelidium Cultivation in the Sea. Hydrobiologia 1991, 221, 91–106. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Kadel, P.; Lüning, K. Obtaining Plantlets from Apical Meristem of the Red Alga Gelidium sp. J. Appl. Phycol. 2006, 18, 167–174. [Google Scholar] [CrossRef]
- Wijayanto, A.; Widowati, I.; Winanto, T. Domestication of Red Seaweed (Gelidium latifolium) in Different Culture Media. J. Mar. Sci./Ilmu Kelaut. 2020, 25, 39–44. [Google Scholar] [CrossRef]
- Boulus, A.; Spaneir, E.; Friedlander, M. Effect of Outdoor Conditions on Growth Rate and Chemical Composition of Gelidium Crinale in Culture. J. Appl. Phycol. 2007, 19, 471–478. [Google Scholar] [CrossRef]
- Salinas, J.M.; Valdés, L. Influence of Temperature and Photoperiod on the Re-Attachment Process of Gelidium Sesquipedale. J. Appl. Phycol. 1993, 5, 317–326. [Google Scholar] [CrossRef]
- Rojas, H.R.; León, M.N.; Rojas, O.R. Practical and Descriptive Techniques for Gelidium Rex (Gelidiales, Rhodophyta) Culture. Hydrobiologia 1996, 326/327, 367–370. [Google Scholar] [CrossRef]
- Chiheb, H.; García-Jiménez, P.; Robaina, R.R.; Hassoun, M.; Riadi, H. Développement d’un Stock de Semences (Seedstocks) de l’algue Rouge Gelidium Corneum (Gelidiaceae, Rhodophyta). Eur. Sci. J. 2018, 14, 1857–7881. [Google Scholar] [CrossRef]
- Duarte, P.; Ferreira, J.G. A Model for the Simulation of Macroalgal Population Dynamics and Productivity. Ecol. Model. 1997, 98, 199–214. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, L.; Pang, T.; Qin, R. Effects of Temperature on the Photosynthetic Performance in Mature Thalli of the Red Alga Gelidium Amansii (Gelidiaceae). Aquaculture 2019, 512, 734320. [Google Scholar] [CrossRef]
- Sousa-Pinto, I.; Murano, E.; Coelho, S.; Felga, A.; Pereira, R. The Effect of Light on Growth and Agar Content of Gelidium Pulchellum (Gelidiaceae, Rhodophyta) in Culture. Hydrobiologia 1999, 398–399, 329–338. [Google Scholar] [CrossRef]
- Vergara, J.J.; Niell, F.X.; Torres, M. Culture of Gelidium Sesquipedale in a Chemostat System: Biomass Production and Metabolic Responses Affected by N Flow. J Appl Phycol 1993, 5, 405–415. [Google Scholar] [CrossRef]
- Hurtado, A.Q. Genetic Resources for Farmed Seaweeds—Thematic Background Study; Food & Agriculture Org.: Rome, Italy, 2022. [Google Scholar]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Elalami, D.; Monlau, F.; Carrere, H.; Abdelouahdi, K.; Charbonnel, C.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Evaluation of Agronomic Properties of Digestate from Macroalgal Residues Anaerobic Digestion: Impact of Pretreatment and Co-Digestion with Waste Activated Sludge. Waste Manag. 2020, 108, 127–136. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Gomez, L.P.; Senthamaraikannan, R.; Padamati, R.B.; O’Donnell, C.P.; Tiwari, B.K. Investigation of Enzyme-Assisted Methods Combined with Ultrasonication under a Controlled Alkali Pretreatment for Agar Extraction from Gelidium Sesquipedale. Food Hydrocoll. 2021, 120, 106905. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.L. Factors Affecting Yield and Gelling Properties of Agar. J. Appl. Phycol. 2017, 29, 1527–1540. [Google Scholar] [CrossRef]
- Ait Mohamed, L.; Ethmane Kane, C.S.; Kouhila, M.; Jamali, A.; Mahrouz, M.; Kechaou, N. Thin Layer Modelling of Gelidium Sesquipedale Solar Drying Process. Energy Convers. Manag. 2008, 49, 940–946. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Ström, A.; Lopez-Sanchez, P.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Sokolova, A.; Gilbert, E.P.; López-Rubio, A. Advanced Structural Characterisation of Agar-Based Hydrogels: Rheological and Small Angle Scattering Studies. Carbohydr. Polym. 2020, 236, 115655. [Google Scholar] [CrossRef]
- Hnini, M.C.; Benchanaa, M.; el Hammioui, M. Study of the Interaction between Water and Gelidium Sesquipedale (Rhodophyta). Part I: Thermodynamic Aspect of the Sorption Equilibrium. J. Taiwan Inst. Chem. Eng. 2013, 44, 795–801. [Google Scholar] [CrossRef]
- Mouradi-Givernaud, A.; Hassani, L.A.; Givernaud, T.; Lemoine, Y.; Benharbet, O. Biology and Agar Composition of Gelidium Sesquipedale Harvested along the Atlantic Coast of Morocco. Hydrobiologia 1999, 398, 391–395. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Kouhila, M.; Lahsasni, S.; Jamali, A.; Idlimam, A.; Rhazi, M.; Aghfir, M.; Mahrouz, M. Equilibrium Moisture Content and Heat of Sorption of Gelidium Sesquipedale. J. Stored Prod. Res. 2005, 41, 199–209. [Google Scholar] [CrossRef]
- Djaeni, M.; Sari, D.A. Low Temperature Seaweed Drying Using Dehumidified Air. Procedia Environ. Sci. 2015, 23, 2–10. [Google Scholar] [CrossRef]
- Domínguez-González, R.; Romarís-Hortas, V.; García-Sartal, C.; Moreda-Piñeiro, A.; Barciela-Alonso, M.D.C.; Bermejo-Barrera, P. Evaluation of an in Vitro Method to Estimate Trace Elements Bioavailability in Edible Seaweeds. Talanta 2010, 82, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Romarís-Hortas, V.; Bermejo-Barrera, P.; Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Speciation of the Bio-Available Iodine and Bromine Forms in Edible Seaweed by High Performance Liquid Chromatography Hyphenated with Inductively Coupled Plasma-Mass Spectrometry. Anal. Chim. Acta 2012, 745, 24–32. [Google Scholar] [CrossRef]
- de La Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.v.; Herrera, E. Antioxidant Activity of Mycosporine-like Amino Acids Isolated from Three Red Macroalgae and One Marine Lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the Protein Fraction with High Antioxidant Activity from Red Seaweed Industrial Solid Residue after Agar Extraction by Subcritical Water Treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- el Wahidi, M.; el Amraoui, B.; el Amraoui, M.; Bamhaoud, T. Screening of Antimicrobial Activity of Macroalgae Extracts from the Moroccan Atlantic Coast. Ann. Pharm. Françaises 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M.D. Marine Algal Carbohydrates as Carbon Sources for the Production of Biochemicals and Biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef]
- Tůma, S.; Izaguirre, J.K.; Bondar, M.; Marques, M.M.; Fernandes, P.; da Fonseca, M.M.R.; Cesário, M.T. Upgrading End-of-Line Residues of the Red Seaweed Gelidium Sesquipedale to Polyhydroxyalkanoates Using Halomonas Boliviensis. Biotechnol. Rep. 2020, 27, e00491. [Google Scholar] [CrossRef]
- Baptista, S.L.; Romaní, A.; Oliveira, C.; Ferreira, S.; Rocha, C.M.R.; Domingues, L. Galactose to Tagatose Isomerization by the L-Arabinose Isomerase from Bacillus Subtilis: A Biorefinery Approach for Gelidium Sesquipedale Valorisation. LWT—Food Sci. Technol. 2021, 151, 112199. [Google Scholar] [CrossRef]
- Amamou, S.; Sambusiti, C.C.; Monlau, F.; Dubreucq, E.; Barakat, A.; Barakat Mechano, A.; Sambusiti, C.C. Mechano-Enzymatic Deconstruction with a New Enzymatic Cocktail to Enhance Enzymatic Hydrolysis and Bioethanol Fermentation of Two Macroalgae Species. Molecules 2018, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Alehosseini, A.; Gomez del Pulgar, E.M.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Fabra, M.J.; Sanz, Y.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Unpurified Gelidium-Extracted Carbohydrate-Rich Fractions Improve Probiotic Protection during Storage. LWT 2018, 96, 694–703. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar Films Containing Green Tea Extract and Probiotic Bacteria for Extending Fish Shelf-Life. LWT—Food Sci. Technol. 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Giménez, B.; López de Lacey, A.; Pérez-Santín, E.; López-Caballero, M.E.; Montero, P. Release of Active Compounds from Agar and Agar–Gelatin Films with Green Tea Extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Ku, K.J.; Hong, Y.H.; Song, K.B. Mechanical Properties of a Gelidium Corneum Edible Film Containing Catechin and Its Application in Sausages. J. Food Sci. 2008, 73, C217–C221. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.O.; Hong, Y.H.; Song, K.B. Application of Gelidium Corneum Edible Films Containing Carvacrol for Ham Packages. J. Food Sci. 2010, 75, C90–C93. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Lim, G.-O.; Song, K.B. Physical Properties of Gelidium Corneum-Gelatin Blend Films Containing Grapefruit Seed Extract or Green Tea Extract and Its Application in the Packaging of Pork Loins. J. Food Sci. 2009, 74, C6–C10. [Google Scholar] [CrossRef]
- Lim, G.O.; Jang, S.A.; Song, K. bin Physical and Antimicrobial Properties of Gelidium Corneum/Nano-Clay Composite Film Containing Grapefruit Seed Extract or Thymol. J. Food Eng. 2010, 98, 415–420. [Google Scholar] [CrossRef]
- Jo, W.; Song, N.; Lee, J.; Song, B. Physical Properties and Antimicrobial Activities of a Persimmon Peel/Red Algae Composite Film Containing Grapefruit Seed Extract. Food Sci. Biotechnol. 2014, 23, 1169–1172. [Google Scholar] [CrossRef]
- Song, N.B.; Song, H.Y.; Jo, W.S.; Song, K. bin Physical Properties of a Composite Film Containing Sunflower Seed Meal Protein and Its Application in Packaging Smoked Duck Meat. J. Food Eng. 2013, 116, 789–795. [Google Scholar] [CrossRef]
- Guerrero, P.; Garrido, T.; Leceta, I.; de La Caba, K. Films Based on Proteins and Polysaccharides: Preparation and Physical–Chemical Characterization. Eur. Polym. J. 2013, 49, 3713–3721. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; Fabra, M.J.; da Rosa Zavareze, E.; López-Rubio, A.; Martínez-Sanz, M. Development of Food Packaging Bioactive Aerogels through the Valorization of Gelidium Sesquipedale Seaweed. Food Hydrocoll. 2019, 89, 337–350. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Martínez-Abad, A.; López-Rubio, A. Cost-Efficient Bio-Based Food Packaging Films from Unpurified Agar-Based Extracts. Food Packag. Shelf Life 2019, 21, 100367. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Antimicrobial and Physical-Mechanical Properties of Agar-Based Films Incorporated with Grapefruit Seed Extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef]
- Rhim, J.W. Effect of Clay Contents on Mechanical and Water Vapor Barrier Properties of Agar-Based Nanocomposite Films. Carbohydr. Polym. 2011, 86, 691–699. [Google Scholar] [CrossRef]
- Yoon, M.H.; Lee, Y.W.; Lee, C.H.; Seo, Y.B. Simultaneous Production of Bio-Ethanol and Bleached Pulp from Red Algae. Bioresour. Technol. 2012, 126, 198–201. [Google Scholar] [CrossRef]
- Seo, Y.B.; Lee, Y.W.; Lee, C.H.; You, H.C. Red Algae and Their Use in Papermaking. Bioresour. Technol. 2010, 101, 2549–2553. [Google Scholar] [CrossRef]
- Mukherjee, P.; Prakash Keshri, J. Present Status and Development of Algal Pulp for Hand-Made Paper Making Technology: A Review. Adv. Plants Agric. Res. 2018, 8, 10–18. [Google Scholar] [CrossRef][Green Version]
- Rajak, R.C.; Jacob, S.; Kim, B.S. A Holistic Zero Waste Biorefinery Approach for Macroalgal Biomass Utilization: A Review. Sci. Total Environ. 2020, 716, 137067. [Google Scholar] [CrossRef]
- el Achaby, M.; Kassab, Z.; Aboulkas, A.; Gaillard, C.C.; Barakat, A.; Barakat Reuse, A. Reuse of Red Algae Waste for the Production of Cellulose Nanocrystals and Its Application in Polymer Nanocomposites. Int. J. Biol. Macromol. 2018, 106, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Cebrián-Lloret, V.; Mazarro-Ruiz, J.; López-Rubio, A. Improved Performance of Less Purified Cellulosic Films Obtained from Agar Waste Biomass. Carbohydr. Polym. 2020, 233, 115887. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Yusoff, S.; Ng, C.G.; Lim, P.E.; Ching, Y.C. Bioplastic Made from Seaweed Polysaccharides with Green Production Methods. J. Environ. Chem. Eng. 2021, 9, 105895. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Guerrero, P.; de La Caba, K. Characterization of Agar/Soy Protein Biocomposite Films: Effect of Agar on the Extruded Pellets and Compression Moulded Films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef]
- Guerrero, P.; Etxabide, A.; Leceta, I.; Peñalba, M.; de La Caba, K. Extraction of Agar from Gelidium Sesquipedale (Rodhopyta) and Surface Characterization of Agar Based Films. Carbohydr. Polym. 2014, 99, 491–498. [Google Scholar] [CrossRef]
- Méndez, A.; Gascó, G.; Ruiz, B.; Fuente, E. Hydrochars from Industrial Macroalgae “Gelidium Sesquipedale” Biomass Wastes. Bioresour. Technol. 2019, 275, 386–393. [Google Scholar] [CrossRef]
- Ferrera-Lorenzo, N.; Fuente, E.; Suárez-Ruiz, I.; Ruiz, B. Sustainable Activated Carbons of Macroalgae Waste from the Agar–Agar Industry. Prospects as Adsorbent for Gas Storage at High Pressures. Chem. Eng. J. 2014, 250, 128–136. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A. Valorization of Algal Waste via Pyrolysis in a Fixed-Bed Reactor: Production and Characterization of Bio-Oil and Bio-Char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef]
- Tayibi, S.; Monlau, F.; Fayoud, N.E.; Oukarroum, A.; Zeroual, Y.; Hannache, H.; Barakat, A. One-Pot Activation and Pyrolysis of Moroccan Gelidium Sesquipedale Red Macroalgae Residue: Production of an Efficient Adsorbent Biochar. Biochar 2019, 1, 401–412. [Google Scholar] [CrossRef]
- Ferrera-Lorenzo, N.; Fuente, E.; Suárez-Ruiz, I.; Gil, R.R.; Ruiz, B. Pyrolysis Characteristics of a Macroalgae Solid Waste Generated by the Industrial Production of Agar-Agar. J. Anal. Appl. Pyrolysis 2014, 105, 209–216. [Google Scholar] [CrossRef]
- Sidi, O.A.; Ben, M. Preparation of an Antifungal Soap from Gelidium Sesquipedale Waste. Bothalia J. 2014, 44, 9–16. [Google Scholar]
- Trioui, L.; Byoud, F.; Channaoui, S.; Belghmi, K.; Azzi, M.; Blaghen, M. Study of Chromium Biosorption by Four Atlantic Algae: Gelidium Sesquipedale, Gelidium Corneum, Corallina Officinalis, and Ulva Lactuca. Am. J. Eng. Res. (AJER) 2017, 6, 103–108. [Google Scholar]
- Vilar, V.J.P.; Loureiro, J.M.; Botelho, C.M.S.; Boaventura, R.A.R. Continuous Biosorption of Pb/Cu and Pb/Cd in Fixed-Bed Column Using Algae Gelidium and Granulated Agar Extraction Algal Waste. J. Hazard. Mater. 2008, 154, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Vilar, V.J.P.; Botelho, C.M.S.; Loureiro, J.M.; Boaventura, R.A.R. Biosorption of Copper by Marine Algae Gelidium and Algal Composite Material in a Packed Bed Column. Bioresour. Technol. 2008, 99, 5830–5838. [Google Scholar] [CrossRef]
- Carbó, R.; Molero, A.C. Scattering Strength of a Gelidium Biomass Bottom. Appl. Acoust. 1997, 51, 343–351. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Pinheiro, J.P.S.; Domingos, R.F.; Boaventura, R.A.R. Copper Removal by Algal Biomass: Biosorbents Characterization and Equilibrium Modelling. J. Hazard. Mater. 2009, 163, 1113–1122. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Sebesta, F.; Botelho, C.M.S.; Boaventura, R.A.R. Equilibrium and Kinetic Modelling of Pb2+ Biosorption by Granulated Agar Extraction Algal Waste. Process Biochem. 2005, 40, 3276–3284. [Google Scholar] [CrossRef]
- Faraj, A.; Lebbar, T.; Debry, G.; Najim, L. Protein and Amino Acids Analysis during Alimentary-Agar Extraction from Gelidium Sesquipedale. Hydrobiologia 1987, 151, 513–522. [Google Scholar] [CrossRef]
- Gomes-Dias, J.S.; Romaní, A.; Teixeira, J.J.; Rocha, C.M.R. Valorization of Seaweed Carbohydrates: Autohydrolysis as a Selective and Sustainable Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 17143–17153. [Google Scholar] [CrossRef]
- Trigueros, E.; Alonso-Riaño, P.; Ramos, C.; Diop, C.I.K.; Beltrán, S.; Sanz, M.T. Kinetic Study of the Semi-Continuous Extraction/Hydrolysis of the Protein and Polysaccharide Fraction of the Industrial Solid Residue from Red Macroalgae by Subcritical Water. J. Environ. Chem. Eng. 2021, 9, 106768. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, F.L.; Flores-Moya, A.; Wiencke, C. An Inventory of UV-Absorbing Mycosporine-like Amino Acids in Macroalgae from Polar to Warm-Temperate Regions. Bot. Mar. 1998, 41, 443–453. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Rotterdam, S.; Altmeyer, P.; Hoffmann, K. Protection against Ultraviolet Radiation by Commercial Summer Clothing: Need for Standardised Testing and Labelling. BMC Dermatol. 2001, 1, 6. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Morabito, K.; Shapley, N.C.; Mello, C.M.; Calvert, P.; Tripathi, A. Nanoparticles and Their Applications in Ultraviolet Protection: A Review. Anal. Chem. 2009, 1, 1–10. [Google Scholar]
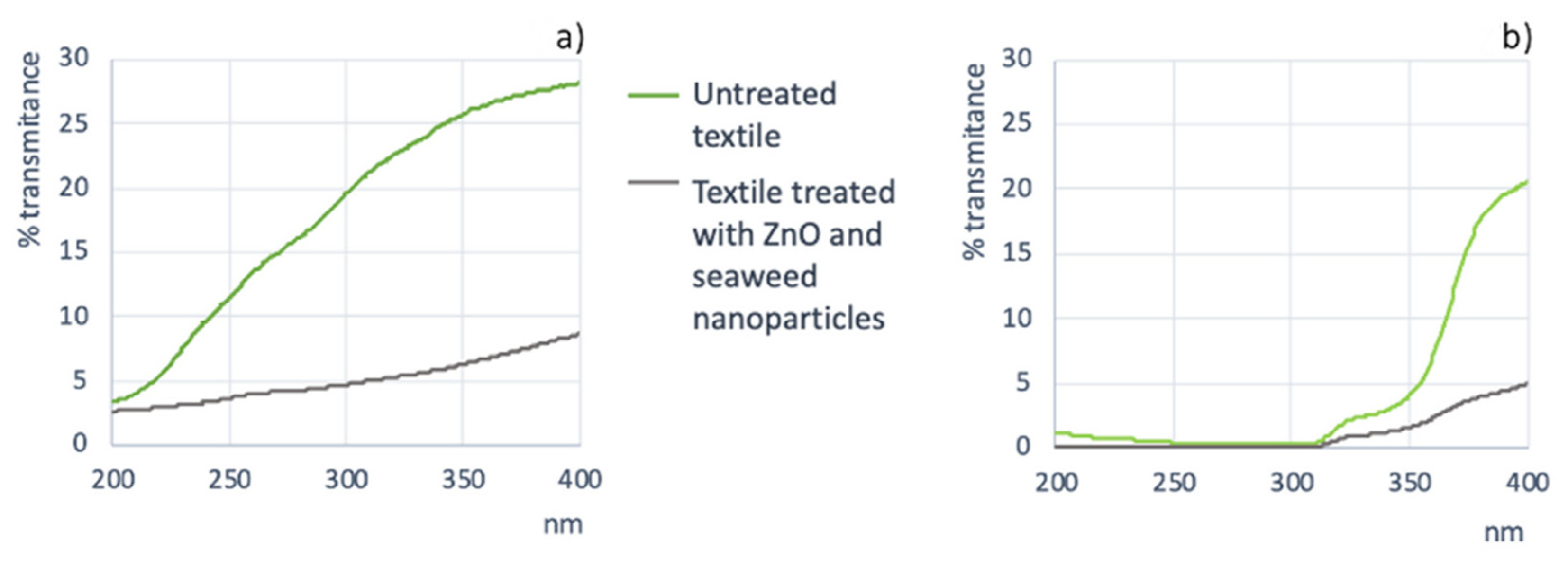
- Kathirvelu, S.; D’Souza, L.; Dhurai, B. UV Protection Finishing of Textiles Using ZnO Nanoparticles. Indian J. Fibre Text. Res. 2009, 34, 267–273. [Google Scholar]
- Abdelhady, M.M. Preparation and Characterization of Chitosan/Zinc Oxide Nanoparticles for Imparting Antimicrobial and UV Protection to Cotton Fabric. Int. J. Carbohydr. Chem. 2012, 2012, 840591. [Google Scholar] [CrossRef]
- Gregersen, Ó.; Bak, G.; Zwaanenburg, L.; Gunnarsdóttir, K.; Bonefeld, B. A Feasibility Study on Blue Fashion Using Cultivated Seaweed for Textile Production Final Report; Kolbrun: Reykjavík, Iceland, 2019. [Google Scholar]
- Fuchs, H.; Schönberger, C.; Kroner, G.; Schobesberger, H. Modified Viscose Fiber. U.S. Patent No. 16/095, 2017. [Google Scholar]
- Hussein, M.H.; Eltanahy, E.; al Bakry, A.F.; Elsafty, N.; Elshamy, M.M. Seaweed Extracts as Prospective Plant Growth Bio-Stimulant and Salinity Stress Alleviator for Vigna Sinensis and Zea Mays. J. Appl. Phycol. 2021, 33, 1273–1291. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Critchley, A.T. Time for Applications of Biostimulants in Phyconomy: Seaweed Extracts for Enhanced Cultivation of Seaweeds (SEECS). In Sustainable Seaweed Technologies—Cultivation, Biorefinery, and Applications; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 103–127. ISBN 9780128179437. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of Liquid Seaweed Extracts on Growth of Tomato Seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Buono, D. del The Opportunity of Valorizing Agricultural Waste, Through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Arora, N.K.; Mehnaz, S.; Balestrini, R. Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; ISBN 9788132227779. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Dangariya, M.; Agarwal, P. Seaweed Extracts: Potential Biodegradable, Environmentally Friendly Resources for Regulating Plant Defence. Algal Res. 2021, 58, 102363. [Google Scholar] [CrossRef]
- Mohanty, D.; Adhikary, S.P. Standardization of Protocol for Preparation of Liquid Extracts from Seaweeds, Quantification of Their Phytohormones and Application for Crop Improvement. Indian J. Geo Mar. Sci. 2018, 47, 1364–1372. [Google Scholar]
- Mandal, S.; Verma, B.C.; Ramkrushna, G.I.; Singh, R.K.; Rajkhowa, D.J. Characterization of Biochar Obtained from Weeds and Its Effect on Soil Properties of North Eastern Region of India. J. Environ. Biol. 2015, 36, 499–505. [Google Scholar] [PubMed]
- Katiyar, R.; Patel, A.K.; Nguyen, T.B.; Singhania, R.R.; Chen, C.W.; Dong, C. di Adsorption of Copper (II) in Aqueous Solution Using Biochars Derived from Ascophyllum Nodosum Seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, B.A.; Adesina, A. Recent Advancements in the Use of Biochar for Cementitious Applications: A Review. J. Build. Eng. 2020, 32, 101705. [Google Scholar] [CrossRef]
- Gade, R.; Tulasi, M.S.; Bhai, V.A. Seaweeds: A Novel Biomaterial. Int. J. Pharm. Pharm. Sci. 2013, 5, 40–44. [Google Scholar]
- Freile-Pelegrín, Y.; Madera-Santana, T.J. Characterization Techniques for Algae-Based Materials. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 18, pp. 649–670. ISBN 9780128123607. [Google Scholar]
- Mostafavi, F.S.; Zaeim, D. Agar-Based Edible Films for Food Packaging Applications—A Review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Pei, J.; Lin, A.; Zhang, F.; Zhu, D.; Li, J.; Wang, G. Using Agar Extraction Waste of Gracilaria lemaneiformis in the Papermaking Industry. J. Appl. Phycol. 2013, 25, 1135–1141. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.S. Marine Macroalgae: An Untapped Resource for Producing Fuels and Chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.M. From First- to Third-Generation Biofuels: Challenges of Producing a Commodity from a Biomass of Increasing Complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Kraan, S. Mass-Cultivation of Carbohydrate Rich Macroalgae, a Possible Solution for Sustainable Biofuel Production. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Gengiah, K.; Moses, G.L.P.; Baskar, G. Bioethanol Production from Codium Tomentosum Residue. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–10. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Marhaeni, B.; Oktaviani, D.F.; Jeong, G.T.; Hong, Y.K. Comparison of Bioethanol Production from Cultivated versus Wild Gracilaria Verrucosa and Gracilaria Gigas. J. Appl. Phycol. 2018, 30, 143–147. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Cook, D.J. Bioethanol Production from UK Seaweeds: Investigating Variable Pre-Treatment and Enzyme Hydrolysis Parameters. Bioenergy Res. 2020, 13, 271–285. [Google Scholar] [CrossRef]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae Biorefinery: Review on a Broad Spectrum of Downstream Processes and Products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Ra, C.H.; Lee, H.J.; Shin, M.K.; Kim, S.-K. Bioethanol Production from Seaweed Gelidium Amansii for Separated Hydrolysis and Fermentation (SHF). KSBB J. 2013, 28, 282–286. [Google Scholar] [CrossRef][Green Version]
- Meinita, M.D.N.; Marhaeni, B.; Hong, Y.K.; Jeong, G.T. Enzymatic Saccharification of Agar Waste from Gracilaria Verrucosa and Gelidium Latifolium for Bioethanol Production. J. Appl. Phycol. 2017, 29, 3201–3209. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.J.; Park, H.D.; Lim, D.J.; Kim, S.H. Anaerobic Digestibility of Algal Bioethanol Residue. Bioresour. Technol. 2012, 113, 78–82. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel Macroalgae (Seaweed) Biorefinery Systems for Integrated Chemical, Protein, Salt, Nutrient and Mineral Extractions and Environmental Protection by Green Synthesis and Life Cycle Sustainability Assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Rybicki, E.; Pienkos, P.T.; Stockenreiter, M.; Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine Macroalgae in a Circular Economy Context: A Comprehensive Analysis Focused on Residual Biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed Biorefinery: A Sustainable Process for Valorising the Biomass of Brown Seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Flórez-Fernández, N.; Dolores Torres, M.; Domínguez, H.; Rodríguez-Jasso, R.M.; Ruiz, H.A. Hydrothermal Systems to Obtain High Value-Added Compounds from Macroalgae for Bioeconomy and Biorefineries. Bioresour. Technol. 2022, 343, 126017. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, P.; Xiao, L.; Liang, Y.; Huang, Y.; Ye, H.; Wu, K.; Lu, Y. Enrichment of Lipids from Agar Production Wastes of Gracilaria Lemaneiformis by Ultrasonication: A Green Sustainable Process. Biomass Convers. Biorefinery 2021, 11, 2899–2908. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound Assisted Methods for Enhanced Extraction of Phycobiliproteins from Marine Macro-Algae, Gelidium Pusillum (Rhodophyta). Ultrason. Sonochemistry 2017, 38, 92–103. [Google Scholar] [CrossRef]
- de Jong, E.; Stichnothe, H.; Bell, G.; Henning Jørgensen, M.; de Bari, I.; Jacco van Haveren, E.; Lindorfer, J.; an der Johannes Kepler, E. Bio-Based Chemicals a 2020 Update (PDF Version); IEA Bioenergy: Paris, France, 2020. [Google Scholar]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Braungart, M.; McDonough, W. Cradle to Cradle: Remaking the Way We Make Things; North Point Press: New York, NY, USA, 2002; ISBN 0-86547-587-3. [Google Scholar]


| References Retrieved | Science Direct | Google Scholar |
|---|---|---|
| Gelidium corneum | 192 | 1320 |
| Gelidium corneum AND Agar | 98 | 584 |
| Gelidium sesquipedale | 222 | 1900 |
| Gelidium sesquipedale AND Agar | 123 | 963 |
| Shared references G. corneum and G. sesquipedale | 25 | 196 |
| Total references | 399 | 3220 |
| Type of Resource | Properties and Applications | References |
|---|---|---|
| Gelidium corneum wild biomass or extracts applications | ||
| Raw biomass chemical composition Raw biomass elements’ bioavailability Raw dry biomass and storage Raw biomass yield improvement Raw biomass Process optimization | Agar industry | [26,34,35,37,38,127,128,129,130,131,132,133,134,135,136] |
| EtOH/Aq extracts Chf extracts MAA | Antioxidant | [48,49,54,137,138,139] |
| MeOH extracts DCM-MeOH extracts DCM-EtOH extracts | Antimicrobial | [6,14,51,140] |
| Ag nanoparticles | Antimicrobial Antifouling | [52] |
| EtOH extracts | anti-inflammatory | [53] |
| Aq extracts DCM-methanol extracts MeOH/Aq extract | Anti-proliferative Cytotoxicity | [53,54,55] |
| Elemental analysis | Food, food supplement | [69] |
| Waste biomass hydrolysates | Source of biochemicals for biomaterials, biofuels, and fine chemicals, such as poly-3 hydroxybutyrate and D-tagatose | [141,142,143] |
| Biomass mechano-enzymatic deconstruction of sugar and bioethanol | Biofuels | [144] |
| Agarose and agar-based matrices | Probiotics encapsulation | [145] |
| Oligosaccharides | Phyto-stimulant | [64] |
| Agar-based hydrogels and aerogels Enzyme immobilization in nanoflowers for lactose breakdown | UV protection | [50] |
| Agar, agar-gelatines Polysaccharides, fibres Nanocellulose biofilms | Antimicrobial/Antioxidant Edible biofilms for food packaging Biodegradable biocomposites | [146,147,148,149,150,151,152,153,154,155,156,157] |
| Agar/clay nanocomposite films | Biodegradable packaging | [153,158] |
| Heated mucilaginous carbohydrates | Paper | [159,160,161] |
| Biomass fermentation for ethanol production Cellulosic ethanol | Biofuel | [159,162] |
| Gelidium corneum waste biomass applications | ||
| Waste chemical characterization | Antioxidant | [48] |
| Cellulose nanocrystals | Polymer composites Bioplastics | [163] |
| Biodegradable biofilms | Bioplastics for packaging | [164,165,166,167] |
| Biochar | Biofuel Adsorbent | [168,169,170,171,172] |
| Full waste biomass | Antifungal soap for cosmetics | [173] |
| Full waste biomass | Biofertilizer | [126] |
| Full waste biomass | Bioremediation Biosorption | [56,57,58,59,61,62,63,174,175,176,177,178,179] |
| Gelidium corneum Chemical Composition and Antioxidant Activity | Wild Harvested Biomass | Agar Industry Residue |
|---|---|---|
| Proximate Analysis (% dry weight) | ||
| Protein | 14.19 ± 0.33 | 20.59 ± 0.79 |
| Lipids | 2.10 ± 0.11 | 0.95 ± 0.04 |
| Carbohydrates | 33.30 ± 1.25 | 36.40 ± 3.96 |
| Antioxidant Analysis (% Inhibition) | ||
| ABTS | 12.31 ± 1.39 | 9.79 ± 0.15 |
| DPPH | 8.50 ± 1.32 | 7.53 ± 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouga, T.; Fernandes, I.B. The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework. Earth 2022, 3, 788-813. https://doi.org/10.3390/earth3030045
Mouga T, Fernandes IB. The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework. Earth. 2022; 3(3):788-813. https://doi.org/10.3390/earth3030045
Chicago/Turabian StyleMouga, Teresa, and Isabel Barreto Fernandes. 2022. "The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework" Earth 3, no. 3: 788-813. https://doi.org/10.3390/earth3030045
APA StyleMouga, T., & Fernandes, I. B. (2022). The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework. Earth, 3(3), 788-813. https://doi.org/10.3390/earth3030045







