Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development
Abstract
1. Introduction
2. Research Method in Reviewed Studies
3. Review of Existing Studies
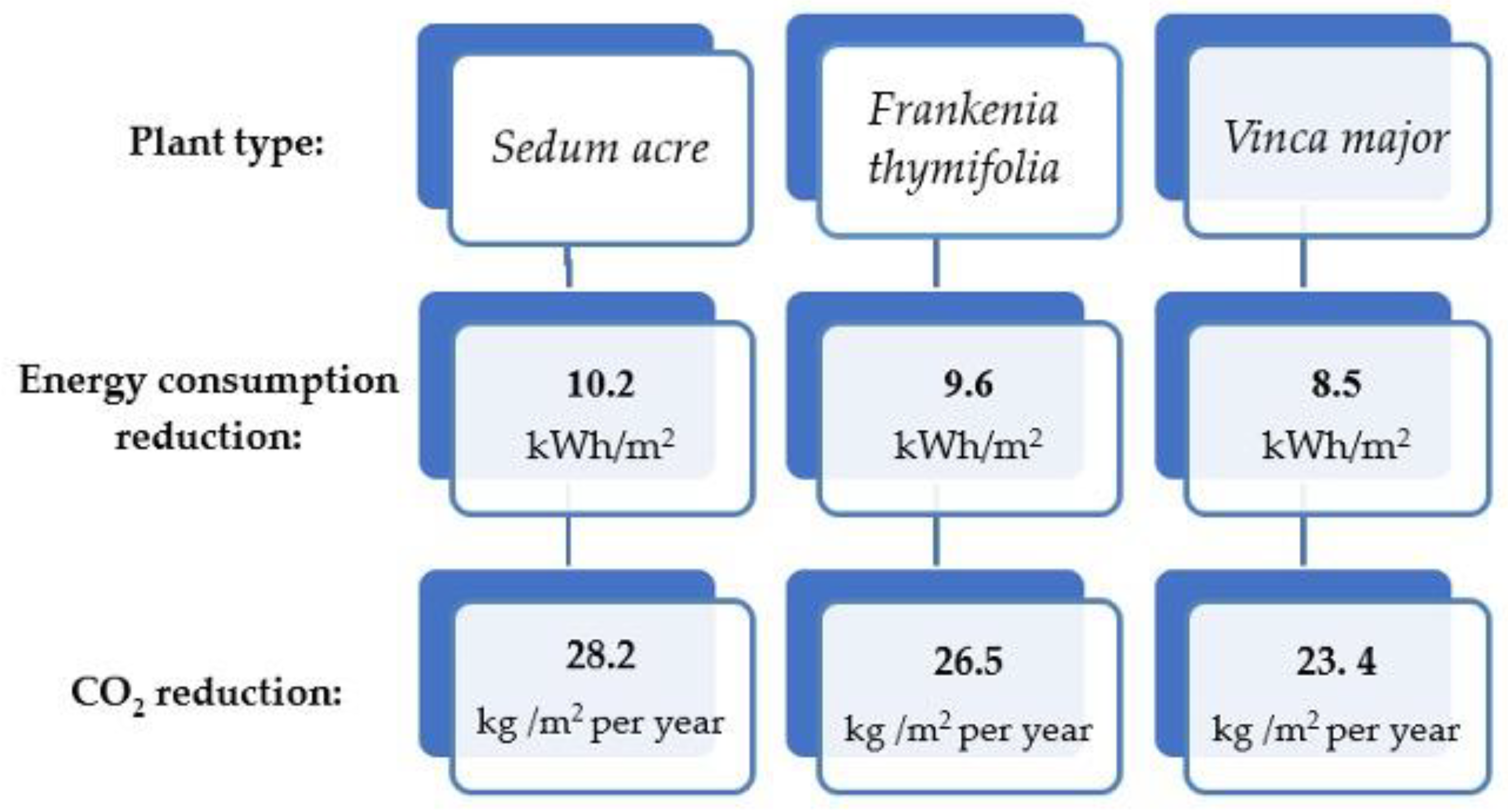
3.1. Types of Green Walls and Their Comparison in Terms of Energy Saving
3.2. The Effect of an Internal Green Wall in Reducing Emissions CO2
3.3. Urban Gardening in Green Walls to Reduce CO2 Emissions
3.4. Safe Urban Gardening through the Application of PGPR
3.5. Indirect Effects of Anoxia on Plants
| Names of Plants | CO2 Absorption Amount | Description | Source | |||
| Plants green structures | Sedum acre L. | 0.143 μmol CO2 m−2 s−1 | Plants exposed to high levels of sunlight (around noon) and during hotter seasons (summer) are more efficient at absorption. | [194] | ||
| Sedum pectabile Boreau | - | Less light intensity (1000–1500 µmol/m2/s) = CO2 absorption with increasing light intensity | ||||
| Frankenia laevis | 2.070 μmol CO2 m−2 s−1 | Higher light intensity (1500–2000 μmol/m2 s−1) = 5-foldincrease | ||||
| Vinca major | 0.607 μmol CO2 m−2 s−1 | The plants exposed to high levels of sunlight (around noon) and during hotter seasons (summer) are more efficient at absorption. | ||||
| Carpobrotus edulis | Almost proportional increase in CO2 absorption with increasing light intensity | - | ||||
| Aptenia cordifolia | - | |||||
| Alysicrpus vaginalis | High carbon absorption capacity | They are not suitable for planting on green roofs since they do not tolerate direct sunlight. | [195] | |||
| Baccharis bilularis | ||||||
| Dichondra repens | ||||||
| Gallium odoratum | ||||||
| Sarcococca hookeriana var. humilis | ||||||
| Sedum dendroideum | Compared to Sedum dendroideum, Sedum acre has wider leaves, which absorb less CO2. | [56] | ||||
| Portulaca grandiflora | 20.22 μmol CO2 m−2 s−1 | As a result, heat-resistant plants can absorb more CO2 during photosynthesis. | [195] | |||
| Alternanthera paronychioides | 23.59 μmol CO2 m−2 s−1 | |||||
| Sansevieria trifasciata | 8.77 μmol CO2 m−2 s−1 | |||||
| Tradescantia spathacea | 7.65 μmol CO2 m−2 s−1 | |||||
| Chrysothemis pulchella | 10.72 μmol CO2 m−2 s−1 | |||||
| Sansevieria trifasciata var. laurentii | 12.43 μmol CO2 m−2 s−1 | |||||
| Plectranthus barbatus | 8.21 μmol CO2 m−2 s−1 | |||||
| Episcia cupreata | 14.32 μmol CO2 m−2 s−1 | |||||
| Sphagneticola trilobata | 6.58 μmol CO2 m−2 s−1 | |||||
| Mentha spicata | 19.58 μmol CO2 m−2 s−1 | |||||
| Potted plants | Areca palm | According to site changes | Related to the article titled “reduction of indoor air pollutants using potted plants of areca palm in real environments”. | [196] | ||
| Dracaena “Janet Craig” | Reduction of up to 10% in CO2 levels | The CO2 level is reduced by 10%, and the level of CO2 in the air-conditioned and naturally ventilated buildings is decreased by up to 86–92% and 25%, respectively. | [172] | |||
| Epipremnum aureum | 0.31 ppm cm−2 | About 1328 cm2 of leaf area is present in an Epipremnum aureum pot. This indicates that the plant can absorb 412 ppm of CO2 from 7 mornings to noon. | [197] | |||
| spathiphyllum wallisei | 0.1 ppm cm−2 | About 2438 cm2 of leaf area is present in a spathiphyllum wallisei pot. Accordingly, the plant is capable of absorbing 244 ppm in the morning. | ||||
| Dieffenbachia sp. | 0.19 ppm cm−2 | About 535 cm2 of leaf area is present in a dieffenbachia sp. pot. Therefore, the plant is capable of absorbing 102 ppm in the morning. | ||||
| Aloe vera | 487 ppm cm−2 | In addition to reducing CO2 concentration and humidity, aloe vera also released less CO2 after absorbing CO2 for eight hours than SMTA and TNF. | [173] | |||
| Spanish moss Tillandsia Aerobic (SMTA) | 276 ppm m−2 | The efficiency of TNF is higher according to volume and weight than that of SMTA, even though SMTA can reduce CO2 concentration more than TNF can (the initial CO2 concentration is between 4750 and 4990 ppm). | ||||
| Thailandia Native Fulia (TNF) | 185 ppm m−2 | (Concentration of density CO2 between 4750 and 4990 ppm) | ||||
| Ficus benjamina | 657 ppm m−2 | Multi-fold decrease in CO2 concentration in small chambers. | [173,174] | |||
| Fuchsia magellanica | 252 ppm m−2 | |||||
| Schefflera arboricola | 1252 ppm m−2 | |||||
| Epipremnum aureum | natural light ppm | Artificial light 1000 lux | Artificial light 2000 lux | Total leaf area 1814 cm2 | [198] | |
| 1058 | 407 | 467 | ||||
| Spathiphyllum spp. | 1036 | 390 | 450 | Total leaf area 1796 cm2 | ||
| Ficus lyrata | 947 | 376 | 429 | Total leaf area 1840 cm2 | ||
| Syngonium podophyllum | 827 | 350 | 401 | Total leaf area 1791 cm2 | ||
| Sansevieria trifasciata prain | 718 | 329 | 392 | Total leaf area 1771 cm2 | ||
| Calathea makoyana (E.Morr.) | 426 | 114 | 215 | Total leaf area 1665 cm2 | ||
| Bromus tomentellus | Root organs (g/m2) | Aerial organs (g/m2) | Storage capacity of carbon (carbon stored) in the organs of some important pasture plants in grams per square metre | [199] | ||
| 12.68 | 1.96 | |||||
| Agropyron trichophorum | 1.74 | 4.63 | ||||
| Astragalus verus | 1.48 | 15.90 | ||||
| Astragalus cephalantus | 0.69 | 5.80 | ||||
| Prangos ferulacea | 0.56 | 2.99 | ||||
| Scariola orientalis | 0.39 | 2.41 | ||||
| Cousinia cylindracea | 0.29 | 1.44 | ||||
| Echinops leiopolycerus | 0.26 | 1.79 | ||||
| Plant shrubs | Magnolia denudate | 0.92 (gC m−2 d−1) | Poor carbon sequestration efficiency (various deciduous tree species) | [182] | ||
| Acer truncatum | 0.94 (gC m−2 d−1) | |||||
| Berberis thunbergii | 0.47 (gC m−2 d−1) | |||||
| Weigela florida | 0.89 (gC m−2 d−1) | |||||
| Clerodendrum trichotomum | 0.93 (gC m−2 d−1) | |||||
| Viburnum opulus | 0.98 (gC m−2 d−1) | |||||
| Platycladus orientalis | 2.92 (gC m−2 d−1) | Semi-open grey spaces need to be planted with large plants with a long lifespan and high carbon sequestration efficiency. | ||||
| Ginkgo biloba | 1.51 (gC m−2 d−1) | |||||
| Microalgae strains | Botryococcus braunii Chlorella vulgaris | Initial (v/v) CO2 (%) | CO2 biofixation rate (g/L/d) | Biomass yield (g/L) | Plant cultivation system | [200,201,202,203,204] |
| 5 | 0.5 | 3.11 | Fermenter | |||
| 0.03 | - | ~0.32 | Sequential bioreactor and spiral tubular | |||
| 0.09 | 3.45 | 0.9 | bioreactor with membrane and membrane processes | |||
| Chlorella sp. | 2 | 0.43 | 2.03 | Vertical tubular bioreactor | [205,206,207] | |
| 5 | 0.25 | 1.94 | Fermenter | |||
| 6 | 2.22 | 10.02 | Glass bubble column | |||
| 2 | 0.86 | - | bubble column | |||
| 5 | 0.7 | - | Vertical tubular bioreactor | |||
| 10 | - | 2.25 | Laboratory scale flask method | |||
| 10 | - | 5.15 | Aerial lift photobioreactor | |||
| Nannochloropsis oculata Scenedesmus dimorphus | 2.0–15.0 | - | 0.25–1.32 | Cylindrical glass photobioreactor | [207,208] | |
| 2 | - | 5.17 | - | |||
| 10 | - | 4.51 | - | |||
| 20 | - | 3.82 | - | |||
| Scenedesmus obliquus Spirulina sp. | 10 | 0.55 | 3.51 | Glass jar | [209,210] | |
| 6 | - | 3.4 | Serial tubular photobioreactor | |||
| 12 | - | 3.5 | Serial tubular photobioreactor | |||
| Bacterial strains | Bacillus subtilis | The use of bacteria that secrete the 1 CA enzyme is an alternative method [211]. CA * has been extracted from these bacteria in different species. | [139,206] | |||
| Enterobacter sp. | ||||||
| Citrobacter freundii | ||||||
| Stenotrophomonas | ||||||
| Acidophilia | ||||||
| Staphylococcus spp. | ||||||
| Proteus vulgaris | ||||||
| Bacillus pasteurii | CO2 can be absorbed through pores of aerated concrete as CaCO3. Therefore, 2 B-ACB may be able to be used as a biodegradation technology. Pre-precipitation properties of CaCO3 have been enhanced by named bacterial species and ureolytic bacteria. | [212,213,214,215,216] | ||||
| Pseudomonas aeruginosa | ||||||
| Bacillus alkalinitrilicus | ||||||
| Bacillus sphaericus | ||||||
| Bacillus subtilis | ||||||
| Enterococcus faecalis | ||||||
| Shewanella sp. | ||||||
| Sporosarcina pasteurii | ||||||
| Arthronema sp. | 52.64 (mg L−1 d−1) | Product formation 3 | [217] | |||
| Biodiesel | ||||||
| Chlorella sp. | 31.02 (mg L−1 d−1) | Biodiesel | ||||
| Bacillus cereus SS105 | 287.21 (mg L−1 d−1) | Calcite, biofuels, biopolymers | ||||
| Bacillus sp. | not reported | Polyhydroxyalkanoates | [218] | |||
| Nannochloropsis gaditana | 1700 (mg L−1 d−1) | Biodiesel | [219] | |||
| Serratia sp. | not reported | Biodiesel | [220,221] | |||
| Scenedesmus sp. IMMTCC-6 | 85.7 (mg L−1 d−1) | Biodiesel | [211] | |||
| Scenedesmus obliquus CNW-N | 549.90 (mg L−1 d−1) | Biodiesel | [222] | |||
| Thiomicrospira crunogena | 41.28 (mg L−1 d−1) | Biodiesel | [223] | |||
| Food products and vegetables | Spinach | 15.57 t/ha | Land type: agricultural land | [224] | ||
| Chili | 27.60 t/ha | |||||
| Eggplant | 23.13 t/ha | |||||
| Chinese cabbage | 24.75 t/ha | |||||
| Tomato | 19.68 t/ha | Land type: non-agricultural land | ||||
| Okra | 24.38 t/ha | |||||
| Chili | 16.41 t/ha | |||||
| Eggplant | 15.46 t/ha | |||||
3.6. CO2 Absorption by Plants in Green Wall Systems
4. Key Takeaways
5. Research Recommendations
- Further studies should be conducted to investigate the most effective methods for building and implementing green walls in different climates and regions;
- More research is needed to understand the morphological, anatomical, and physiological characteristics of different plant species to ensure optimal selection for green walls;
- The potential health benefits of green walls, including the impact of bacterial strains, should be studied further;
- The impact of various factors on carbon sequestration in urban green spaces, such as plant species, size, canopy cover, and dominant categories, should be investigated in more detail;
- Bylaws and regulations for green walls should be designed using science-based solutions to maximise their impact on mitigating climate change while enabling adaptation.
6. Conclusions
Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Fenner, A.E.; Kibert, C.J.; Woo, J.; Morque, S.; Razkenari, M.; Hakim, H.; Lu, X. The carbon footprint of buildings: A review of methodologies and applications. Renew. Sustain. Energy Rev. 2018, 94, 1142–1152. [Google Scholar] [CrossRef]
- Dai, W.L.; Luo, S.L.; Yin, S.F.; Au, C.T. The Direct Transformation of Carbon Dioxide to Organic Carbonates over Heterogeneous Catalysts. Appl. Catal. A 2009, 366, 2–12. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Heterogeneous Catalysts for Production of Chemicals Using Carbon Dioxide as Raw Material: A Review. Renew. Sustain. Energy Rev. 2012, 16, 4951–4964. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Rajendran, S.; Hoang, T.K.; Ahmad, A.; Luque, R. Photobiological effects of converting biomass into hydrogen-Challenges and prospects. Bioresour. Technol. 2023, 367, 128278. [Google Scholar] [CrossRef] [PubMed]
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogen Energy 2023, 48, 21088–21109. [Google Scholar] [CrossRef]
- Kanwal, F.; Torriero, A.A. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783. [Google Scholar] [CrossRef]
- Malla, F.A.; Mushtaq, A.; Bandh, S.A.; Qayoom, I.; Hoang, A.T. Understanding climate change: Scientific opinion and public perspective. In Climate Change: The Social and Scientific Construct; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–20. [Google Scholar]
- Huang, G.; Xu, Z.; Qu, X.; Cao, J.; Long, S.; Yang, K.; Hou, H.; Wang, Y.; Ma, X. Critical climate issues toward carbon neutrality targets. Fundam. Res. 2022, 2, 396–400. [Google Scholar] [CrossRef]
- Yoon, I.S.; Çopuroğlu, O.; Park, K.B. Effect of global climatic change on carbonation progress of concrete. Atmos. Environ. 2007, 41, 7274–7285. [Google Scholar] [CrossRef]
- Alshalif, A.F.; Irwan, J.M.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S. A systematic review on bio-sequestration of carbon dioxide in bio-concrete systems: A future direction. Eur. J. Environ. Civ. Civil. Eng. 2022, 26, 1209–1228. [Google Scholar] [CrossRef]
- Sutton, R.K. Introduction to green roof ecosystems. In Green Roof Ecosystems; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–25. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2014 Revision, CD-ROM ed.; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2014. [Google Scholar]
- United Nations. World Urbanization Prospects: The 2005 Revision. 2006. Available online: http://www.un.org/esa/population/publications/WUP2005/2005wup.htm. (accessed on 14 June 2018).
- Un DESA. World Urbanization Prospects: The 2018 Revision. 2018. Available online: https://population.un.org/wup/DataQuery/. (accessed on 26 March 2020).
- Gogoi, P.P.; Vinoj, V.; Swain, D.; Roberts, G.; Dash, J.; Tripathy, S. Land use and land cover change effect on surface temperature over Eastern India. Sci. Rep. 2019, 9, 8859. [Google Scholar] [CrossRef]
- Jozay, M. Combining Green Space and Urban Gardening in External Green Wall under the Influence of Growth-Promoting Bacteria and Types of Recycled Water; Gorgan University of Agricultural Sciences and Natural Resources: Gorgan, Iran, 2024. [Google Scholar]
- Rosenzweig, C.; Solecki, W.D.; Romero-Lankao, P.; Mehrotra, S.; Dhakal, S.; Ali Ibrahim, S. Climate Change and Cities: Second Assessment Report of the Urban Climate Change Research Network; Cambridge University Press: New York, NY, USA, 2018. [Google Scholar]
- Seto, K.C.; Sanchez-Rodríguez, R.; Fragkias, M. The new geography of contemporary urbanization and the environment. Annu. Rev. Environ. Resour. 2010, 35, 167–194. [Google Scholar] [CrossRef]
- Mitchell, M.; Johansen, K.; Maron, M.; McAlpine, C.A.; Wu, D.; Rhodes, J.R. Identification of fine scale and landscape scale drivers of urban aboveground carbon stocks using high-resolution modeling and mapping. Sci. Total Environ. 2018, 622–623, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S. GHG emissions from urbanization and opportunities for urban carbon mitigation. Curr. Opin. Environ. Sustain. 2010, 2, 277–283. [Google Scholar] [CrossRef]
- Teixeira, C.P.; Fernandes, C.O.; Ahern, J. Adaptive planting design and management framework for urban climate change adaptation and mitigation. Urban For. Urban Green. 2022, 70, 127548. [Google Scholar] [CrossRef]
- Zhou, Y. Low-carbon transition in smart city with sustainable airport energy ecosystems and hydrogen-based renewable-grid-storage-flexibility. Energy Rev. 2022, 100001. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials and hybrid nanocomposites for CO2 capture and utilization: Environmental and energy sustainability. RSC Adv. 2022, 12, 23869–23888. [Google Scholar] [CrossRef] [PubMed]
- Nadoushani, Z.S.M.; Akbarnezhad, A. Effects of structural system on the life cycle carbon footprint of buildings. Energy Build. 2015, 102, 337–346. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Lee, S.E.; Huh, J.H. Estimation of CO2 emission of apartment buildings due to major construction materials in the Republic of Korea. Energy Build. 2012, 49, 437–442. [Google Scholar] [CrossRef]
- Su, J.G.; Jerrett, M.; de Nazelle, A.; Wolch, J. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ. Res. 2011, 111, 319–328. [Google Scholar] [CrossRef]
- Giuliano, W.M.; Accamando, A.K.; Mcadams, E.J. Lepidoptera-habitat relationships in urban parks. Urban Ecosyst. 2004, 7, 361–370. [Google Scholar] [CrossRef]
- Wang, R.; Helbich, M.; Yao, Y.; Zhang, J.; Liu, P.; Yuan, Y. Urban greenery and mental wellbeing in adults: Cross-sectional mediation analyses on multiple pathways across different greenery measures. Environ. Res. 2019, 176, 108535. [Google Scholar] [CrossRef] [PubMed]
- Shafique, M.; Kim, R.; Rafiq, M. Green roof benefits, opportunities and challenges—A review. Renew. Sustain. Energy Rev. 2018, 90, 757–773. [Google Scholar] [CrossRef]
- Brudermann, T.; Sangkakool, T. Green buildings in temperate climate cities in Europe—An analysis of key decision factors. Urban For. Urban Green. 2017, 21, 224–234. [Google Scholar] [CrossRef]
- Zeng, C.; Bai, X.; Sun, L.; Zhang, Y.; Yuan, Y. Optimal parameters of green buildings in representative cities of four climate zones in China: A simulation study. Energy Build. 2017, 150, 118–131. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zhu, M.; Yin, J. Role of plant leaves in removing airborne dust and associated metals on Beijing roadsides. Aerosol Air Qual. Res. 2017, 17, 2566–2584. [Google Scholar] [CrossRef]
- Manso, M.; Teotónio, I.; Silva, C.M.; Cruz, C.O. Green roof and green wall benefits and costs: A review of the quantitative evidence. Renew. Sustain. Energy Rev. 2021, 135, 110111. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, T.; Jing, L. China’s energy transition towards carbon neutrality with minimum cost. J. Clean. Prod. 2023, 388, 135904. [Google Scholar] [CrossRef]
- Coma, J.; Pérez, G.; Gracia, A.; Burés, S.; Urrestarazu, M.; Cabeza, L.F. Vertical greenery systems for energy savings in buildings: A comparative study between green walls and green facades. Build. Environ. 2017, 111, 228–237. [Google Scholar] [CrossRef]
- Zuo, J.; Zhao, Z.Y. Green building research–current status and future agenda: A review. Renew. Sustain. Energy Rev. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Shahteymuri, A.; Naaranoja, M. Analysis of Diffusion of Biomass Energy Utilization. Comput. Res. Prog. Appl. Sci. Eng. 2016, 2, 101–105. [Google Scholar]
- Whittinghill, L.J.; Rowe, D.B.; Schutzki, R.; Cregg, B.M. Quantifying carbon sequestration of various green roof and ornamental landscape systems. Landsc. Urban Plan. 2014, 123, 41–48. [Google Scholar] [CrossRef]
- Agra, H.; Klein, T.; Vasl, A.; Shalom, H.; Kadas, G.; Blaustein, L. Sedum-dominated green-roofs in a semi-arid region increase CO2 concentrations during the dry season. Sci. Total Environ. 2017, 584, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Blanusa, T.; Monteiro, M.M.V.; Fantozzi, F.; Vysini, E.; Li, Y.; Cameron, R.W. Alternatives to Sedum on green buildings: Can broad leaf perennial plants offer better ‘cooling service’? Build. Environ. 2013, 59, 99–106. [Google Scholar] [CrossRef]
- Akbari, H. Measured energy savings from the application of reflective roofs in two small non-residential buildings. Energy 2003, 28, 953–967. [Google Scholar] [CrossRef]
- Kohler, M. Green facades—A view back and some visions. Urban Ecosyst. 2008, 11, 423. [Google Scholar] [CrossRef]
- Gandy, M. The ecological facades of Patrick Blanc. Arch. Des. 2010, 80, 28–33. [Google Scholar] [CrossRef]
- Larson, D.; Matthes, U.; Lundholm, J.; Gerrath, J.; Kelly, P.E. The Urban Cliff Revolution: New Findings on the Origins and Evolution of Human Habitats; Fitzhenry & Whiteside: East York, ON, Canada, 2004. [Google Scholar]
- Jonathan, A. Vegetation ± Climate Interaction: How Vegetation Makes the Global Environment; Springer: New York, NY, USA, 2003. [Google Scholar]
- Yeang, K. Eco Skyscraper; Image Publishing: Chadstone, VIC, Australia, 2007; ISBN 9781864702682. [Google Scholar]
- Wilmers, F. Effects of vegetation on urban climate and buildings. Energy Build. 1990–1991, 15, 507–514. [Google Scholar] [CrossRef]
- Jozay, M. The Effect of Growing Media on the Morphophysiological Characteristics of Some Cover Plants for Use in An External Green Wall System. Master’s Thesis, Ferdowsi University of Mashhad, Mashhad, Iran, 2020. [Google Scholar]
- Besir, A.B.; Cuce, E. Green buildings and facades: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 915–939. [Google Scholar] [CrossRef]
- Li, J.F.; Wei, O.W.H.; Li, Y.S.; Zhan, J.; Ho, Y.A.; Li, J.; Lam, E. Effect of green roof on ambient CO2 concentration. Build. Environ. 2010, 45, 2644–2651. [Google Scholar] [CrossRef]
- Cole, R.J. Energy and greenhouse gas emissions associated with the construction of alternative structural systems. Build. Environ. 1998, 34, 335–348. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. ‘Renewable’ Hydrogen: Prospects and Challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Huang, W.; Li, F.; Cui, S.; Huang, L.; Lin, J. Carbon footprint and carbon emission reduction of urban buildings: A case in Xiamen City. China. Procedia Eng. 2017, 198, 1007–1017. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Sun, J. Evaluation and application of an online coupled modeling system to assess the interaction between urban vegetation and air quality. Aerosol Air Qual. Res. 2018, 18, 693–710. [Google Scholar] [CrossRef]
- Collazo-Ortega, M.; Rosas, U.; Reyes-Santiago, J. Towards providing solutions to the air quality crisis in the mexico city metropolitan area: Carbon sequestration by succulent species in green buildings. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Ahirwal, J.; Sahoo, U.K. Plant biodiversity and carbon sequestration potential of the planted forest in Brahmaputra flood plains. J. Environ. Manag. 2021, 280, 111671. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, A.; Istrate, I.; Herrera, I.; Lago, C.; Lizana, J.; Lechón, Y. Energy and water consumption and carbon footprint of school buildings in hot climate conditions. Results from life cycle assessment. J. Clean. Prod. 2018, 195, 1326–1337. [Google Scholar] [CrossRef]
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2016; EPA: Washington, DC, USA, 2018.
- Seyedabadi, M.R.; Karrabi, M.; Nabati, J. Investigating green roofs’ CO2 sequestration with cold-and drought-tolerant plants (a short-and long-term carbon footprint view). Environ. Sci. Pollut. Res. 2022, 29, 14121–14130. [Google Scholar] [CrossRef]
- Ascione, F.; De Masi, R.F.; Mastellone, M.; Ruggiero, S.; Vanoli, G.P. Green walls, a critical review: Knowledge gaps, design parameters, thermal performances and multi-criteria design approaches. Energies 2020, 13, 2296. [Google Scholar] [CrossRef]
- Almeida, C.; Teotónio, I.; Silva, C.M.; Cruz, C.O. Socioeconomic feasibility of green buildings and walls in public buildings: The case study of primary schools in Portugal. Eng. Econ. 2021, 66, 27–50. [Google Scholar] [CrossRef]
- Liberalesso, T.; Oliveira Cruz, C.; Matos Silva, C.; Manso, M. Green infrastructure and public policies: An international review of green buildings and green walls incentives. Land Use Pol. 2020, 96, 104693. [Google Scholar] [CrossRef]
- Wilkinson, S.; Ghosh, S.; Pelleri, N. Green Walls and Roofs: A Mandatory or Voluntary Approach for Australia? Literature. Green Walls and Roofs: A Mandatory or Voluntary Approach for Australia? Lit. Rev. 2017. Available online: https://opus.lib.uts.edu.au/handle/10453/119485 (accessed on 30 January 2024).
- Irga, P.J.; Braun, J.T.; Douglas, A.N.J.; Pettit, T.; Fujiwara, S.; Burchett, M.D.; Torpy, F.R. The distribution of green walls and green roofs throughout Australia: Do policy instruments influence the frequency of projects? Urban For. Urban Green. 2017, 24, 164–174. [Google Scholar] [CrossRef]
- Manso, M.; Castro-Gomes, J. Green wall systems: A review of their characteristics. Renew. Sustain. Energy Rev. 2015, 41, 863–871. [Google Scholar] [CrossRef]
- Jozay, M.; Rabbani Khairkhah, S. Vertical Green Systems (Green Wall); Chaponashr Iran (Arasto) Publications: Kaveh Industrial City, Iran, 2020. [Google Scholar]
- Dover, J.W. Green Infrastructure: Incorporating Plants and Enhancing Biodiversity in Buildings and Urban Environments; Routledge: London, UK, 2015. [Google Scholar]
- Bustamiq, B.; Belusko, M.; Ward, A.; Beecham, S. Vertical greenery systems: A systematic review of research trends. Build. Environ. 2018, 146, 226–237. [Google Scholar] [CrossRef]
- Ottelé, M.; Perini, K.; Fraaij, A.L.A.; Haas, E.M.; Raiteri, R. Comparative life cycle analysis for green façades and living wall systems. Energy Build. 2001, 43, 3419–3429. [Google Scholar] [CrossRef]
- Baumgartner, J.; Schauer, J.J.; Ezzati, M.; Lu, L.; Cheng, C.; Patz, J.; Bautista, L.E. Patterns and predictors of personal exposure to indoor air pollution from biomass combustion among women and children in rural China. Indoor Air 2011, 21, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.S.; Louis, V.R.; Sié, A.; Sauerborn, R. Biomass smoke in Burkina Faso: What is the relationship between particulate matter, carbon monoxide, and kitchen characteristics? Environ. Sci. Pollut. Res. 2014, 21, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Indoor Air Quality Guidelines: Household Fuel Combustion, Review 2: Emissions of Health-Damaging Pollutants from Household Stoves; WHO: Geneva, Switzerland, 2014; pp. 1–42. [Google Scholar]
- IEA. CO2 Emissions from Fossil-Fuel Combustion; OECD/IEA: Paris, France, 2016. [Google Scholar]
- Fisk, W.J. Health and productivity gains from better indoor environments and their relationship with building energy efficiency. Annu. Rev. Energy Environ. 2000, 25, 537–566. [Google Scholar] [CrossRef]
- Sakai, K.; Norbäck, D.; Mi, Y.; Shibata, E.; Kamijima, M.; Yamada, T.; Takeuchi, Y. A comparison of indoor air pollutants in Japan and Sweden: Formaldehyde, nitrogen dioxide, and chlorinated volatile organic compounds. Environ. Res. 2004, 94, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Shrubsole, C.; Dimitroulopoulou, S.; Foxall, K.; Gadeberg, B.; Doutsi, A. IAQ guidelines for selected volatile organic compounds (VOCs) in the UK. Build. Environ. 2019, 165, 106382. [Google Scholar] [CrossRef]
- Rosch, C.; Kohajda, T.; Roder, S.; von Bergen, M.; Schlink, U. Relationship between sources and patterns of VOCs in indoor air. Atmos. Pollut. Res. 2014, 5, 129–137. [Google Scholar] [CrossRef]
- Amoatey, P.; Omidvarborna, H.; Baawain, M.S.; Al-Mamun, A. Indoor air pollution and exposure assessment of the gulf cooperation council countries: A critical review. Environ. Int. 2018, 121, 491–506. [Google Scholar] [CrossRef]
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Rev. Environ. Sci. Biotechnol. 2018, 17, 395–415. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.J.; Torpy, F.R. Towards practical indoor air phytoremediation: A review. Chemosphere 2018, 208, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Papinchak, H.L.; Holcomb, E.; Best, T.; Decoteau, D.R. Effectiveness of Houseplants in Reducing the Indoor Air Pollutant Ozone. HortTechnology 2009, 19, 286–290. [Google Scholar] [CrossRef]
- Soreanu, G.; Dixon, M.; Darlington, A. Botanical biofiltration of indoor gaseous pollutants—A mini-review. Chem. Eng. J. 2013, 229, 585–594. [Google Scholar] [CrossRef]
- Aydogan, A.; Montoya, L.D. Formaldehyde removal by common indoor plant species and various growing media. Atmos. Environ. 2011, 45, 2675–2682. [Google Scholar] [CrossRef]
- Wolverton, B.C.; Johnson, A.; Bounds, K. Interior Landscape Plants for Indoor Air Pollution Abatement; NASA/ALCA Final Report; Plants for Clean Air Council: Mitchellville, MD, USA, 1989.
- Treesubsuntorn, C.; Thiravetyan, P. Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmos. Environ. 2012, 57, 317–321. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Khalekuzzaman, M.; Yoo, E.; Jung, H.; Jang, H. Removal ratio of gaseous toluene and xylene transported from air to root zone via the stem by indoor plants. Environ. Sci. Pollut. Res. 2016, 23, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Cáceres, G.P.; Fernández-Cañero, R.; Fernández-Espinosa, A.J.; Rossini-Oliva, S.; Franco-Salas, A.; Pérez-Urrestarazu, L. Volatile organic compounds removal by means of a felt-based living wall to improve indoor air quality. Atmos. Pollut. Res. 2021, 12, 224–229. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco-Salas, A.; Egea, G. Vertical greening systems and sustainable cities. J. Urban Technol. 2015, 22, 65–85. [Google Scholar] [CrossRef]
- Irga, P.J.; Pettit, T.; Irga, R.F.; Paull, N.J.; Douglas, A.N.; Torpy, F.R. Does plant species selection in functional active green walls influence VOC phytoremediation efficiency? Environ. Sci. Pollut. Res. 2019, 26, 12851–12858. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Rabbani, M.; Jozay, M. Investigating plant and air-quality performances of an internal greenwall system under hydroponic conditions. Environ. Manag. 2020, 275, 111230. [Google Scholar]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality a critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Artmann, M.; Sartison, K. The role of urban agriculture as a nature-based solution: A review for developing a systemic assessment framework. Sustainability 2018, 10, 1937. [Google Scholar] [CrossRef]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.I.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture production as a major driver of the earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22, 8. [Google Scholar] [CrossRef]
- Parajuli, R.; Matlock, M.D.; Thoma, G. Cradle to grave environmental impact evaluation of the consumption of potato and tomato products. Sci. Total Environ. 2021, 758, 143662. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Rovira-Val, M.R. A longitudinal literature review of life cycle costing applied to urban agriculture. Int. J. Life Cycle Assess. 2020, 25, 1418–1435. [Google Scholar] [CrossRef]
- Zasada, I. Multifunctional peri-urban agriculture-A review of societal demands and the provision of goods and services by farming. Land. Use Policy 2011, 28, 639–648. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.D.; Meijer, N.; Gerrits, E.W.J.; Appel, M.J. New approaches for safe use of food by-products and biowaste in the feed production chain. J. Clean. Prod. 2023, 388, 135954. [Google Scholar] [CrossRef]
- Specht, K.; Siebert, R.; Hartmann, I.; Freisinger, U.B.; Sawicka, M.; Werner, A.; Thomaier, S.; Henckel, D.; Walk, H.; Dierich, A. Urban agriculture of the future: An overview of sustainability aspects of food production in and on buildings. Agric. Hum. Human. Values 2014, 31, 33–51. [Google Scholar] [CrossRef]
- Polling, B.; Prados, M.-J.; Torquati, B.M.; Giacch, G.; Recasens, X.; Paffarini, C.; Alfranca, O.; Lorleberg, W. Business models in urban farming: A comparative analysis of case studies from Spain. Italy and Germany. Morav. Geogr. Rep. 2017, 25, 166–180. [Google Scholar] [CrossRef]
- Langemeyer, J.; Latkowska, M.J.; Gomez-Baggethun, E.; Voigt, A.; Calvet-Mir, L.; Pourias, J.; Camps-Calvet, M.; Orsini, F.; Breuste, J.; Artmann, M.; et al. Ecosystem services from urban gardens. In Urban Allotment Gardens in Europe; Bell, S., Fox-Kämper, R., Keshavarz, N., Benson, M., Caputo, S., Noori, S., Voigt, A., Eds.; Routledge: London, UK, 2016; pp. 115–141. ISBN 978-1-138-92109-2. [Google Scholar]
- García-Nieto, A.P.; Geijzendorffer, I.R.; Baró, F.; Roche, P.K.; Bondeau, A.; Cramer, W. Impacts of urbanization around Mediterranean cities: Changes in ecosystem service supply. Ecol. Indic. 2018, 91, 589–606. [Google Scholar] [CrossRef]
- Besthorn, F.H. Vertical farming: Social work and sustainable urban agriculture in an age of global food crises. Aust. Soc. Work. 2013, 66, 187–203. [Google Scholar] [CrossRef]
- Blanc, P. The Vertical Garden from Nature to the City; Norton & Company: New York, NY, USA, 2008. [Google Scholar]
- Saxena, N.N. The Review on Techniques of Vertical Farming. J. Mod. Agric. 2021, 10, 732–738. [Google Scholar]
- Malisia, W.; Allison, K. Onward & Upward: Filling the Hunger Gaps with Vertical Farming. Hort 408 Literature Review. Clemson University, 2013, p. 50. Available online: http://www.clemson.edu/cafls/vincent/articles/lit_review.pdf (accessed on 23 January 2023).
- Rabaia, M.K.H.; Semeraro, C.; Olabi, A.G. Modeling photovoltaics’ waste projection and waste management optimization. J. Clean. Prod. 2023, 388, 135947. [Google Scholar] [CrossRef]
- Jozay, M.; Kazemi, F.; Fotovat, A. Evaluating the environmental performance of the growing media in a green wall system in a dry climate region. Desert 2019, 20, 217–230. [Google Scholar]
- Cabannes, Y.; Marocchino, C. Food and urban planning: The missing link. In Integrating Food into Urban Planning; Cabannes, Y., Marocchino, C., Eds.; UCL Press: London, UK; FAO: Rome, Italy, 2018; pp. 18–59. [Google Scholar]
- Blay-Palmer, A.; Santini, G.; Dubbeling, M.; Renting, H.; Taguchi, M.; Giordano, T. Validating the City Region Food System Approach: Enacting Inclusive, Transformational City Region Food Systems. Sustainability 2018, 10, 1680. [Google Scholar] [CrossRef]
- Lamond, J.; Everett, G. Sustainable blue-green infrastructure: A social practice approach to understanding community preferences and stewardship. Landsc. Urban Plan. 2019, 191, 103639. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Brunnhofer, N.; Petit-Boix, A.; Gabarrell, X.; Guisasola, A.; Villalba, G. Can wastewater feed cities? Determining the feasibility and environmental burdens of struvite recovery and reuse for urban regions. Sci. Total Environ. 2020, 737, 139783. [Google Scholar] [CrossRef]
- Francesco, O.; Remi, K.; Remi, N.-W.; Giorgio, G. Urban agriculture in the developing world: A review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Reetz, H.F. Fertilizers and Their Efficient Use; International Fertilizer industry Association: Paris, France, 2016; ISBN 979-10-92366-04-4. [Google Scholar]
- Elmqvist, T.; Andersson, E.; Frantzeskaki, N.; McPhearson, T.; Olsson, P.; Gaffney, O. Sustainability and resilience for transformation in the urban century. Nat. Sustain. 2019, 2, 267–273. [Google Scholar] [CrossRef]
- Jozay, M.; Zarei, H.; Khorasaninejad, S.; Miri, T. Advantages and disadvantages of organic production of garden products on external green walls. In Proceedings of the 15th International Conference on Food Industry Sciences, Organic Farming and Food Security, Spain, 18 August 2023. [Google Scholar]
- Jansma, J.E.; Wertheim-Heck, S.C. Thoughts for urban food: A social practice perspective on urban planning for agriculture in Almere, the Netherlands. Landsc. Urban Plan. 2021, 206, 103976. [Google Scholar] [CrossRef]
- Safitri, K.I.; Abdoellah, O.S.; Gunawan, B. Urban Farming as Women Empowerment: Case Study Sa’uyunan Sarijadi Women’s Farmer Group in Bandung City. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 249, p. 01007. [Google Scholar]
- Jozay, M.; Zarei, H.; Khorasaninejad, S.; Miri, T. Urban gardening on the green wall. In Proceedings of the 10th International Conference on Agricultural Science, Environment, Urban and Rural Development, Tbilisi, Georgia, 24–25 November 2022; Available online: https://civilica.com/doc/1541998 (accessed on 30 January 2024).
- Jozay, M.; Zarei, H.; Khorasaninejad, S.; Miri, T. Investigating the morpho-physiological characteristics of Ophiopogon japonicus, Festuca ovina glauca, Aptenia cordifolia and Carpobrotus edulis under the influence of unconventional water and plant growth promoting bacteria in external green walls. J. Plant Process. Funct. 2023. ready to print for publication. [Google Scholar]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, e963401. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Jozay, M. Remediation of Cadmium and Its’ Human Health Effects on Urban Agricultural Vertical Systems. Electron. J. 2022. [Google Scholar] [CrossRef]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef]
- FAO. New Standards to Curb the Global Spread of Plant Pests and Diseases; UN Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- Arora, N.K. Bioremediation: A green approach for restoration of polluted ecosystems. Environ. Sustain. 2018, 1, 305–307. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Zhang, Y.; Fu, X.; Tsang, Y.; Wu, J.; Le, Y. Variable decomposition of two plant litters and their effects on the carbon sequestration ability of wetland soil in the Yangtze River estuary. Geoderma 2018, 319, 230–238. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, S.; Kamyab, H.; Kumar, A. Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: A review. J. Clean. Prod. 2020, 247, 19–138. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F. Microalgae biofuels production: A systematic review on socioeconomic prospects of microalgae biofuels and policy implications. Environ. Chall. 2021, 5, 100207. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.M.; Kumar, G.; Yang, Y.H. Carbon dioxide capture and bioenergy production using biological system—A review. Renew. Sustain. Energy Rev. 2019, 110, 143–158. [Google Scholar] [CrossRef]
- Jajesniak, P.; Omar Ali, H.E.M.; Wong, T.S. Carbon Dioxide Capture and Utilization using Biological Systems: Opportunities and Challenges. J. Bioprocess. Biotech. 2014, 4, 1–15. [Google Scholar]
- Maheshwari, N.; Kumar, M.; Thakur, I.S.; Srivastava, S. Production, process optimization and molecular characterization of polyhydroxyalkanoate (PHA) by CO2 sequestering B. cereus SS105. Bioresour. Technol. 2018, 254, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.S.; Zhou, P.P.; Yu, L.J. Influence of enzyme concentration on bio sequestration of CO2 in carbonate form using bacterial carbonic anhydrase. Chem. Eng. J. 2013, 232, 149–156. [Google Scholar] [CrossRef]
- Fulke, A.B.; Mudliar, S.N.; Yadav, R.; Shekh, A.; Srinivasan, N.; Ramanan, R.; Krishnamurthi, K.; Saravana Devi, S.; Chakrabarti, T. Biomitigation of CO2, calcite formation and simultaneous biodiesel precursors production using Chlorella sp. Bioresour. Technol. 2010, 101, 8473–8476. [Google Scholar] [CrossRef]
- Goldberg, D.; Aston, L.; Bonneville, A.; Demirkanli, I.; Evans, C.; Fisher, A.; White, S. Geological storage of CO2 in sub-seafloor basalt: The CarbonSAFE pre-feasibility study offshore Washington State and British Columbia. Energy Procedia 2018, 146, 158–165. [Google Scholar] [CrossRef]
- Kumar, K.; Das, D. Carbon dioxide sequestration by biological processes. In Transformation and Utilization of Carbon Dioxide; Springer: Berlin/Heidelberg, Germany, 2014; pp. 303–334. [Google Scholar]
- Jaya, P.; Nathan, V.K.; Ammini, P. Characterization of marine bacterial carbonic anhydrase and their CO2 sequestration abilities based on a soil microcosm. Prep. Biochem. Biotechnol. 2019, 49, 891–899. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A.; DULCIS (D-dimer-ULtrasonography in Combination Italian Study) Investigators. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chang, E.E.; Chiang, P.C. CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Cizer, O.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Kinetic effect of carbonic anhydrase enzyme on the carbonation reaction of lime mortar. Int. J. Archit. Herit. 2018, 12, 779–789. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jo, Y.S.; Park, C.S.; Kang, C.H.; So, J.S. Biocementation of concrete pavements using microbially induced calcite precipitation. J. Microbiol. Biotechnol. 2017, 27, 1331–1335. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Anne Cotton, T.E.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils—An Ontario field study. Int. J. Greenh. Gas. Control 2020, 97, 103017. [Google Scholar] [CrossRef]
- Shi, A.; Chakrawal, A.; Manzoni, S.; Fischer, B.M.; Nunan, N.; Herrmann, A.M. Substrate spatial heterogeneity reduces soil microbial activity. Soil Biol. Biochem. 2021, 152, 108068. [Google Scholar] [CrossRef]
- Li, C.X.; Jiang, X.C.; Qiu, Y.J.; Xu, J.H. Identification of a new thermostable and alkali-tolerant α-carbonic anhydrase from Lactobacillus delbrueckii as a biocatalyst for CO2 biomineralization. Bioresour. Bioprocess. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Battisti, D.S.; Naylor, R.L. Historical Warnings of Future Food Insecurity with Unprecedented Seasonal Heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef]
- Albo, A.; Luis, P.; Irabin, A. Carbon Dioxide Capture from Flue Gases Using a Cross-Flow Membrane Contactor and the Ionic Liquid 1-Ethyl-3- methylimidazolium Ethylsulfate. Ind. Eng. Chem. Res. 2010, 49, 11045–11051. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook; IEA: Paris, France, 2012. [Google Scholar]
- Oh, T.H. Carbon Capture and Storage Potential in Coal-fired Plant in Malaysia-a Review. Renew. Sustain. Energy Rev. 2010, 14, 2697–2709. [Google Scholar] [CrossRef]
- Hertel, S.; Navarro, P.; Deegener, S.; Körner, I. Biogas and nutrients from blackwater, lawn cuttings and grease trap residues—Experiments for Hamburg’s Jenfelder Au district. Energy Sustain. Soc. 2015, 5, 29. [Google Scholar] [CrossRef]
- Barreca, F. Rooftop gardening. A solution for energy saving and landscape enhancement in Mediterranean urban areas. ProcediaSoc. Behav. Sci. 2016, 223, 720–725. [Google Scholar]
- Jouhara, H.; Czajczyńska, D.; Ghazal, H.; Krzyżyńska, R.; Anguilano, L.; Reynolds, A.J.; Spencer, N. Municipal waste management systems for domestic use. Energy 2017, 139, 485–506. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Thakur, N. Organic farming, food quality, and human health: A trisection of sustainability and a move from pesticides to eco-friendly biofertilizers. In Probiotics in Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2017; pp. 491–515. [Google Scholar]
- Stoknes, K.; Scholwin, F.; Krzesiński, W.; Wojciechowska, E.; Jasińska, A. Efficiency of a novel “Food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Manag. 2016, 56, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Liedl, B.E.; Cummins, M.; Young, A.; Williams, M.L.; Chatfield, J.M. Liquid Effluent from Poultry Waste Bioremediation as a Potential Nutrient Source for Hydroponic Tomato Production. Acta Hortic. 2004, 659, 647–652. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, M. Effects of intermittent aeration on the performance of partial nitrification treating fertilizer wastewater. China Environ. Sci. 2017, 37, 146–153. [Google Scholar]
- Lombardi, L. Life cycle assessment comparison of technical solutions for CO2 emissions reduction in power generation. Energy Convers. Manag. 2003, 44, 93–108. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Kazemi, F.; Mahdizadeh, H. A Cost-benefit Analysis of Applying Urban Agriculture in Sustainable Park Design. Land Use Policy 2022, 112, 105834. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Chen, R.; Ma, Y.; Yang, B.; Chen, Z.; Liang, Y.; Fang, J.; Xiao, Y. Role of Two Plant Growth-Promoting Bacteria in Remediating Cadmium-Contaminated Soil Combined with Miscanthus floridulus (Lab.). Plants 2021, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Jozay, M.; Rabbani, M.; Kazemi, F. The impact of humic acid solutions and types of growing media on some morphophysiological and biochemical features of Syngonium sp. and Pothos sp. plants in interior green wall conditions. Plant Arshive 2021, 21, 2240–2252. [Google Scholar] [CrossRef]
- Santoso, E.I. Improving the thermal comfort of room through a combination of outdoor and indoor parks: Evidence from Indonesia. Acad. Strateg. Manag. J. 2021, 20, 1–14. [Google Scholar]
- Tian, Z.; Yang, L.; Wu, X.; Guan, Z. A field study of occupant thermal comfort with radiant ceiling cooling and overhead air distribution system. Energy Build. 2020, 223, 109949. [Google Scholar] [CrossRef]
- Thornley, J.H.M. Respiration, growth and maintenance in plants. Nature 1970, 227, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J.S. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Ann. Bot. 2000, 86, 1–20. [Google Scholar] [CrossRef]
- Loveys, B.R.; Atkinson, L.J.; Sherlock, D.J.; Roberts, R.L.; Fitter, A.H.; Atkin, O.K. Thermal acclimation of leaf and root respiration: An investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. 2003, 9, 895–910. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Pregitzer, K.S.; Wright, I.J.; Oleksyn, J.; Machado, J.L. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008, 11, 793–801. [Google Scholar] [CrossRef]
- Pons, T.L.; Welschen, R.A.M. Overestimation of respiration rates in commercially available clamp-on leaf chambers: Complications with measurements of net photosynthesis. Plant Cell Environ. 2002, 25, 1367–1372. [Google Scholar] [CrossRef]
- Smith, A.; Pitt, M. Healthy workplaces: Plantscaping for indoor environmental quality. Facilities 2011, 29, 169–187. [Google Scholar] [CrossRef]
- Bidwell, R.G.S.; Bebee, G.P. Carbon monoxide fixation by plants. Can. J. Bot. 1974, 52, 1841–1847. [Google Scholar] [CrossRef]
- Tarran, J.; Torpy, F.; Burchett, M.D. Use of living pot-plants to cleanse indoor air. Research Review. In Proceedings of the 6th International Conference on Indoor Air Quality, Ventilation & Energy Conservation, -Sustainable Built, Sendai, Japan, 28–31 October 2007; pp. 249–256. [Google Scholar]
- Sevik, H.; Cetin, M.; Belkayali, N.; Guney, K. The effect of some indoor plants of the amount of CO2 in the internal environment. Result TUBITAK 2015, 3001. [Google Scholar]
- Sevik, H.; Cetin, M.; Guney, K.; Belkayali, N. The influence of house plants on indoor CO2. Pol. J. Environ. Stud. 2017, 26, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.M.M.; Braendle, R. Oxygen deprivation stress in a changing environment. J. Exp. Bot. 1996, 47, 145–159. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimation and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Beckett, P.M. Experimental and modeling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytol. 2011, 190, 431–441. [Google Scholar] [CrossRef]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef]
- Gupta, K.J.; Zabalza, A.; Van Dongen, J.T. Regulation of respiration when the oxygen availability changes. Physiol. Plant 2009, 137, 383–391. [Google Scholar] [CrossRef]
- Gliński, J.; Stępniewski, W. Soil Aeration and Its Role for Plants; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Simojoki, A.; Jaakkola, A. Effect of nitrogen fertilization, cropping and irrigation on soil air composition and nitrous oxide emission in a loamy clay. Eur. J. Soil Sci. 2000, 51, 413–424. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Q.; Li, X. Promoting sustainable carbon sequestration of plants in urban greenspace by planting design: A case study in parks of Beijing. Urban For. Urban Green. 2021, 64, 127291. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Liu, M.; Li, C. Impact of urban expansion on forest carbon sequestration: A study in northeastern China. Pol. J. Environ. Stud. 2020, 29, 451–461. [Google Scholar] [CrossRef]
- Foster, J.; Lowe, A.; Winkelman, S. The value of green infrastructure for urban climate adaptation. Cent. Clean. Air Policy 2011, 750, 1–52. [Google Scholar]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.C.; Hobbs, R.J. Cultural ecosystem services: Characteristics, challenges and lessons for urban green space research. Ecosyst. Serv. 2017, 25, 179–194. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P. Valuation of the ecosystem services: A psycho-cultural perspective. Ecol. Econ. 2008, 64, 808–819. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Donahue, M.L.; Keeler, B.L.; Wood, S.A.; Fisher, D.M.; Hamstead, Z.A.; McPhearson, T. Using social media to understand drivers of urban park visitation in the Twin Cities. MN. Landsc. Urban Plan. 2018, 175, 1–10. [Google Scholar] [CrossRef]
- Margaritis, E.; Kang, J.; Filipan, K.; Botteldooren, D. The influence of vegetation and surrounding traffic noise parameters on the sound environment of urban parks. Appl. Geogr. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B.; Robertson, G.P.; Cregg, B.M.; Andresen, J.A. Carbon sequestration potential of extensive green buildings. Environ. Sci. Technol. 2009, 43, 7564–7570. [Google Scholar] [CrossRef]
- Charoenkit, S.; Yiemwattana, S. Role of specific plant characteristics on thermal and carbon sequestration properties of living walls in tropical climate. Build. Environ. 2017, 115, 67–79. [Google Scholar] [CrossRef]
- Eksi, M.; Rowe, D.B.; Wichman, I.S.; Andresen, J.A. Effect of substrate depth, vegetation type, and season on green roof thermal properties. Energy Build. 2017, 145, 174–187. [Google Scholar] [CrossRef]
- Seyedabadi, M.R.; Eicker, U.; Karimi, S. Plant selection for green buildings and their impact on carbon sequestration and the building carbon footprint. Environ. Chall. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Bird, R. Knowing and Growing Annuals and Perennials, An Illustrated Encyclopedia and Complete Practical Gardening Guide; Gardening. Fulcrun Publication: Wheat Ridge, CO, USA, 2004; p. 142. [Google Scholar]
- Bhargava, B.; Malhotra, S.; Chandel, A.; Rakwal, A.; Kashwap, R.R.; Kumar, S. Mitigation of indoor air pollutants using Areca palm potted plants in real-life settings. Environ. Sci. Pollut. Res. 2021, 28, 8898–8906. [Google Scholar] [CrossRef] [PubMed]
- Taemthong, W. Air quality improvement using ornamental plants in classrooms. J. Green. Build. 2021, 16, 201–216. [Google Scholar] [CrossRef]
- Plitsiri, I.; Taemthong, W. Indoor Carbon Dioxide Reduction by Ornamental Plants: Comparison between Natural and Artificial Daylight. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2022, 13, 1–12. [Google Scholar]
- Chitsaz, V.; Parvizi, Y. The impacts of plant species and rangeland practices on the carbon sequestration capacity (Case study: Dehaghan watershed, Isfahan province). Iran. J. Range Desert Res. 2022, 29, 196–110. [Google Scholar]
- Sydney, E.B.; Sturm, W.; de Carvalho, J.C.; Thomaz-Soccol, V.; Larroche, C.; Pandey, A.; Soccol, C.R. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas. Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Fan, L.H.; Zhang, Y.T.; Zhang, L.; Chen, H.L. Evaluation of a membranesparged helical tubular photobioreactor for carbon dioxide biofixation by Chlorella vulgaris. J. Membr. Sci. 2008, 325, 336–345. [Google Scholar] [CrossRef]
- Yeh, K.L.; Chang, J.S. Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: Implications for biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Optimization of CO2 bio mitigation by Chlorella vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Kao, C.Y.; Tsai, M.T.; Ong, S.C.; Chen, C.H.; Lin, C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kannan, K.; Deshkar, A.; Yadav, R.; Chakrabarti, T. Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour. Technol. 2010, 101, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Tsai, M.T.; Kao, C.Y.; Ong, S.C.; Lin, C.S. The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng. Life Sci. 2009, 9, 254–260. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Pagarette, A.; Rooks, P.; Ali, S.T. The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J. Appl. Phycol. 2013, 25, 153–165. [Google Scholar] [CrossRef]
- Alonso, M.J.C.; Ortiz, C.E.L.; Perez, S.O.G.; Narayanasamy, R.; Fajardo San Miguel, G.D.J.; Hernandez, H.H.; Balagurusamy, N. Improved strength and durability of concrete through metabolic activity of ureolytic bacteria. Environ. Sci. Pollut. Res. 2017, 25, 1–8. [Google Scholar] [CrossRef]
- Alshalif, A.F.; Juki, M.I.; Othman, N.; Al-Gheethi, A.A. Improvement of mechanical properties of bio-concrete using Enterococcus faecalis and Bacillus cereus. Environ. Eng. Res. 2019, 24, 630–637. [Google Scholar] [CrossRef]
- Al-Thawadi, S.M. Ureolytic bacteria and calcium carbonate formation as a mechanism of strength enhancement of sand. J. Adv. Sci. Eng. Res. 2011, 1, 98–114. [Google Scholar]
- Pacheco-Torgal, F.; Labrincha, J.A. Biotechconcrete: An innovative approach for concrete with enhanced durability. In Eco-Efficient Concrete; Torgal, F.P., Jalali, S., Labrincha, J., John, V.M., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 565–576. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Maheshwari, N.; Kumar, M.; Thakur, I.S.; Srivastava, S. Cloning, expression and characterization of β- and γ-carbonic anhydrase from Bacillus sp. SS105 for biomimetic sequestration of CO2. Int. J. Biol. Macromol. 2019, 131, 445–452. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, A.; Thakur, I.S. Carbon dioxide sequestration by chemolithotrophic oleaginous bacteria for production and optimization of polyhydroxyalkanoate. Bioresour. Technol. 2016, 213, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Lasek, J.; Skawinska, A. CO2 biofixation and growth kinetics of Chlorella vulgaris and nannochloropsis gaditana. Appl. Biochem. Biotechnol. 2016, 179, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.K.; Srivastava, S.; Thakur, I.S. Production and characterization of biodiesel from carbon dioxide concentrating chemolithotrophic bacteria, Serratia sp. ISTD04. Bioresour. Technol. 2014, 53, 189–197. [Google Scholar] [CrossRef]
- Kumar, M.; Morya, R.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Characterization of carbon dioxide concentrating chemolithotrophic bacterium Serratia sp. ISTD04 for production of biodiesel. Bioresour. Technol. 2017, 243, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Chen, W.M.; Chang, J.S. Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour. Technol. 2010, 101, 8725–8730. [Google Scholar] [CrossRef]
- Gaddy, G.L.; Ko, C.W. CO2 Sequestration in Cell Biomass of Chlorobium Thiosulphatophilum; Bioengineering Resource Inc.: Fayetteville, AR, USA, 2009. [Google Scholar]
- Hastuti, D.R.D.; Darma, R.; Salman, D.; Santoso, S.; Rahim, A. June. Carbon sequestration of city agriculture: Between farming and non-farming land. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1041, p. 012009. [Google Scholar]
- Carmona-Salazar, L.; El Hafidi, M.; Enriquez-Arredondo, C.; Vazquez Vazquez, C.; Gonzalez De La Vara, L.E.; Gavilanes-Ruiz, M. Isolation of detergent-resistant membranes from plant photosynthetic and non-photosynthetic tissues. Anal. Biochem. 2011, 417, 220–227. [Google Scholar] [CrossRef]
- Idi, A.; Md Nor, M.H.; Abdul Wahab, M.F.; Ibrahim, Z. Photosynthetic bacteria: An eco-friendly and cheap tool for bioremediation. Rev. Environ. Sci. Bio/Technol. 2015, 14, 271–285. [Google Scholar] [CrossRef]
- Srivastava, S.; Bharti, R.K.; Thakur, I.S. Characterization of bacteria isolated from palaeoproterozoic metasediments for sequestration of carbon dioxide and formation of calcium carbonate. Environ. Sci. Pollut. Res. 2015, 22, 1499–1511. [Google Scholar] [CrossRef]
- Shafique, M.; Xue, X.; Luo, X. An overview of carbon sequestration of green roofs in urban areas. Urban For. Urban Green. 2020, 47, 126515. [Google Scholar] [CrossRef]
- Goudarzi, H.; Mostafaeipour, A. Energy saving evaluation of passive systems for residential buildings in hot and dry regions. Renew. Sustain. Energy Rev. 2017, 68, 432–446. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-López, A.; Barrera, G.M.; Suzán-Azpiri, H.; Bolaños, M.Q. Photosynthesis. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Southton, UK, 2019; pp. 47–72. [Google Scholar]
- Guo, W.J.; Lee, N. Effect of leaf and plant age, and day/night temperature on net CO2 uptake in Phalaenopsis amabilis var. Formosa. J. Am. Soc. Hortic. Sci. 2006, 131, 320–326. [Google Scholar] [CrossRef]
- EPA. Energy Resources. 2010. Available online: http://www.epa.gov/cleanenergy/energyresources/refs.html (accessed on 27 January 2015).
- Cilliers, E.J.; Lategan, L.; Cilliers, S.S.; Stander, K. Reflecting on the Potential and Limitations of Urban Agriculture as an Urban Greening Tool in South Africa. Front. Sustain. Cities Soc. 2020, 2, 43. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jozay, M.; Zarei, H.; Khorasaninejad, S.; Miri, T. Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development. Pollutants 2024, 4, 91-116. https://doi.org/10.3390/pollutants4010007
Jozay M, Zarei H, Khorasaninejad S, Miri T. Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development. Pollutants. 2024; 4(1):91-116. https://doi.org/10.3390/pollutants4010007
Chicago/Turabian StyleJozay, Mansoure, Hossein Zarei, Sarah Khorasaninejad, and Taghi Miri. 2024. "Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development" Pollutants 4, no. 1: 91-116. https://doi.org/10.3390/pollutants4010007
APA StyleJozay, M., Zarei, H., Khorasaninejad, S., & Miri, T. (2024). Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development. Pollutants, 4(1), 91-116. https://doi.org/10.3390/pollutants4010007