Copper in Commercial Marine Fish: From Biomonitoring to the ESG (Environment, Social, and Governance) Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.3. Cu Analysis
2.4. Cu Data Treatment for Human Health Risk Assessment
- (a)
- Direct MPL comparisons
- (b)
- THQ estimation
- (c)
- EWI vs. PTWI comparisons
3. Results and Discussion
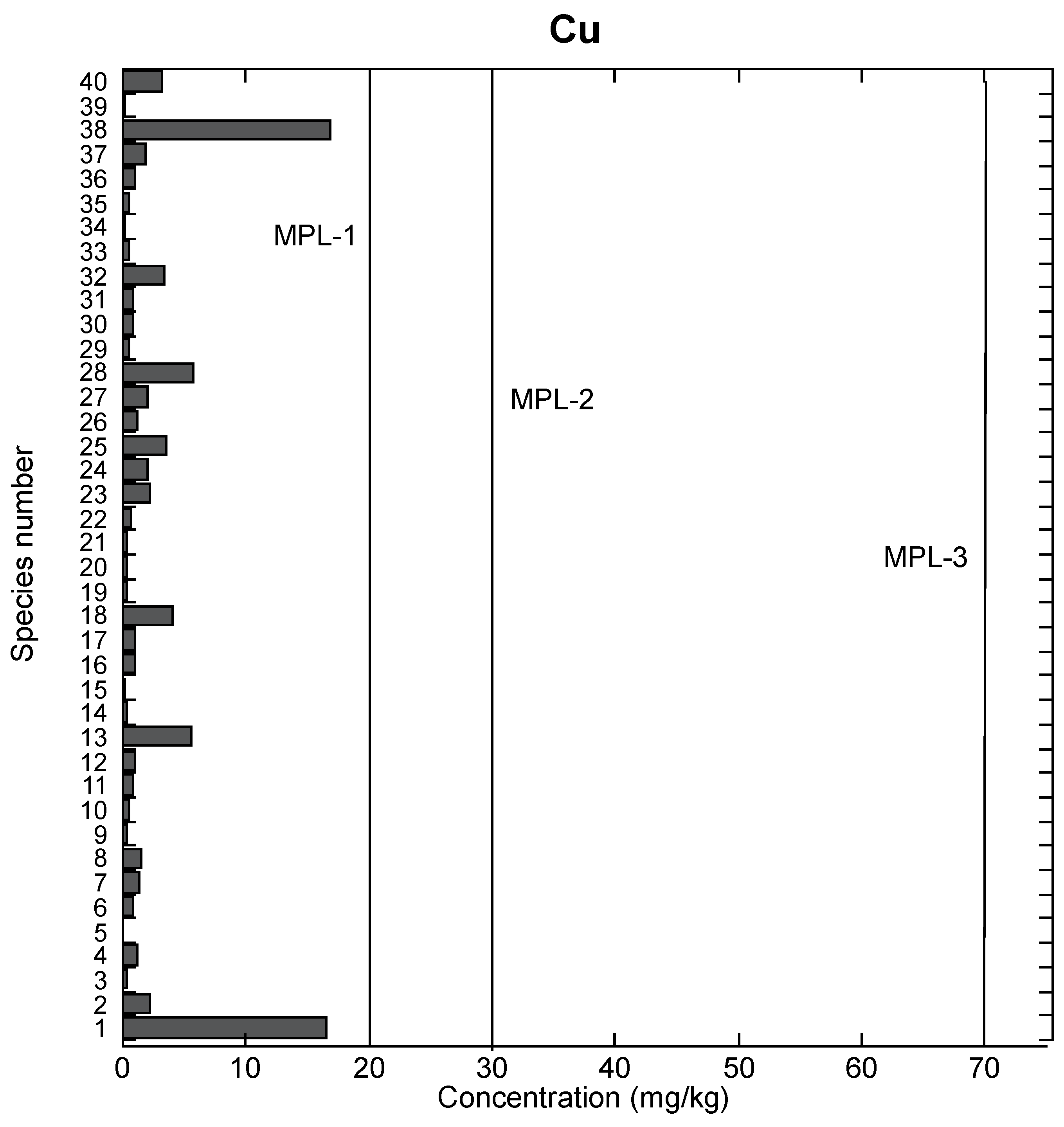
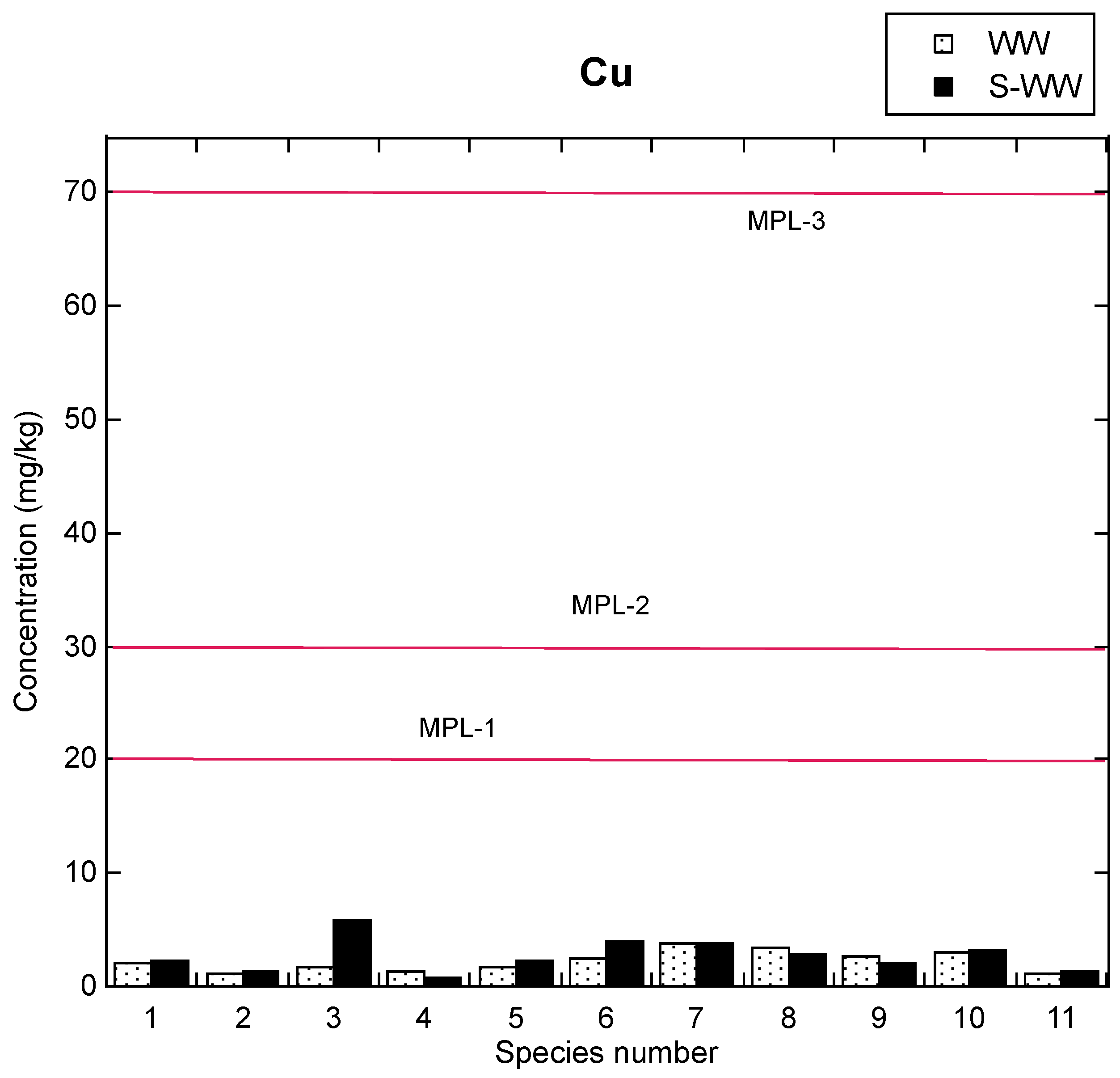
3.1. Comparison of Cu Food Safety Recommendations and Reported Cu Amounts in Fish Species
3.2. Comparison to Reported Studies
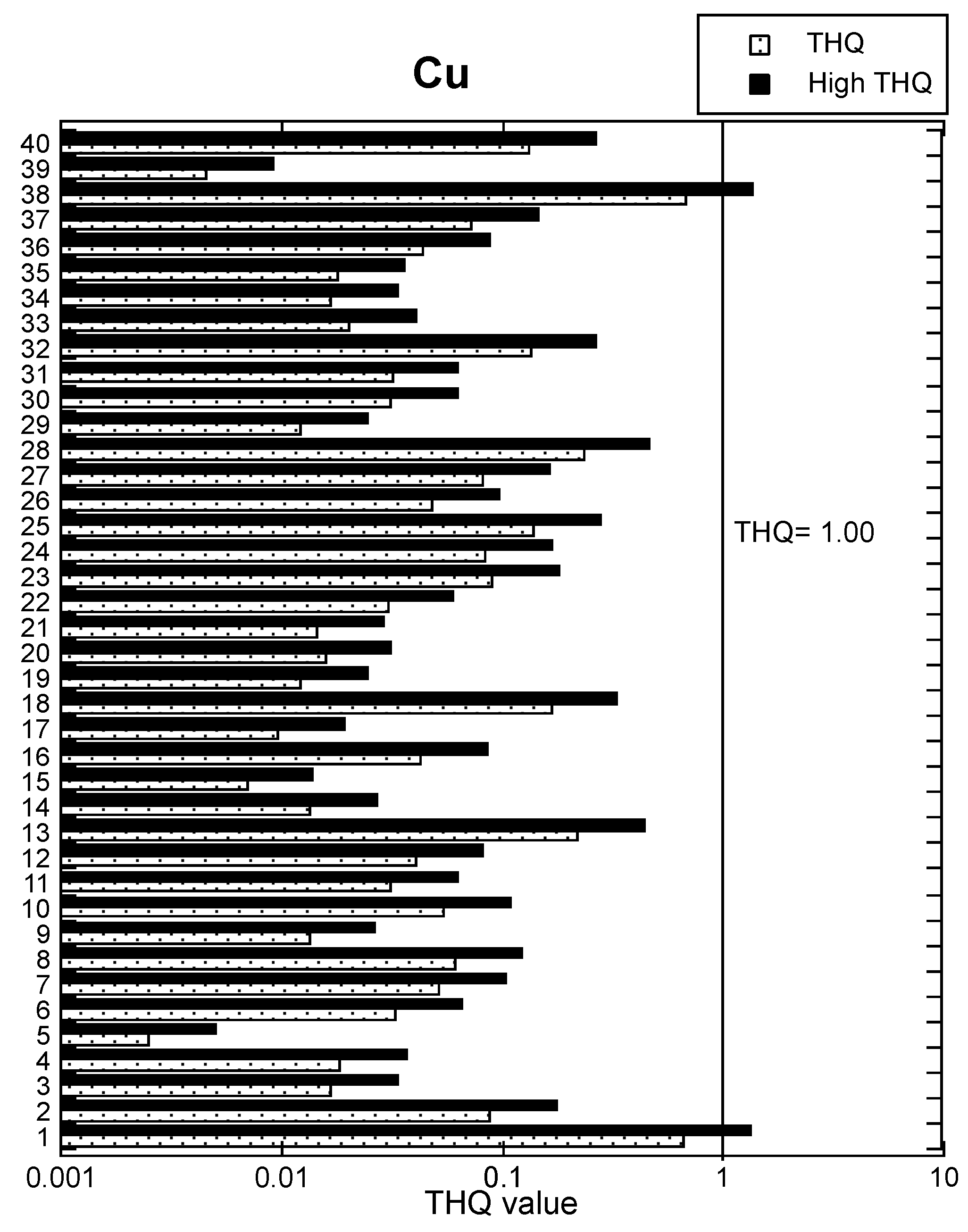
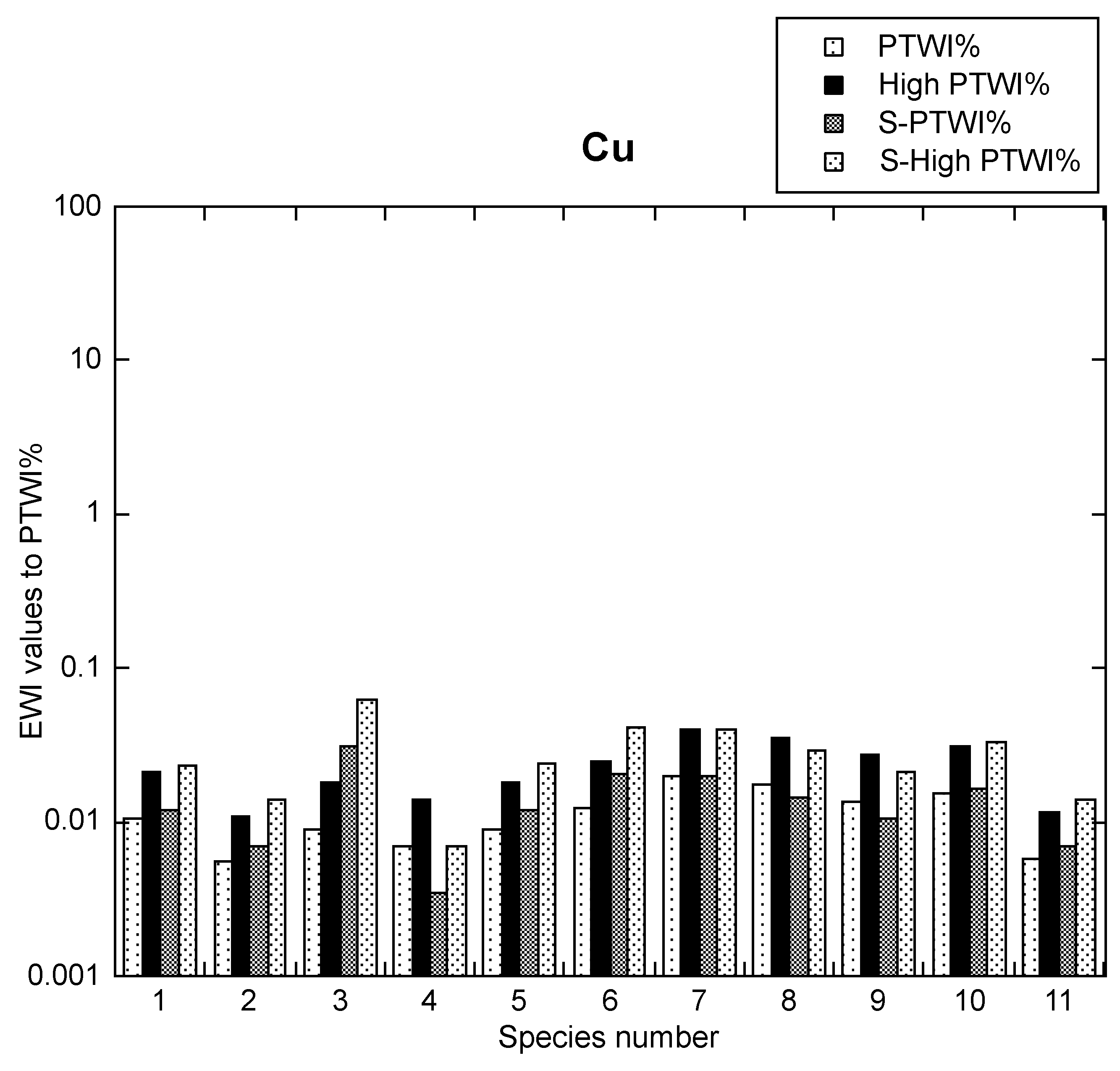
3.3. Cu Health Risk Assessment
3.4. Correlations of Cu Concentrations and Body Size of Fish
3.5. Monitoring of Cu as an ESG Requirement to Safeguard Consumers’ Health
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy Metals in Marine Fish Meat and Consumer Health: A Review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Dorsey, A.; Ingerman, L.; Swarts, S. Toxicological Profile for Copper. 2021. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp132.pdf (accessed on 15 December 2021).
- Roney, N.; Cassandra, V.; Williams, M.; Osier, M.; Paikoff, S.J. Toxicological Profile for Zinc; U.S. Department of Health and Human Services Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- WHO. Environmental Health Criteria 200: Copper; Joint Sponsorship of the UNEP, IPO, and WHO. Produced within the Framework of the Inter-Organization Programme for the Sound Management of Chemicals; World Health Organization: Geneva, Switzerland, 1998; ISBN 978-0-429-13978-9. [Google Scholar]
- Lall, S.P. Macro and Trace Elements in Fish and Shellfish. In Fish and Fishery Products: Composition, Nutritive Properties and Stability; Ruiter, A., Ed.; CAB Internat: Wallingford, UK, 1995; pp. 187–213. ISBN 978-0-85198-927-3. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Atlanta. Toxicological Profile for Copper; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004.
- Sivaperumal, P.; Sankar, T.V.; Viswanathan Nair, P.G. Heavy Metal Concentrations in Fish, Shellfish and Fish Products from Internal Markets of India Vis-a-Vis International Standards. Food Chem. 2007, 102, 612–620. [Google Scholar] [CrossRef]
- Demirezen, D.; Uruç, K. Comparative Study of Trace Elements in Certain Fish, Meat and Meat Products. Meat Sci. 2006, 74, 255–260. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Lloret, J.; Marin, A. Carbon and Nitrogen Stable Isotopes and Metal Concentration in Food Webs from a Mining-Impacted Coastal Lagoon. Sci. Total Environ. 2008, 393, 118–130. [Google Scholar] [CrossRef]
- Solami, L.A. Heavy metal content in coral reef-associated fish collected from the central Red Sea, Saudi Arabia. Oceanol. Hydrobiol. Stud. 2022, 51, 149–157. [Google Scholar] [CrossRef]
- Momčilović, B. Copper. In Elements and Their Compounds in the Environment: Occurrence, Analysis and Biological Relevance, 2nd ed.; Merian, E., Anke, M., Ihnat, M., Stoeppler, M., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2004; Chapter 8.1; pp. 731–750. [Google Scholar] [CrossRef]
- Han, J.L.; Pan, X.D.; Chen, Q.; Huang, B.F. Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci. Rep. 2021, 1, 11079. [Google Scholar] [CrossRef]
- Dimari, G.A.; Abdulrahman, F.I.; Akan, J.C.; Garba, S.H. Metals Concentrations in Tissues of Tilapia gallier, Crarias lazera and Osteoglossidae Caught from Alau Dam, Maiduguri, Borno State, Nigeria. Am. J. Environ. Sci. 2008, 4, 373. [Google Scholar] [CrossRef]
- Ebrahimpour, M.; Idris, M. Seasonal Variation of Cadmium, Copper, and Lead Concentrations in Fish from a Freshwater Lake. Biol. Trace Elem. Res. 2010, 138, 190–201. [Google Scholar] [CrossRef]
- Dineva, S. Ecotoxicity of Purified Industrial Waste Water. Environ. Ecol. Res. 2021, 7, 208–219. [Google Scholar] [CrossRef]
- Saleh, S.; Abdulnabi, Z.; Mizhir, A.; Hantoush, A.; Hassan, W.; Al-Khion, D. Evaluation of levels of some heavy metals in marine environment, southern Iraq. Mesopotamian J. Mar. Sci. 2021, 36, 96–105. [Google Scholar] [CrossRef]
- Daniszewski, P.; Konieczny, R. Heavy Metal Content in Water of Resko Lake (North-West Poland). Int. Lett. Chem. Phys. Astron. 2013, 8, 279–287. [Google Scholar] [CrossRef]
- Ouda, Y.W.; Kadhim, K.F.; Amer, A.M. Study of some toxic metals in parts from catfish (Silurus triostegus) in Shatt Al-Arab river. Iraqi J. Vet. Sci. 2023, 37, 459–467. [Google Scholar] [CrossRef]
- El-Moselhy, K.M.; Othman, A.I.; Abd El-Azem, H.; El-Metwally, M.E.A. Bioaccumulation of Heavy Metals in Some Tissues of Fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Khalaf, M.; Al-Najjar, T.; Alawi, M.; Disi, A.A. Levels of Trace Metals in Three Fish Species Decapterus macrellus, Decapterus macrosomos and Decapterus russelli of the Family Carangidae from the Gulf of Aqaba, Red Sea, Jordan. Nat. Sci. 2012, 4, 362–367. [Google Scholar] [CrossRef][Green Version]
- Younis, E.M.; Abdel-Warith, A.-W.A.; Al-Asgah, N.A.; Elthebite, S.A.; Mostafizur Rahman, M. Nutritional Value and Bioaccumulation of Heavy Metals in Muscle Tissues of Five Commercially Important Marine Fish Species from the Red Sea. Saudi J. Biol. Sci. 2021, 28, 1860–1866. [Google Scholar] [CrossRef]
- Kureishy, T.W.; Sanzgiry, S.; Braganca, A. Some Heavy Metals in Fishes from the Andaman Sea. Indian J. Mar. Sci. 1981, 10, 303–307. [Google Scholar]
- Nair, M.; Balachandran, K.K.; Sankaranarayanan, V.N.; Joseph, T. Heavy Metals in Fishes from Coastal Waters of Cochin, Southwest Coast of India. Int. J. Mar. Sci. 1997, 26, 98–100. [Google Scholar]
- Mohan, M.; Deepa, M.; Ramasamy, E.V.; Thomas, A.P. Accumulation of Mercury and Other Heavy Metals in Edible Fishes of Cochin Backwaters, Southwest India. Environ. Monit. Assess. 2012, 184, 4233–4245. [Google Scholar] [CrossRef]
- Raza, R.; Sayeed, S.A.; Siddiqi, R.; Naz, S. Trace Metal Contents in Selected Marine Fish Species of Northwest Coastal Area of Karachi, Pakistan. J. Chem. Soc. Pak. 2003, 25, 313–316. [Google Scholar]
- Ahmed, Q.; Khan, D.; Mohammad, Q. Heavy Metals (Fe, Mn, Pb, Cd, and Cr) Concentrations in Muscles, Liver, Kidneys, and Gills of Torpedo Scad (Megalaspis cordyla (Linnaeus, 1758)) from Karachi Waters of Pakistan. Int. J. Biol. Biotech. 2014, 11, 517–524. [Google Scholar]
- Velusamy, A.; Satheesh Kumar, P.; Ram, A.; Chinnadurai, S. Bioaccumulation of Heavy Metals in Commercially Important Marine Fishes from Mumbai Harbor, India. Mar. Pollut. Bull. 2014, 81, 218–224. [Google Scholar] [CrossRef]
- Kakar, A.; Hayat, M.T.; Abbasi, A.M.; Pervez, A.; Mahmood, Q.; Farooq, U.; Akbar, T.A.; Ali, S.; Rizwan, M.; El-Serehy, H.A.; et al. Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan. Animals 2020, 10, 1738. [Google Scholar] [CrossRef]
- Yap, C.K.; Al-Mutairi, K.A. Copper and Zinc levels in commercial marine fish from Setiu, East Coast of Peninsular Malaysia. Toxics 2022, 10, 52. [Google Scholar] [CrossRef]
- Takarina, N.D.; Purwiyanto, A.I.S.; Suteja, Y. Cadmium (Cd), Copper (Cu), and Zinc (Zn) Levels in Commercial and Non-Commercial Fishes in the Blanakan River Estuary, Indonesia: A Preliminary Study. Mar. Pollut. Bull. 2021, 170, 112607. [Google Scholar] [CrossRef]
- Abdolahpur Monikh, F.; Safahieh, A.; Savari, A.; Ronagh, M.T.; Doraghi, A. The Relationship between Heavy Metal (Cd, Co, Cu, Ni and Pb) Levels and the Size of Benthic, Benthopelagic and Pelagic Fish Species, Persian Gulf. Bull. Environ. Contam. Toxicol. 2013, 90, 691–696. [Google Scholar] [CrossRef]
- Abadi, D.R.V.; Dobaradaran, S.; Nabipour, I.; Lamani, X.; Ravanipour, M.; Tahmasebi, R.; Nazmara, S. Comparative Investigation of Heavy Metal, Trace, and Macro Element Contents in Commercially Valuable Fish Species Harvested off from the Persian Gulf. Environ. Sci. Pollut. Res. Int. 2015, 22, 6670–6678. [Google Scholar] [CrossRef]
- Hosseini, M.; Nabavi, S.M.B.; Nabavi, S.N.; Pour, N.A. Heavy Metals (Cd, Co, Cu, Ni, Pb, Fe, and Hg) Content in Four Fish Commonly Consumed in Iran: Risk Assessment for the Consumers. Environ. Monit. Assess. 2015, 187, 237. [Google Scholar] [CrossRef]
- Janadeleh, H.; Jahangiri, S. Risk Assessment and Heavy Metal Contamination in Fish (Otolithes ruber) and Sediments in Persian Gulf. J. Community Health Res. 2016, 5, 169–181. [Google Scholar]
- Agah, H.; Saleh, A.; Bastami, K.; Fumani, N. Ecological Risk, Source and Preliminary Assessment of Metals in the Surface Sediments of Chabahar Bay, Oman Sea. Mar. Pollut. Bull. 2016, 107, 383–388. [Google Scholar] [CrossRef]
- Rahman, M.S.; Molla, A.H.; Saha, N.; Rahman, A. Study on Heavy Metals Levels and Its Risk Assessment in Some Edible Fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem. 2012, 134, 1847–1854. [Google Scholar] [CrossRef]
- Lakshmanasenthil, S.; Vinothkumar, T.; AjithKumar, T.T.; Marudhupandi, T.; Veettil, D.K.; Ganeshamurthy, R.; Ghosh, S.; Balasubramanian, T. Harmful Metals Concentration in Sediments and Fishes of Biologically Important Estuary, Bay of Bengal. J. Environ. Health Sci. Eng. 2013, 11, 33. [Google Scholar] [CrossRef]
- Jahangir Sarker, M.; Naher Rima, N.; Sultana, N. Human Health Risk Assessment with Reference to the Consumption of Shrimp and Marine Fish. Pak. J. Biol. Sci. 2020, 23, 1291–1302. [Google Scholar] [CrossRef]
- Mziray, P.; Kimirei, I. Bioaccumulation of Heavy Metals in Marine Fishes (Siganus sutor, Lethrinus harak, and Rastrelliger kanagurta) from Dar Es Salaam Tanzania. Reg. Stud. Mar. Sci. 2016, 7, 72–80. [Google Scholar] [CrossRef]
- Ahmad, S.; Al-Ghais, S.M. Metal Contents in the Tissues of Lutjanus fulviflamma (Smith 1949) and Epinephelus tauvina (Forskål 1775) Collected from the Arabian Gulf. Bull. Environ. Contam. Toxico. 1996, 57, 957–963. [Google Scholar] [CrossRef]
- Deshpande, A.; Bhendigeri, S.; Shirsekar, T.; Dhaware, D.; Khandekar, R.N. Analysis of heavy metals in marine fish from Mumbai Docks. Environ. Monitor. Assess. 2008, 159, 493–500. [Google Scholar] [CrossRef]
- Ebrahimi Yazdanabad, T.; Emtyazjoo, M.; Ghavam Mostafavi, P.; Sahebi, Z. Evaluation of copper and vanadium concentration in the soft tissue of Tylosurus crocodilusin Bahregan region, Persian Gulf. Chem. Spec. Bioavail. 2014, 26, 92–98. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Lin, Q.; Wang, X.-H.; Du, F.-Y.; Yu, Z.-L.; Huang, H.-H. Heavy metal concentrations in wild fishes captured from the South China Sea and associated health risks. Mar. Pollut. Bull. 2015, 96, 508–512. [Google Scholar] [CrossRef]
- Jaziri, A.A.; Hasanuddin, H.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Nutritional composition and mineral analysis of the by-products from tropical marine fish, purple-spotted bigeye (Priacanthus tayenus Richardson, 1846) and barracuda (Sphyraena obtusata Cuvier, 1829). Earth Environ. Sci. 2022, 967, 1. [Google Scholar] [CrossRef]
- Krishnamurti, A.J.; Nair, V.R. Concentration of metals in fishes from Thane and Bassein creeks of Bombay, India. Indian J. Mar. Sci. 1999, 28, 39–44. [Google Scholar]
- Ong, C.; Aziz, N.M.; Shazili, N.A.; Yunus, K. Selected heavy metals content in commercial fishes at different season landed at Fisheries Development Authority of Malaysia Complex (LKIM) Complex, Kuala Terengganu, Malaysia. Sustain. Sci. Manag. 2018, 13, 29–38. [Google Scholar]
- Salam, M.A.; Paul, S.C.; Noor, S.N.B.M.; Siddiqua, S.A.; Aka, T.D.; Wahab, R.; Aweng, E.R. Contamination profile of heavy metals in marine fish and shellfish. Glob. J. Environ. Sci. Manag. 2019, 5, 225–236. [Google Scholar] [CrossRef]
- Sarker, M.J.; Polash, A.U.; Islam, M.A.; Rima, N.N.; Farhana, T. Heavy metals concentration in native edible fish at upper Meghna River and its associated tributaries in Bangladesh: A prospective human health concern. SN Appl. 2020, 2, 1667. [Google Scholar] [CrossRef]
- Tabezar, N.; Sadeghi, P.; Attaran Fariman, G. Monsoon effect on heavy metal and chemical composition in Parastromateus niger of the Oman Sea: Health risk assessment of fish consumption. Biol. Trace Elem. Res. 2023, 201, 4093–4102. [Google Scholar] [CrossRef]
- Babji, A.S.; Embong, M.S.; Woon, W.W. Heavy Metal Contents in Coastal Water Fishes of West Malaysia. Bull. Environ. Contam. Toxicol. 1979, 23, 830–836. [Google Scholar] [CrossRef]
- Fathi, B.; Othman, M.; Mazlan, A.G.; Idris, G.; Arshad, A.; Amin, S.M.N.; Simon, D. Trace Metals in Muscle, Liver and Gill Tissues of Marine Fishes from Mersing, Eastern Coast of Peninsular Malaysia: Concentration and Assessment of Human Health Risk. Asian J. Anim. Vet. Adv. 2013, 8, 227–236. [Google Scholar] [CrossRef][Green Version]
- Alipour, M.; Sarafraz, M.; Chavoshi, H.; Bay, A.; Nematollahi, A.; Sadani, M.; Fakhri, Y.; Vasseghian, Y.; Mousavi Khaneghah, A. The Concentration and Probabilistic Risk Assessment of Potentially Toxic Elements in Fillets of Silver Pomfret (Pampus argenteus): A Global Systematic Review and Meta-Analysis. J. Environ. Sci. 2021, 100, 167–180. [Google Scholar] [CrossRef]
- Ahmad Kamal, N.H.; James Noik, V.; Chu, P.; Yuh, L.; Yin, T. A Comparative Study of Cd and Pb Concentration in Selected Commercial Marine Fishes from Wet Markets and Supermarkets in Klang Valley, Malaysia. Health Environ. J. 2012, 3, 25–37. [Google Scholar]
- Agusa, T.; Kunito, T.; Yasunaga, G.; Iwata, H.; Subramanian, A.; Ismail, A.; Tanabe, S. Concentrations of Trace Elements in Marine Fish and Its Risk Assessment in Malaysia. Mar. Pollut. Bull. 2005, 51, 896–911. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Sudaryanto, A.; Monirith, I.; Kan-Atireklap, S.; Iwata, H.; Ismail, A.; Sanguansin, J.; Muchtar, M.; Tana, T.S.; et al. Exposure Assessment for Trace Elements from Consumption of Marine Fish in Southeast Asia. Environ. Pollut. 2007, 145, 766–777. [Google Scholar] [CrossRef]
- Rosli, M.N.R.; Samat, S.B.; Yasir, M.S.; Yusof, M.F.M. Analysis of Heavy Metal Accumulation in Fish at Terengganu Coastal Area, Malaysia. Sains Malays. 2018, 47, 1277–1283. [Google Scholar] [CrossRef]
- Irwandi, J.; Farida, M. Mineral and Heavy Metal Contents of Marine Fin Fish in Langkawi Island, Malaysia. Int. Food Res. J. 2009, 16, 105–112. [Google Scholar]
- Wan Azmi, W.N.F.; Nurul Izzah, A.; Wan Mahiyuddin, W.R. Heavy Metal Levels and Risk Assessment from Consumption of Marine Fish in Peninsular Malaysia. J. Environ. Prot. 2019, 10, 1450–1471. [Google Scholar] [CrossRef]
- Salam, M.A.; Dayal, S.R.; Siddiqua, S.A.; Muhib, M.I.; Bhowmik, S.; Kabir, M.M.; Rak, A.A.E.; Srzednicki, G. Risk Assessment of Heavy Metals in Marine Fish and Seafood from Kedah and Selangor Coastal Regions of Malaysia: A High-Risk Health Concern for Consumers. Environ. Sci. Pollut. Res. Int. 2021, 28, 55166–55175. [Google Scholar] [CrossRef]
- Kamaruzzaman, Y.; Rina, Z.; John, B.A.; Jalal, K.C.A. Heavy Metal Accumulation in Commercially Important Fishes of South West Malaysian Coast. Res. J. Environ. Sci. 2011, 5, 595–602. [Google Scholar] [CrossRef]
- Kamaruzzaman, Y.; Ong, M.C.; Rina, S.Z. Concentration of Zn, Cu and Pb in Some Selected Marine Fishes of the Pahang Coastal Waters, Malaysia. Am. J. Appl. Sci. 2010, 7, 309–314. [Google Scholar] [CrossRef]
- Kamaruzzaman, B.Y.; Ong, M.C.; Jalal, K.C.A. Levels of Copper, Zinc and Lead in Fishes of Mengabang Telipot River, Terengganu, Malaysia. J. Biol. Sci. 2008, 8, 1181–1186. [Google Scholar] [CrossRef][Green Version]
- Mohsin, A.K.M.; Ambak, M.A. Marine Fishes & Fisheries of Malaysia and Neighbouring Countries; Universiti Pertanian Malaysia Press: Serdang, Malaysia, 1996; ISBN 978-983-9319-04-0. [Google Scholar]
- Matsunuma, M.; Motomura, H.; Matsuura, K.; Shazili, N.A.M.; Ambak, M. Fishes of Terengganu, East Coast of Malay Peninsular, Malaysia; National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum: Kagoshima, Japan, 2011. [Google Scholar]
- Yap, C.K.; Jusoh, A.; Leong, W.J.; Karami, A.; Ong, G.H. Potential Human Health Risk Assessment of Heavy Metals via the Consumption of Tilapia Oreochromis mossambicus Collected from Contaminated and Uncontaminated Ponds. Environ. Monit. Assess. 2015, 187, 584. [Google Scholar] [CrossRef]
- Nauen, C. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Products; FAO Fishery Circular No. 464; Food and Agriculture Organization: Rome, Italy, 1983; pp. 5–100. [Google Scholar]
- MAFF. Monitoring and Surveillance of Non-Radioactive Contaminants in the Aquatic Environment and Activities Regulating the Disposal of Wastes at Sea. In Aquatic Environment Monitoring Report No. 52; Center for Environment, Fisheries and Aquaculture Science, Ministry of Agriculture, Fisheries and Food: Lowestoft, UK, 2000. [Google Scholar]
- Malaysian Food Regulations (MFR). Malaysian Law on Food and Drugs; Malaysian Law Publishers: Kuala Lumpur, Malaysia, 1985; pp. 43–44. [Google Scholar]
- Sai-Ut, S.; Jongjareonrak, A.; Rawdkuen, S. Re-extraction, recovery, and characteristics of skin gelatin from farmed giant catfish. Food Bioprocess Technol. 2012, 5, 1197–1205. [Google Scholar] [CrossRef]
- Nurul Izzah, A.; Wan Rozita, W.M.; Tengku Rozaina, T.M.; Cheong, Y.L.; Daud, S.F.; Nasriyah, C.H.; Nor Aini, A.; Rafiza, S.; Lokman, H.S. Fish Consumption Pattern among Adults of Different Ethnics in Peninsular Malaysia. Food Nutr. Res. 2016, 60, 32697. [Google Scholar] [CrossRef]
- Idriss, A.A.; Ahmad, A.K. Heavy Metal Concentrations in Fishes from Juru River, Estimation of the Health Risk. Bull. Environ. Contam. Toxicol. 2015, 94, 204–208. [Google Scholar] [CrossRef]
- US EPA. Human Health Risk Assessment., Regional Screening Level (RSL)—Summary Table. 2021. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 1 January 2024).
- JECFA. Summary and Conclusions of the Seventy-Third Meeting of the JECFA. JECFA/73/SC. 2010. Available online: https://www.fao.org/3/at862e/at862e.pdf (accessed on 1 January 2024).
- WHO/JECFA. List of Chemicals in Functional Category Food Contaminant. 2022. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/search.aspx?fcc=2 (accessed on 15 January 2022).
- Nurnadia, A.A.; Azrina, A.; Amin, I.; Mohd Yunus, A.S.; Effendi Halmi, M. Mineral Contents of Selected Marine Fish and Shellfish from the West Coast of Peninsular Malaysia. Int. Food Res. J. 2013, 20, 431–437. [Google Scholar]
- Celik, U.; Oehlenschläger, J. Determination of Zinc and Copper in Fish Samples Collected from Northeast Atlantic by DPSAV. Food Chem. 2004, 87, 343–347. [Google Scholar] [CrossRef]
- Simanjuntak, C.; Djumanto, D.; Rahardjo, M.; Zahid, A. Assessment of Heavy Metals (Al, Zn, Cu, Cd, Pb and Hg) in Demersal Fishes of Kuala Tanjung Coast, North Sumatera. In Proceedings of the FAPERIKA-UNRI; Riau University: Riau, Indonesia, 2012; pp. 178–196. [Google Scholar]
- Tuzen, M. Toxic and Essential Trace Elemental Contents in Fish Species from the Black Sea, Turkey. Food Chem. Toxicol. 2009, 47, 1785–1790. [Google Scholar] [CrossRef]
- Blevins, R.; Pancorbo, O. Metal Concentrations in Muscle of Fish from Aquatic Systems in East Tennessee, U.S.A. Water Air Soil Pollut. 1986, 29, 361–371. [Google Scholar] [CrossRef]
- Rejomon, G.; Nair, M.; Joseph, T. Trace Metal Dynamics in Fishes from the Southwest Coast of India. Environ. Monit. Assess. 2010, 167, 243–255. [Google Scholar] [CrossRef]
- Anandkumar, R.N.; Prabakaran, K.; Chua, H.B.; Rajaram, D.R. Human Health Risk Assessment and Bioaccumulation of Trace Metals in Fish Species Collected from the Miri Coast, Sarawak, Borneo. Mar. Pollut. Bull. 2018, 133, 655–663. [Google Scholar] [CrossRef]
- Kalay, M.; Ay, O.; Canli, M. Heavy Metal Concentrations in Fish Tissues from the Northeast Mediterranean Sea. Bull. Environ. Contam. Toxicol. 1999, 63, 673–681. [Google Scholar] [CrossRef]
- Ates, A.; Türkmen, M.; Tepe, Y. Assessment of Heavy Metals in Fourteen Marine Fish Species of Four Turkish Seas. Indian J. Geo-Mar. Sci. 2015, 44, 49–55. [Google Scholar]
- Arulkumar, A.; Paramasivam, S.; Rajaram, R. Toxic Heavy Metals in Commercially Important Food Fishes Collected from Palk Bay, Southeastern India. Mar. Pollut. Bull. 2017, 119, 454–459. [Google Scholar] [CrossRef]
- Prasath, P.M.D.; Khan, T.H. Impact of Tsunami on the Heavy Metal Accumulation in Water, Sediments and Fish at Poompuhar Coast, Southeast Coast of India. J. Chem. 2008, 5, 16–22. [Google Scholar] [CrossRef]
- Dural Eken, M.; Ziya Lugal Göksu, M.; Akif Özak, A. Investigation of Heavy Metal Levels in Economically Important Fish Species Captured from the Tuzla Lagoon. Food Chem. 2007, 102, 415–421. [Google Scholar] [CrossRef]
- Hellou, J.; Fancey, L.L.; Payne, J.F. Concentrations of Twenty-Four Elements in Bluefin Tuna, Thunnus Thynnus from the Northwest Atlantic. Chemosphere 1992, 24, 211–218. [Google Scholar] [CrossRef]
- Yabanli, M.; Alparslan, Y. Potential Health Hazard Assessment in Terms of Some Heavy Metals Determined in Demersal Fishes Caught in Eastern Aegean Sea. Bull. Environ. Contam. Toxicol. 2015, 95, 494–498. [Google Scholar] [CrossRef]
- Praveena, S.; Lin, C.L.S. Assessment of Heavy Metal in Self-Caught Saltwater Fish from Port Dickson Coastal Water, Malaysia. Sains Malays. 2015, 44, 91–99. [Google Scholar] [CrossRef]
- Peycheva, K.; Panayotova, V.; Stancheva, M. Assessment of Human Health Risk for Copper, Arsenic, Zinc, Nickel, and Mercury in Marine Fish Species Collected from Bulgarian Black Sea Coast. Int. J. Fish. Aquat. Stud. 2016, 4, 41–46. [Google Scholar]
- Bashir, F.H.; Othman, M.S.; Mazlan, A.G.; Rahim, S.M.; Simon, K.D. Heavy Metal Concentration in Fishes from the Coastal Waters of Kapar and Mersing, Malaysia. Turk. J. Fish. Aquat. Sci. 2013, 13, 375–382. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Huang, H.-H.; Lin, Q. Concentrations and Human Health Implications of Heavy Metals in Wild Aquatic Organisms Captured from the Core Area of Daya Bay’s Fishery Resource Reserve, South China Sea. Environ. Toxicol. Pharmacol. 2016, 45, 90–94. [Google Scholar] [CrossRef]
- Saghali, M.; Hoseini, S.M.; Hosseini, S.A.; Baqraf, R. Determination of Heavy Metal (Zn, Pb, Cd and Cr) Concentration in Benthic Fauna Tissues Collected from the Southeast Caspian Sea, Iran. Bull. Environ. Contam. Toxicol. 2014, 92, 57–60. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Senthilkumar, D.; Ananthan, G.; Soundarapandian, P.; Khan, A.B. Measurement of Hematological and Biochemical Studies on Wild Marine Carnivorous Fishes from Vellar Estuary, Southeast Coast of India. Comp. Clin. Pathol. 2011, 20, 127–134. [Google Scholar] [CrossRef]
- Chi, Q.; Zhu, G.; Langdon, A. Bioaccumulation of Heavy Metals in Fishes from Taihu Lake, China. J. Environ. Sci. 2007, 19, 1500–1504. [Google Scholar] [CrossRef]
- Singh, R.K.; Chavan, S.L.; Sapkale, P.H. Heavy Metal Concentrations in Water, Sediments and Body Tissues of Red Worm (Tubifex spp.) Collected from Natural Habitats in Mumbai, India. Environ. Monit. Assess. 2007, 129, 471–481. [Google Scholar] [CrossRef]
- Yi, Y.J.; Zhang, S.H. The Relationships between Fish Heavy Metal Concentrations and Fish Size in the Upper and Middle Reach of Yangtze River. Procedia Environ. Sci. 2012, 13, 1699–1707. [Google Scholar] [CrossRef]
- Canli, M.; Atli, G. The Relationships between Heavy Metal (Cd, Cr, Cu, Fe, Pb, Zn) Levels and the Size of Six Mediterranean Fish Species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- Ylitalo, G.M.; Krahn, M.M.; Dickhoff, W.W.; Stein, J.E.; Walker, C.C.; Lassitter, C.L.; Dickey, R.W. Federal seafood safety response to the Deepwater Horizon oil spill. Proc. Nat. Acad. Sci. USA 2012, 109, 20274–20279. [Google Scholar] [CrossRef]
- Cheema, S.; Langa, M. Environment, Social, and Governance (ESG) and Sustainability. In A Director’s Guide to Governance in the Boardroom: Across the Private, Public, and Voluntary Sectors; Routledge: London, UK, 2022; pp. 135–171. [Google Scholar]




| DW | WW | EDI | High EDI | THQ | High THQ | EWI | High EWI | PTWI% | High PTWI% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | 0.72 | 0.06 | 0.10 | 0.20 | 0.00 | 0.01 | 0.70 | 1.41 | 0.00 | 0.00 |
| Maximum | 82.3 | 16.9 | 27.3 | 54.6 | 0.68 | 1.37 | 191 | 382 | 0.09 | 0.18 |
| Mean | 10.2 | 2.17 | 3.50 | 6.89 | 0.09 | 0.17 | 24.1 | 48.2 | 0.01 | 0.02 |
| Median | 4.66 | 0.98 | 1.58 | 2.57 | 0.04 | 0.07 | 9.01 | 18.0 | 0.00 | 0.01 |
| SD | 17.7 | 3.65 | 5.89 | 11.82 | 0.15 | 0.29 | 41.4 | 82.8 | 0.02 | 0.04 |
| Variance | 313 | 13.3 | 34.7 | 139 | 0.02 | 0.09 | 1712 | 6848 | 0.00 | 0.00 |
| SE | 2.80 | 0.58 | 0.93 | 1.87 | 0.02 | 0.05 | 6.54 | 13.1 | 0.00 | 0.01 |
| Skewness | 3.36 | 3.26 | 3.26 | 3.25 | 3.25 | 3.25 | 3.25 | 3.25 | 3.25 | 3.25 |
| Kurtosis | 10.7 | 10.3 | 10.3 | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 |
| DW | WW | EDI | High EDI | THQ | High THQ | EWI | High EWI | PTWI% | High PTWI% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | 0.05 | 0.01 | 0.01 | 0.03 | 0.00 | 0.00 | 0.10 | 0.20 | 0.00 | 0.00 |
| Maximum | 42.8 | 11.2 | 18.1 | 36.1 | 0.45 | 0.90 | 126 | 253 | 0.06 | 0.12 |
| Mean | 4.24 | 0.94 | 1.51 | 3.02 | 0.04 | 0.08 | 10.6 | 21.2 | 0.00 | 0.01 |
| Median | 2.50 | 0.58 | 0.94 | 1.87 | 0.02 | 0.05 | 6.55 | 13.1 | 0.00 | 0.01 |
| SD | 5.45 | 1.33 | 2.15 | 4.30 | 0.05 | 0.11 | 15.05 | 30.1 | 0.01 | 0.01 |
| Variance | 29.7 | 1.78 | 4.62 | 18.5 | 0.00 | 0.01 | 226 | 906 | 0.00 | 0.00 |
| SE | 0.55 | 0.13 | 0.22 | 0.43 | 0.01 | 0.01 | 1.51 | 3.03 | 0.00 | 0.00 |
| Skewness | 4.42 | 5.35 | 5.35 | 5.36 | 5.36 | 5.36 | 5.36 | 5.36 | 5.36 | 5.36 |
| Kurtosis | 25.5 | 35.5 | 35.5 | 35.6 | 35.6 | 35.6 | 35.6 | 35.6 | 35.6 | 35.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, C.K.; Austin Hew, T.Y.; Nulit, R.; Syazwan, W.M.; Okamura, H.; Horie, Y.; Ong, M.C.; Ismail, M.S.; Kumar, K.; Zakaly, H.M.H.; et al. Copper in Commercial Marine Fish: From Biomonitoring to the ESG (Environment, Social, and Governance) Method. Pollutants 2024, 4, 117-135. https://doi.org/10.3390/pollutants4010008
Yap CK, Austin Hew TY, Nulit R, Syazwan WM, Okamura H, Horie Y, Ong MC, Ismail MS, Kumar K, Zakaly HMH, et al. Copper in Commercial Marine Fish: From Biomonitoring to the ESG (Environment, Social, and Governance) Method. Pollutants. 2024; 4(1):117-135. https://doi.org/10.3390/pollutants4010008
Chicago/Turabian StyleYap, Chee Kong, Tze Yik Austin Hew, Rosimah Nulit, Wan Mohd Syazwan, Hideo Okamura, Yoshifumi Horie, Meng Chuan Ong, Mohamad Saupi Ismail, Krishnan Kumar, Hesham M. H. Zakaly, and et al. 2024. "Copper in Commercial Marine Fish: From Biomonitoring to the ESG (Environment, Social, and Governance) Method" Pollutants 4, no. 1: 117-135. https://doi.org/10.3390/pollutants4010008
APA StyleYap, C. K., Austin Hew, T. Y., Nulit, R., Syazwan, W. M., Okamura, H., Horie, Y., Ong, M. C., Ismail, M. S., Kumar, K., Zakaly, H. M. H., & Cheng, W. H. (2024). Copper in Commercial Marine Fish: From Biomonitoring to the ESG (Environment, Social, and Governance) Method. Pollutants, 4(1), 117-135. https://doi.org/10.3390/pollutants4010008







