Sublethal and Lethal Effects of Low-Dose Prothioconazole Alone and in Combination with Low-Dose Lambda-Cyhalothrin on Carabid Beetles in a Field-Realistic Scenario
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Insects
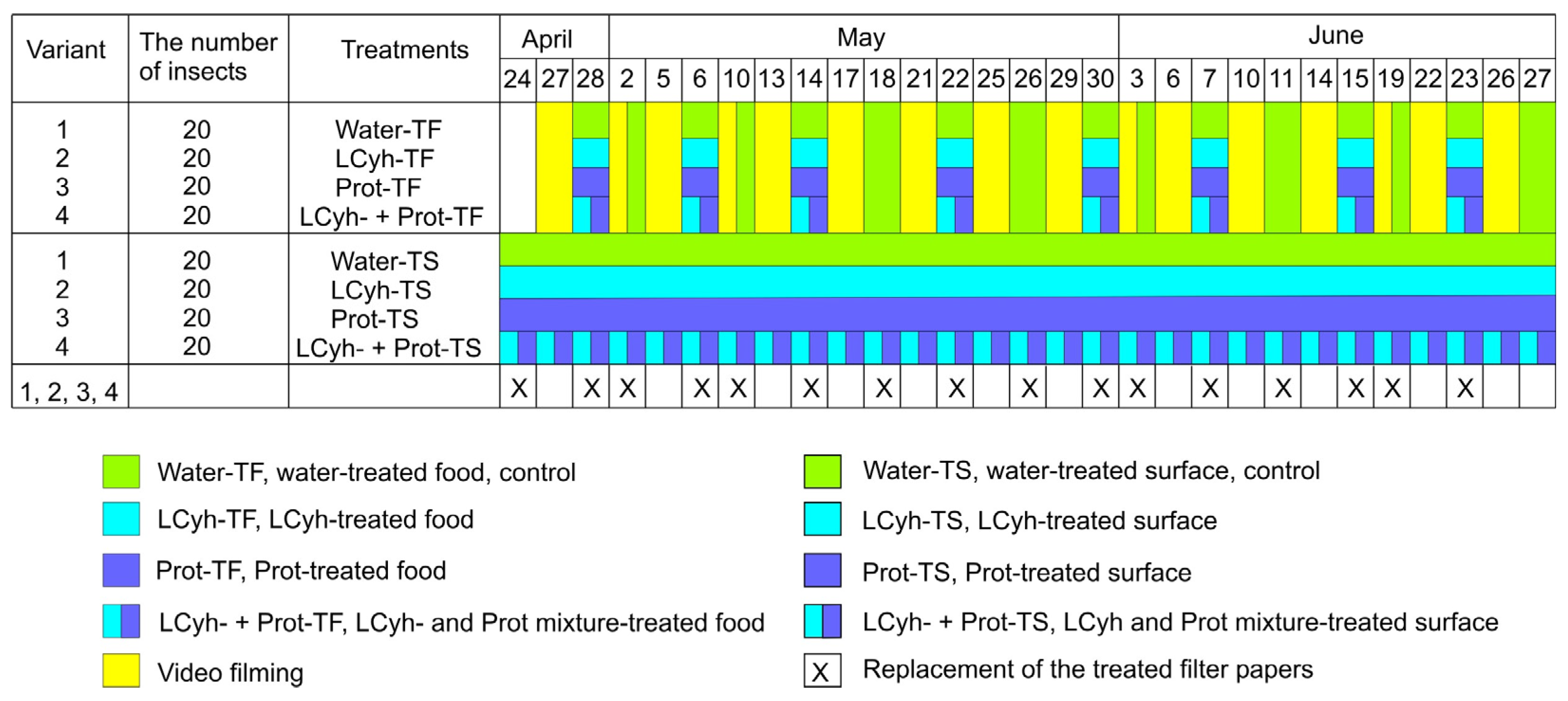
2.2. Treatments
2.2.1. Preparation of Filter Paper Disks for Contact Exposure
2.2.2. Food Contamination for Oral Pesticide Exposure
2.3. Video Recordings and Video Data Analysis
2.4. Mortality Assessment
2.5. Statistical Analyses
3. Results
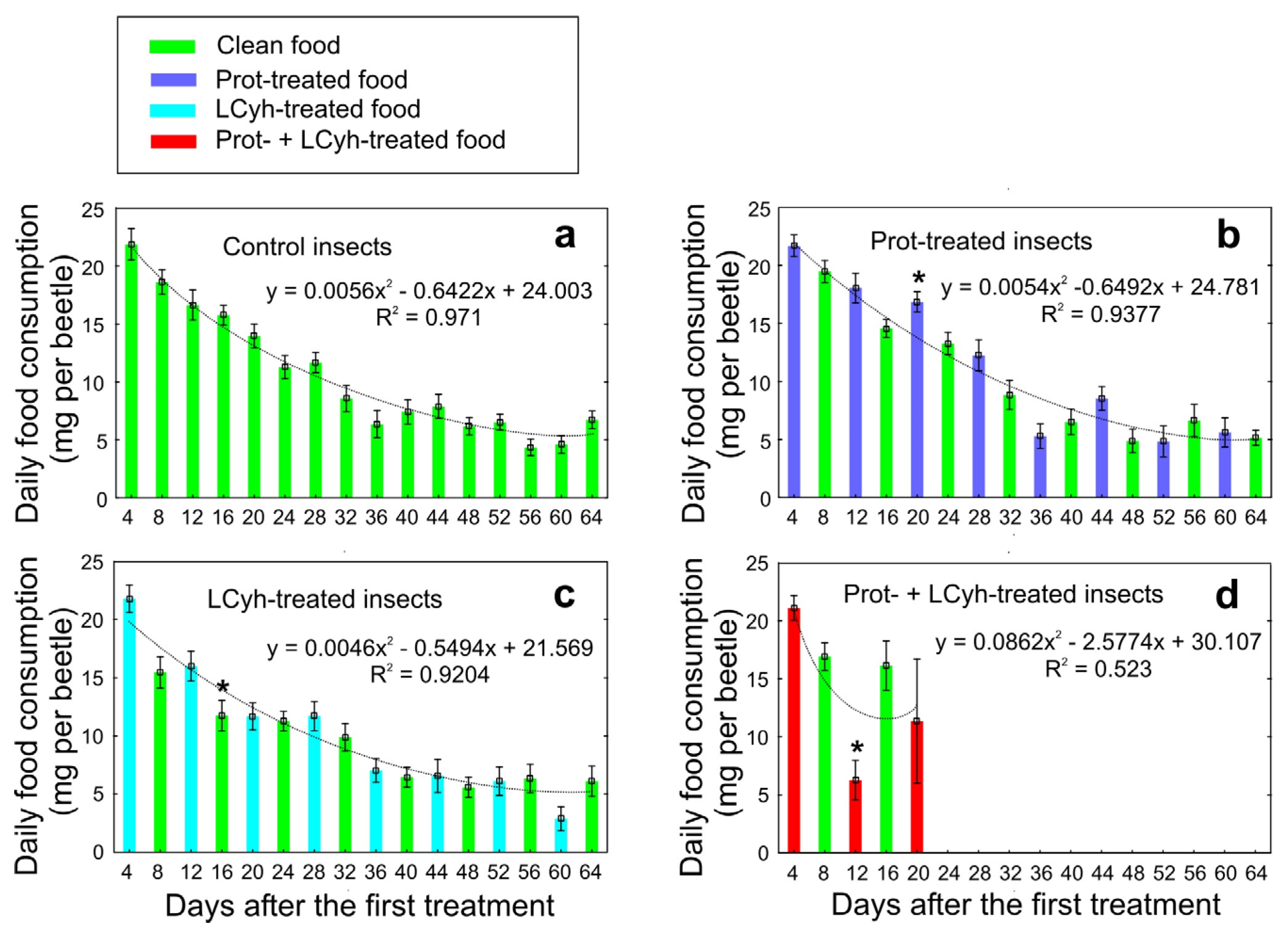
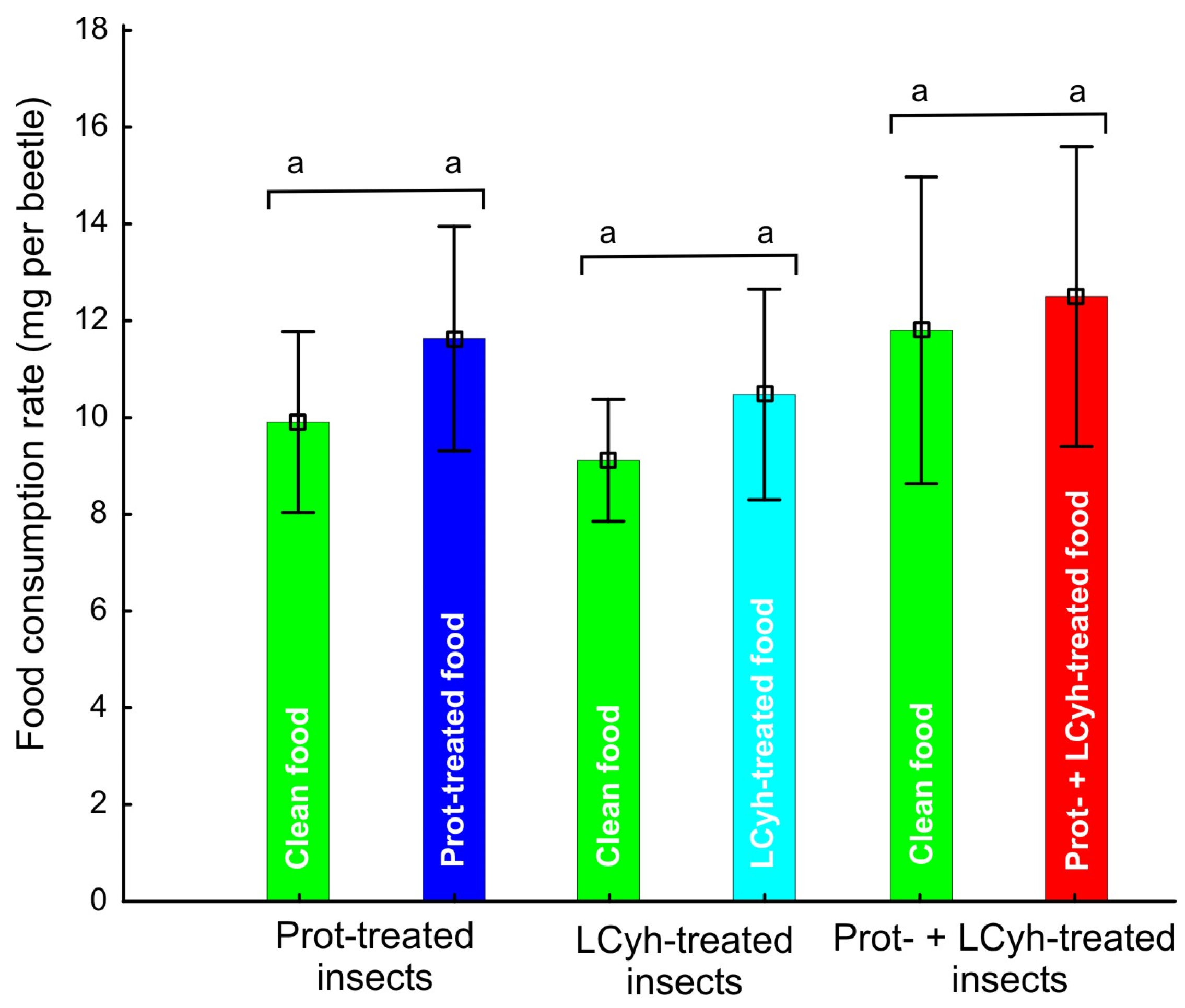
3.1. Food Consumption
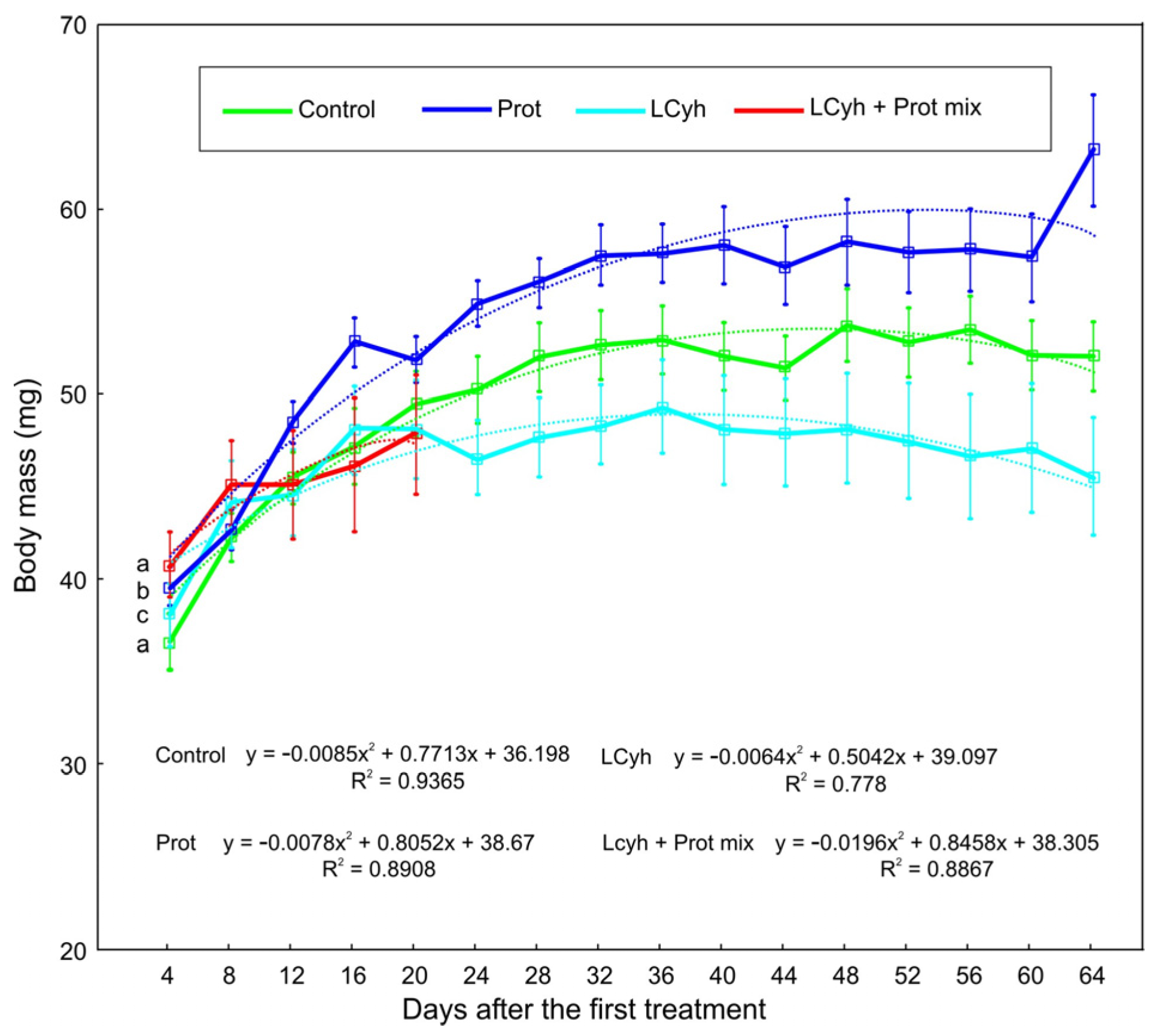
3.2. Body Mass
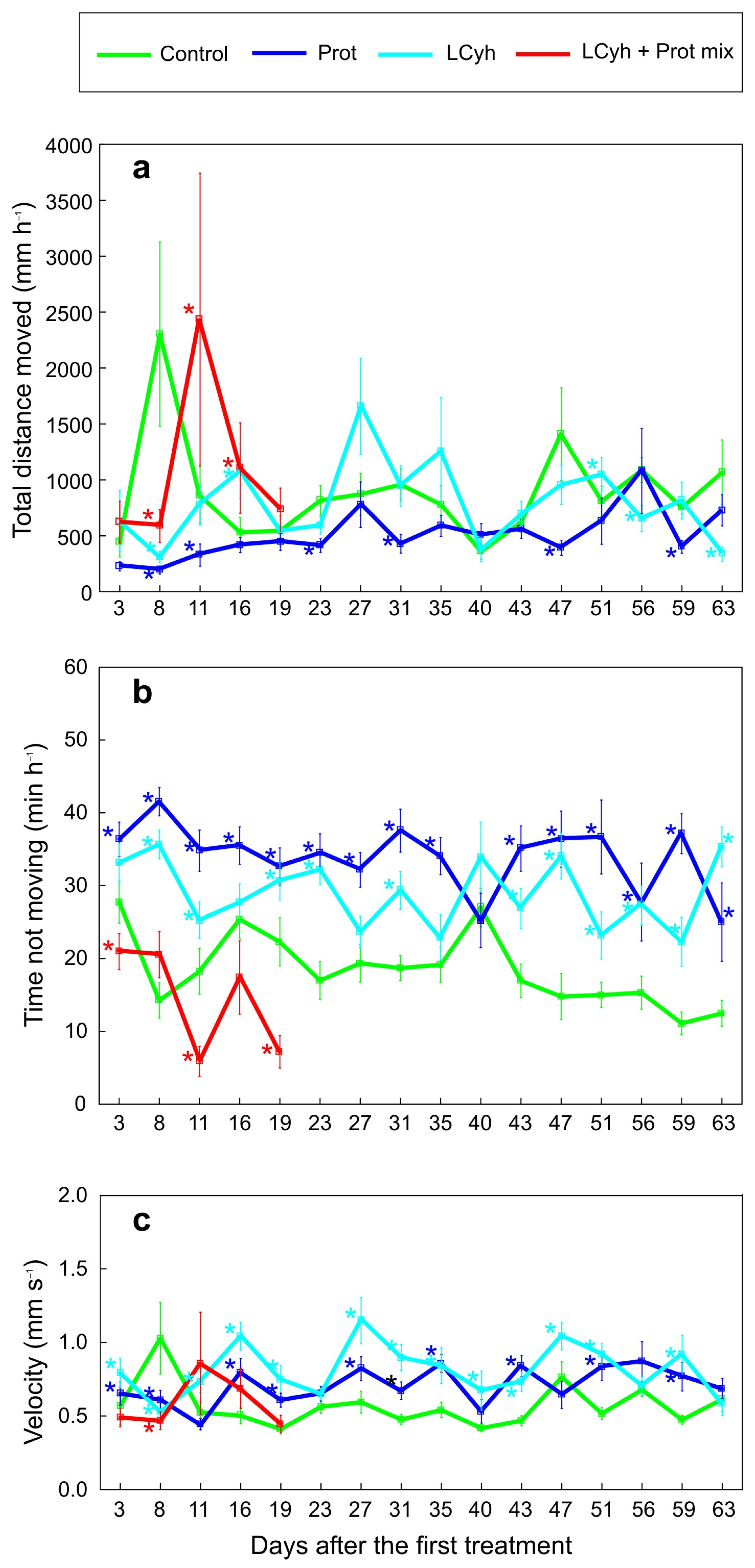
3.3. Locomotor Activity
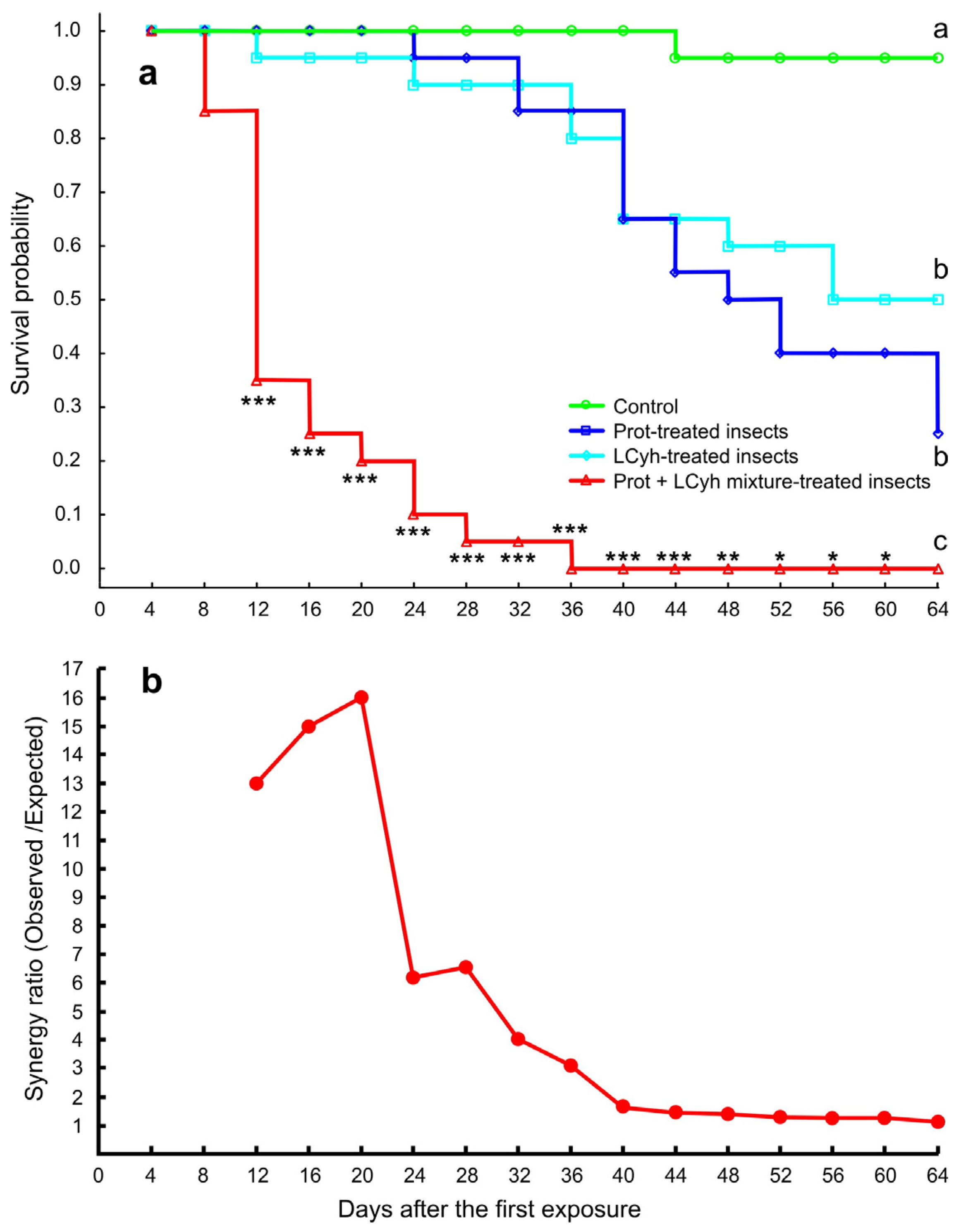
3.4. Mortality
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferrario, C.; Finizio, A.; Villa, S. Legacy and Emerging Contaminants in Meltwater of Three Alpine Glaciers. Sci. Total Environ. 2017, 574, 350–357. [Google Scholar] [CrossRef]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and Recently Used Pesticides in Central European Arable Soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide Residues in European Agricultural Soils—A Hidden Reality Unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- van der Sluijs, J.P. Insect Decline, an Emerging Global Environmental Risk. Reflect. Adv. Health Environ. Res. Context COVID-19. Pandemic 2020, 46, 39–42. [Google Scholar] [CrossRef]
- Quandahor, P.; Kim, L.; Kim, M.; Lee, K.; Kusi, F.; Jeong, I. Effects of Agricultural Pesticides on Decline in Insect Species and Individual Numbers. Environments 2024, 11, 182. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A Global-Scale Expert Assessment of Drivers and Risks Associated with Pollinator Decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Storck, V.; Karpouzas, D.G.; Martin-Laurent, F. Towards a Better Pesticide Policy for the European Union. Sci. Total Environ. 2017, 575, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Zaller, J.G. Biodiversity Decline as a Consequence of an Inappropriate Environmental Risk Assessment of Pesticides. Front. Environ. Sci. 2019, 7, 177. [Google Scholar] [CrossRef]
- Solé, M.; Brendel, S.; Aldrich, A.; Dauber, J.; Ewald, J.; Duquesne, S.; Gottschalk, E.; Hoffmann, J.; Kuemmerlen, M.; Leake, A.; et al. Assessing In-Field Pesticide Effects under European Regulation and Its Implications for Biodiversity: A Workshop Report. Environ. Sci. Eur. 2024, 36, 153. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the Science behind the Development of a Risk Assessment of Plant Protection Products on Bees (Apis Mellifera, Bombus Spp. and Solitary Bees). EFSA J. 2012, 10, 2668. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion Addressing the State of the Science on Risk Assessment of Plant Protection Products for Non-Target Arthropods. EFSA J. 2015, 13, 3996. [Google Scholar] [CrossRef]
- Stehle, S.; Schulz, R. Pesticide Authorization in the EU—Environment Unprotected? Environ. Sci. Pollut. Res. 2015, 22, 19632–19647. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Teder, T.; Knapp, M. Sublethal Effects Enhance Detrimental Impact of Insecticides on Non-Target Organisms: A Quantitative Synthesis in Parasitoids. Chemosphere 2019, 214, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Willow, J.; Silva, A.; Veromann, E.; Smagghe, G. Acute Effect of Low-Dose Thiacloprid Exposure Synergised by Tebuconazole in a Parasitoid Wasp. PLoS ONE 2019, 14, e0212456. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and Pesticide Regulation: Lessons from the Neonicotinoid Experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting Agriculturally Driven Global Environmental Change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Rebek, E.; Frank, S.; Royer, T.; Bogran, C.E. Alternatives to Chemical Control of Insect Pests. In Insecticides—Basic and Other Applications; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Swinton, S.M. Incorporating Natural Enemies in an Economic Threshold for Dynamically Optimal Pest Management. Ecol. Model. 2009, 220, 1315–1324. [Google Scholar] [CrossRef]
- Kromp, B. Carabid Beetles in Sustainable Agriculture: A Review on Pest Control Efficacy, Cultivation Impacts and Enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can Generalist Predators Be Effective Biocontrol Agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Lundgren, J.G. Relationships of Natural Enemies and Non-Prey Foods; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z.; Jarosik, V. Ground Beetles (Carabidae) as Seed Predators. EJE 2003, 100, 531–544. [Google Scholar] [CrossRef]
- Bohan, D.A.; Boursault, A.; Brooks, D.R.; Petit, S. National-Scale Regulation of the Weed Seedbank by Carabid Predators. J. Appl. Ecol. 2011, 48, 888–898. [Google Scholar] [CrossRef]
- Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods: IOBC, BART and EPPO Joint Initiative; Candolfi, M.P., International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palearctic Regional Section (IOBC/WPRS), International Organization for Biological and Integrated Control of Noxious Animals and Plants, Eds.; West Palearctic Regional Section (IOBC/WPRS): Gent, Belgium, 2000. [Google Scholar]
- OECD. Test No. 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding); OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2017. [Google Scholar] [CrossRef]
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Hautier, L. Time-to-Death Approach to Reveal Chronic and Cumulative Toxicity of a Fungicide for Honeybees Not Revealed with the Standard Ten-Day Test. Sci. Rep. 2018, 8, 7241. [Google Scholar] [CrossRef]
- Fisher, A.; Cogley, T.; Ozturk, C.; DeGrandi-Hoffman, G.; Smith, B.H.; Kaftanoglu, O.; Fewell, J.H.; Harrison, J.F. The Active Ingredients of a Mitotoxic Fungicide Negatively Affect Pollen Consumption and Worker Survival in Laboratory-Reared Honey Bees (Apis mellifera). Ecotoxicol. Environ. Saf. 2021, 226, 112841. [Google Scholar] [CrossRef]
- Moncharmont, F.D.; Decourtye, A.; Hennequet-Hantier, C.; Pons, O.; Pham-Delègue, M. Statistical Analysis of Honeybee Survival after Chronic Exposure to Insecticides. Environ. Toxicol. Chem. 2003, 22, 3088–3094. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, G.; Sánchez-Bayo, F.; Tennekes, H.A.; Decourtye, A.; Ramírez-Romero, R.; Desneux, N. Delayed and Time-Cumulative Toxicity of Imidacloprid in Bees, Ants and Termites. Sci. Rep. 2014, 4, 5566. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular Mechanisms of Metabolic Resistance to Synthetic and Natural Xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- du Rand, E.E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.W.; Nicolson, S.W. Detoxification Mechanisms of Honey Bees (Apis mellifera) Resulting in Tolerance of Dietary Nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef]
- Christen, V.; Mittner, F.; Fent, K. Molecular Effects of Neonicotinoids in Honey Bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef]
- Schuhmann, A.; Schmid, A.P.; Manzer, S.; Schulte, J.; Scheiner, R. Interaction of Insecticides and Fungicides in Bees. Front. Insect Sci. 2022, 808335. [Google Scholar] [CrossRef]
- Pilling, E.D.; Bromleychallenor, K.A.C.; Walker, C.H.; Jepson, P.C. Mechanism of Synergism between the Pyrethroid Insecticide λ-Cyhalothrin and the Imidazole Fungicide Prochloraz, in the Honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 1995, 51, 1–11. [Google Scholar] [CrossRef]
- Thompson, H.M.; Fryday, S.L.; Harkin, S.; Milner, S. Potential Impacts of Synergism in Honeybees (Apis mellifera) of Exposure to Neonicotinoids and Sprayed Fungicides in Crops. Apidologie 2014, 45, 545–553. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, M.T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Molowny-Horas, R.; Bosch, J. Synergistic Mortality between a Neonicotinoid Insecticide and an Ergosterol-Biosynthesis-Inhibiting Fungicide in Three Bee Species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef]
- Raimets, R.; Karise, R.; Mänd, M.; Kaart, T.; Ponting, S.; Song, J.; Cresswell, J.E. Synergistic Interactions between a Variety of Insecticides and an Ergosterol Biosynthesis Inhibitor Fungicide in Dietary Exposures of Bumble Bees (Bombus terrestris L.). Pest Manag. Sci. 2018, 74, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C. Lethal and Sublethal Synergistic Effects of a New Systemic Pesticide, Flupyradifurone (Sivanto®), on Honeybees. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190433. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.; Garrido, P.M.; Bruno, D.; Braglia, C.; Alberoni, D.; Baffoni, L.; Tettamanti, G.; Porrini, M.P.; Di Gioia, D.; Angeli, S. Combined Effect of a Neonicotinoid Insecticide and a Fungicide on Honeybee Gut Epithelium and Microbiota, Adult Survival, Colony Strength and Foraging Preferences. Sci. Total Environ. 2023, 905, 167277. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Wen, Z.; Schuler, M.A.; Berenbaum, M.R. Mediation of Pyrethroid Insecticide Toxicity to Honey Bees (Hymenoptera: Apidae) by Cytochrome P450 Monooxygenases. J. Econ. Entomol. 2006, 99, 1046–1050. [Google Scholar] [CrossRef]
- EU Pesticides Database. Available online: https://Food.Ec.Europa.Eu/Plants/Pesticides/Eu-Pesticides-Database_en (accessed on 6 May 2024).
- Syngenta. Available online: https://www.syngenta.com/ (accessed on 6 June 2025).
- Bayer—Global Home|Bayer Global. Available online: https://www.bayer.com/en/ (accessed on 6 June 2025).
- European Food Safety Authority (EFSA). Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Prothioconazole. EFSA J. 2007, 5, 106r. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, L.; Mao, L.; Zhu, L.; Zhang, Y.; Jiang, H.; Zheng, Y.; Liu, X. Sorption and Degradation of Prothioconazole and Its Metabolites in Soils and Water Sediments, and Its Combinative Toxicity to Gobiocypris Rarus. Chemosphere 2022, 303, 135282. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Zhang, L.; Liu, H.; Zhang, C.; Liu, D.; Wang, P.; Zhou, Z. Enantioselective Degradation of Prothioconazole in Soil and the Impacts on the Enzymes and Microbial Community. Sci. Total Environ. 2022, 824, 153658. [Google Scholar] [CrossRef]
- Wood, S.C.; de Mattos, I.M.; Kozii, I.V.; Klein, C.D.; Dvylyuk, I.; Folkes, C.D.A.; de Carvalho Macedo Silva, R.; Moshynskyy, I.; Epp, T.; Simko, E. Effects of Chronic Dietary Thiamethoxam and Prothioconazole Exposure on Apis mellifera Worker Adults and Brood. Pest Manag. Sci. 2020, 76, 85–94. [Google Scholar] [CrossRef]
- Freitas, T.A.D.L.; Alves, T.R.R.; Trivellato, M.F.; Kato, A.Y.; Gomes, C.R.D.A.; Ferraz, Y.M.M.; Nicodemo, D. Food Supplementation Does Not Prevent Oxidative Stress in Forager Honey Bees Exposed to the Fungicides Bixafen, Prothioconazole and Trifloxystrobin. J. Apic. Res. 2024, 63, 1–12. [Google Scholar] [CrossRef]
- Kato, A.Y.; Freitas, T.A.L.; Gomes, C.R.A.; Alves, T.R.R.; Ferraz, Y.M.M.; Trivellato, M.F.; De Jong, D.; Biller, J.D.; Nicodemo, D. Bixafen, Prothioconazole, and Trifloxystrobin Alone or in Combination Have a Greater Effect on Health Related Gene Expression in Honey Bees from Nutritionally Deprived than from Protein Supplemented Colonies. Insects 2024, 15, 523. [Google Scholar] [CrossRef]
- Hornsby, A.G.; Herner, A.E.; Don Wauchope, R. Pesticide Properties in the Environment; Springer: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Lambda-cyhalothrin. EFSA J. 2014, 12, 3677, 1–170. [Google Scholar] [CrossRef]
- Nare, R.W.A.; Savadogo, P.W.; Gajbhiye, V.T.; Singh, S.B.; Mukherjee, I.; Nacro, H.B.; Sedogo, M.P. Dissipation of Lambda-Cyhalothrin under Organic Amendment in Vegetable Soils in Burkina Faso under Laboratory Conditions. J. Environ. Sci. Technol. 2019, 12, 125–130. [Google Scholar] [CrossRef]
- Hill, B.D.; Inaba, D.J. Dissipation of Lambda-Cyhalothrin on Fallow vs Cropped Soil. J. Agric. Food Chem. 1991, 39, 2282–2284. [Google Scholar] [CrossRef]
- Ngaini, Z.; Rudy, C.; Farooq, S.; Henry, M.C.; Chai, A. Differential Degradation Dynamics of λ-Cyhalothrin in Mineral and Peat Soils: A Comparative Study under Laboratory Condition. Discov. Appl. Sci. 2024, 6, 78. [Google Scholar] [CrossRef]
- Thiele, H.-U. Carabid Beetles in Their Environments; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar] [CrossRef]
- Lindroth, C.H. The Carabidae (Coleoptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherland, 1986. [Google Scholar]
- Honek, A.; Kocian, M. Importance of Woody and Grassy Areas as Refugia for Field Carabidae and Staphylinidae (Coleoptera). Acta Soc. Zool. Bohem. Czech Repub. 2003, 67, 71–81. [Google Scholar]
- Purtauf, T.; Dauber, J.; Wolters, V. The Response of Carabids to Landscape Simplification Differs between Trophic Groups. Oecologia 2005, 142, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Purtauf, T.; Roschewitz, I.; Dauber, J.; Thies, C.; Tscharntke, T.; Wolters, V. Landscape Context of Organic and Conventional Farms: Influences on Carabid Beetle Diversity. Agric. Ecosyst. Environ. 2005, 108, 165–174. [Google Scholar] [CrossRef]
- Kosewska, A.; Skalski, T.; Nietupski, M. Effect of Conventional and Non-Inversion Tillage Systems on the Abundance and Some Life History Traits of Carabid Beetles (Coleoptera: Carabidae) in Winter Triticale Fields. EJE 2014, 111, 669–676. [Google Scholar] [CrossRef]
- Grandchamp, A.-C.; Bergamini, A.; Stofer, S.; Niemelä, J.; Duelli, P.; Scheidegger, C. The Influence of Grassland Management on Ground Beetles (Carabidae, Coleoptera) in Swiss Montane Meadows. Agric. Ecosyst. Environ. 2005, 110, 307–317. [Google Scholar] [CrossRef]
- Larochelle, A. The Food of Carabid Beetles. Fabreries 1990, (Suppl. 5), 1–132. [Google Scholar]
- Zalewski, M.; Dudek, D.; Tiunov, A.V.; Godeau, J.-F.; Okuzaki, Y.; Ikeda, H.; Sienkiewicz, P.; Ulrich, W. High Niche Overlap in the Stable Isotope Space Of Ground Beetles. Ann. Zool. Fenn. 2014, 51, 301–312. [Google Scholar] [CrossRef]
- Neudecker, C.; Thiele, H.-U. Die Jahreszeitliche Synchronisation Der Gonadenreifung Bei Agonum Assimile Payk. (Coleopt. Carab.) Durch Temperatur Und Photoperiode. Oecologia 1974, 17, 141–157. [Google Scholar] [CrossRef]
- Tooming, E.; Merivee, E.; Must, A.; Sibul, I.; Williams, I. Sub-Lethal Effects of the Neurotoxic Pyrethroid Insecticide Fastac 50EC on the General Motor and Locomotor Activities of the Non-Targeted Beneficial Carabid Beetle Platynus assimilis (Coleoptera: Carabidae). Pest Manag. Sci. 2014, 70, 959–966. [Google Scholar] [CrossRef]
- Tooming, E.; Merivee, E.; Must, A.; Merivee, M.-I.; Sibul, I.; Nurme, K.; Williams, I.H. Behavioural Effects of the Neonicotinoid Insecticide Thiamethoxam on the Predatory Insect Platynus assimilis. Ecotoxicology 2017, 26, 902–913. [Google Scholar] [CrossRef]
- Merivee, E.; Tooming, E.; Must, A.; Sibul, I.; Williams, I.H. Low Doses of the Common Alpha-Cypermethrin Insecticide Affect Behavioural Thermoregulation of the Non-Targeted Beneficial Carabid Beetle Platynus assimilis (Coleoptera: Carabidae). Ecotoxicol. Environ. Saf. 2015, 120, 286–294. [Google Scholar] [CrossRef]
- Hainzl, D.; Casida, J.E. Fipronil Insecticide: Novel Photochemical Desulfinylation with Retention of Neurotoxicity. Proc. Natl. Acad. Sci. USA 1996, 93, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Iverson, A.; Hale, C.; Richardson, L.; Miller, O.; McArt, S. Synergistic Effects of Three Sterol Biosynthesis Inhibiting Fungicides on the Toxicity of a Pyrethroid and Neonicotinoid Insecticide to Bumble Bees. Apidologie 2019, 50, 733–744. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The Search for Synergy: A Critical Review from a Response Surface Perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [CrossRef] [PubMed]
- Blaney, W.M.; Simmonds, M.S.J.; Ley, S.V.; Anderson, J.C.; Toogood, P.L. Antifeedant Effects of Azadirachtin and Structurally Related Compounds on Lepidopterous Larvae. Entomol. Exp. Appl. 1990, 55, 149–160. [Google Scholar] [CrossRef]
- Han, P.; Niu, C.-Y.; Lei, C.-L.; Cui, J.-J.; Desneux, N. Quantification of Toxins in a Cry1Ac + CpTI Cotton Cultivar and Its Potential Effects on the Honey Bee Apis mellifera L. Ecotoxicology 2010, 19, 1452–1459. Ecotoxicology 2010, 19, 1452–1459. [Google Scholar] [CrossRef]
- Abouelghar, G.E.; Sakr, H.; Ammar, H.A.; Yousef, A.; Nassar, M. Sublethal Effects of Spinosad (Tracer®) on the Cotton Leafworm (Lepidoptera: Noctuidae). J. Plant Prot. Res. 2013, 53, 275–284. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Zheng, Y.; Weng, Q.; Biondi, A.; Desneux, N.; Wu, K. Assessment of Potential Sublethal Effects of Various Insecticides on Key Biological Traits of The Tobacco Whitefly, Bemisia tabaci. Int. J. Biol. Sci. 2013, 9, 246–255. [Google Scholar] [CrossRef]
- van Herk, W.G.; Vernon, R.S.; Vojtko, B.; Snow, S.; Fortier, J.; Fortin, C. Contact Behaviour and Mortality of Wireworms Exposed to Six Classes of Insecticide Applied to Wheat Seed. J. Pest Sci. 2015, 88, 717–739. [Google Scholar] [CrossRef]
- Degrandi-Hoffman, G.; Chen, Y.; Watkins Dejong, E.; Chambers, M.L.; Hidalgo, G. Effects of Oral Exposure to Fungicides on Honey Bee Nutrition and Virus Levels. J. Econ. Entomol. 2015, 108, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Wiles, J.A.; Jepson, P.C. The Dietary Toxicity of Deltamethrin to the Carabid, Nebria brevicollis (F.). Pestic. Sci. 1993, 38, 329–334. [Google Scholar] [CrossRef]
- Luntz, A.J.M.; Cottee, P.K.; Evans, K.A. Azadirachtin: Its Effect on Gut Motility, Growth and Moulting in Locusta. Physiol. Entomol. 1985, 10, 431–437. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Blaney, W.M.; Ley, S.V.; Anderson, J.C.; Toogood, P.L. Azadirachtin: Structural Requirements for Reducing Growth and Increasing Mortality in Lepidopterous Larvae. Entomol. Exp. Appl. 1990, 55, 169–181. [Google Scholar] [CrossRef]
- Rembold, H. Isomeric Azadirachtins and Their Mode of Action. In Focus on Phytochemical Pesticides; Arnason, J.T., Philogène, B.J.R., Morand, P., Eds.; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 47–67. [Google Scholar]
- Koul, O.; Isman, M.B. Effects of Azadirachtin on the Dietary Utilization and Development of the Variegated Cutworm Peridroma saucia. J. Insect Physiol. 1991, 37, 591–598. [Google Scholar] [CrossRef]
- Timmins, W.A.; Reynolds, S.E. Azadirachtin Inhibits Secretion of Trypsin in Midgut of Manduca Sexta Caterpillars: Reduced Growth Due to Impaired Protein Digestion. Entomol. Exp. Appl. 1992, 63, 47–54. [Google Scholar] [CrossRef]
- Sheehan, P.J. Effects on Individuals and Populations. In Effects of Pollutants in Ecosystem Level; Sheehan, P.J., Miller, D.R., Butler, G.C., Bourdeau, P., Eds.; Chichester Jon Willey Sons Ltd.: Chichester, UK, 1984. [Google Scholar]
- Sparks, T.C.; Lockwood, J.A.; Byford, R.L.; Graves, J.B.; Leonard, B.R. The Role of Behavior in Insecticide Resistance. Pestic. Sci. 1989, 26, 383–399. [Google Scholar] [CrossRef]
- Nansen, C.; Baissac, O.; Nansen, M.; Powis, K.; Baker, G. Behavioral Avoidance—Will Physiological Insecticide Resistance Level of Insect Strains Affect Their Oviposition and Movement Responses? PLoS ONE 2016, 11, e0149994. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into Insecticide-Resistance Mechanisms in Invasive Species: Challenges and Control Strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef] [PubMed]
- Baatrup, E.; Bayley, M. Animal Locomotor Behaviour as a Health Biomarker of Chemical Stress. Arch. Toxicol. 1998, 20, 163–178. [Google Scholar]
- Bayley, M. Basic Behaviour: The Use of Animal Locomotion in Behavioural Ecotoxicology. In Behavioural Ecotoxicology; Dell’Omo, G., Ed.; John. Wiley & Sons: Chichester, UK, 2002; pp. 211–230. [Google Scholar]
- Mauchline, A.L.; Osborne, J.L.; Powell, W. Feeding Responses of Carabid Beetles to Dimethoate-Contaminated Prey. Agric. For. Entomol. 2004, 6, 99–104. [Google Scholar] [CrossRef]
- Douglas, M.R.; Rohr, J.R.; Tooker, J.F. Neonicotinoid Insecticide Travels through a Soil Food Chain, Disrupting Biological Control of Non-Target Pests and Decreasing Soya Bean Yield. J. Appl. Ecol. 2015, 52, 250–260. [Google Scholar] [CrossRef]
- Cutler, G.C.; Astatkie, T.; Chahil, G.S. Weed Seed Granivory by Carabid Beetles and Crickets for Biological Control of Weeds in Commercial Lowbush Blueberry Fields. Agric. For. Entomol. 2016, 18, 390–397. [Google Scholar] [CrossRef]
- Šerić Jelaska, L.; Jelic, M.; Andelic Dmitrovic, B.; Kos, T. Bioaccumulation of Pesticides in Carabid Beetles in a Vineyard and Olive Grove under Integrated Pest Management. EJE 2024, 121, 269–279. [Google Scholar] [CrossRef]
- Vinha, G.L.; Plata-Rueda, A.; Soares, M.A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Deltamethrin-Mediated Effects on Locomotion, Respiration, Feeding, and Histological Changes in the Midgut of Spodoptera frugiperda Caterpillars. Insects 2021, 12, 483. [Google Scholar] [CrossRef]
- Quellhorst, H.E.; Arthur, F.H.; Bruce, A.; Zhu, K.Y.; Morrison, W.R. Exposure to a Novel Insecticide Formulation on Maize and Concrete Reduces Movement by the Stored Product Pests, Prostephanus truncatus (Horn) and Sitophilus zeamais (Motschulsky). Front. Agron. 2022, 4, 868509. [Google Scholar] [CrossRef]
- Merivee, E.; Mürk, A.; Nurme, K.; Koppel, M.; Mänd, M. Automated Video-Tracking Analysis of Agriotes Obscurus Wireworm Behaviour before, during and after Contact with Thiamethoxam- and Imidacloprid-Treated Wheat Seeds. Sci. Rep. 2025, 15, 7218. [Google Scholar] [CrossRef]
- Merivee, E.; Must, A.; Nurme, K.; Koppel, M. Automated computer-centred video tracking analysis of wireworm behaviour in the soil bioassay arena in response to attractive, repellent and toxic compounds. Int. J. Pest Manag. 2025, 1–23. [Google Scholar] [CrossRef]
- Maul, J.D.; Brennan, A.A.; Harwood, A.D.; Lydy, M.J. Effect of Sediment-Associated Pyrethroids, Fipronil, and Metabolites on Chironomus Tentans Growth Rate, Body Mass, Condition Index, Immobilization, and Survival. Environ. Toxicol. Chem. 2008, 27, 2582–2590. [Google Scholar] [CrossRef]
- Huang, X.; Lv, S.; Zhang, Z.; Chang, B.H. Phenotypic and Transcriptomic Response of the Grasshopper Oedaleus asiaticus (Orthoptera: Acrididae) to Toxic Rutin. Front. Physiol. 2020, 11, 52. [Google Scholar] [CrossRef]
- Singh, D.S.; Alkins-Koo, M.; Rostant, L.V.; Mohammed, A. Sublethal Effects of Malathion Insecticide on Growth of the Freshwater Crab Poppiana Dentata (Randall, 1840) (Decapoda: Trichodactylidae). Nauplius 2021, 29, e2021030. [Google Scholar] [CrossRef]
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The Molecular Basis of Insecticide Resistance in Mosquitoes. Mol. Popul. Biol. Mosquitoes 2004, 34, 653–665. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Cutler, G.C. Insecticide-Induced Hormesis and Arthropod Pest Management. Pest Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef]
- Sial, M.U.; Zhao, Z.; Zhang, L.; Zhang, Y.; Mao, L.; Jiang, H. Evaluation of Insecticides Induced Hormesis on the Demographic Parameters of Myzus persicae and Expression Changes of Metabolic Resistance Detoxification Genes. Sci. Rep. 2018, 8, 16601. [Google Scholar] [CrossRef]
- Nestler, E.J. Transgenerational Epigenetic Contributions to Stress Responses: Fact or Fiction? PLoS Biol. 2016, 14, e1002426. [Google Scholar] [CrossRef]
- Margus, A.; Piiroinen, S.; Lehmann, P.; Tikka, S.; Karvanen, J.; Lindström, L. Sublethal Pyrethroid Insecticide Exposure Carries Positive Fitness Effects Over Generations in a Pest Insect. Sci. Rep. 2019, 9, 11320. [Google Scholar] [CrossRef]
- Margus, A.; Saifullah, S.; Kankare, M.; Lindström, L. Fungicides Modify Pest Insect Fitness Depending on Their Genotype and Population. Sci. Rep. 2023, 13, 17879. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Brandt, A.; Colli, M.; Fourrier, J.; Giffard, H.; Hernández-López, J.; Malagnini, V.; Williams, G.R.; Simon-Delso, N. Long-Term Field-Realistic Exposure to a next-Generation Pesticide, Flupyradifurone, Impairs Honey Bee Behaviour and Survival. Commun. Biol. 2021, 4, 805. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Adriaanse, P.; Arce, A.; Focks, A.; Ingels, B.; Jölli, D.; Lambin, S.; Rundlöf, M.; Süßenbach, D.; Del Aguila, M.; et al. Revised Guidance on the Risk Assessment of Plant Protection Products on Bees (Apis mellifera, Bombus Spp. and Solitary Bees). EFSA J. 2023, 21, e07989. [Google Scholar] [CrossRef]
- Pilling, E.D.; Jepson, P.C. Synergism between EBI Fungicides and a Pyrethroid Insecticide in the Honeybee (Apis mellifera). Pestic. Sci. 1993, 39, 293–297. [Google Scholar] [CrossRef]
- De Armas, F.S.; Dionei Grutzmacher, A.; Edson Nava, D.; Antonio Pasini, R.; Rakes, M.; de Bastos Pazini, J. Non-Target Toxicity of Nine Agrochemicals toward Larvae and Adults of Two Generalist Predators Active in Peach Orchards. Ecotoxicology 2020, 29, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Bartling, M.-T.; Brandt, A.; Hollert, H.; Vilcinskas, A. Current Insights into Sublethal Effects of Pesticides on Insects. Int. J. Mol. Sci. 2024, 25, 6007. [Google Scholar] [CrossRef] [PubMed]
- Blouquy, L.; Mottet, C.; Olivares, J.; Plantamp, C.; Siegwart, M.; Barrès, B. How Varying Parameters Impact Insecticide Resistance Bioassay: An Example on the Worldwide Invasive Pest Drosophila Suzukii. PLoS ONE 2021, 16, e0247756. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]

| Treatment | Final Mortality (%) | Z-Statistic | p |
|---|---|---|---|
| LCyh | 50 | z = 2.67 | p < 0.01 |
| Prot | 75 | z = 3.89 | p < 0.001 |
| LCyh + Prot mixture | 100 | z = 5.66 | p < 0.0001 |
| Treatment | LT50 (Days) | 95% Confidence Interval |
|---|---|---|
| LCyh | 60.26 | 49.52–71.01 |
| Prot | 51.57 | 39.89–63.25 |
| LCyh + Prot mixture | 12.21 | 3.24–21.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merivee, E.; Mürk, A.; Nurme, K.; Koppel, M.; Ploomi, A.; Mänd, M. Sublethal and Lethal Effects of Low-Dose Prothioconazole Alone and in Combination with Low-Dose Lambda-Cyhalothrin on Carabid Beetles in a Field-Realistic Scenario. Pollutants 2025, 5, 24. https://doi.org/10.3390/pollutants5030024
Merivee E, Mürk A, Nurme K, Koppel M, Ploomi A, Mänd M. Sublethal and Lethal Effects of Low-Dose Prothioconazole Alone and in Combination with Low-Dose Lambda-Cyhalothrin on Carabid Beetles in a Field-Realistic Scenario. Pollutants. 2025; 5(3):24. https://doi.org/10.3390/pollutants5030024
Chicago/Turabian StyleMerivee, Enno, Anne Mürk, Karin Nurme, Mati Koppel, Angela Ploomi, and Marika Mänd. 2025. "Sublethal and Lethal Effects of Low-Dose Prothioconazole Alone and in Combination with Low-Dose Lambda-Cyhalothrin on Carabid Beetles in a Field-Realistic Scenario" Pollutants 5, no. 3: 24. https://doi.org/10.3390/pollutants5030024
APA StyleMerivee, E., Mürk, A., Nurme, K., Koppel, M., Ploomi, A., & Mänd, M. (2025). Sublethal and Lethal Effects of Low-Dose Prothioconazole Alone and in Combination with Low-Dose Lambda-Cyhalothrin on Carabid Beetles in a Field-Realistic Scenario. Pollutants, 5(3), 24. https://doi.org/10.3390/pollutants5030024







