Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments
Abstract
1. Introduction
2. Materials and Methods
2.1. Cabbage Field and Experimental Design
2.2. Data Collection and Soil Sampling
2.3. Metal Analysis
2.3.1. Quantification of Metals in Harvested Plants
2.3.2. Evaluation of Soluble Concentrations of Metals in Soil
2.4. Bioaccumulation Factor (BAF)
2.5. Statistical Analysis
3. Results and Discussions
3.1. Yield and Quality
3.2. Total and Soluble Heavy Metal Concentrations in Soil
3.3. BAF Values of Cabbage var. Primo Vantage
3.4. BAF Values of Cabbage var. Tiara
3.5. BAF Values of Cabbage var. Capture
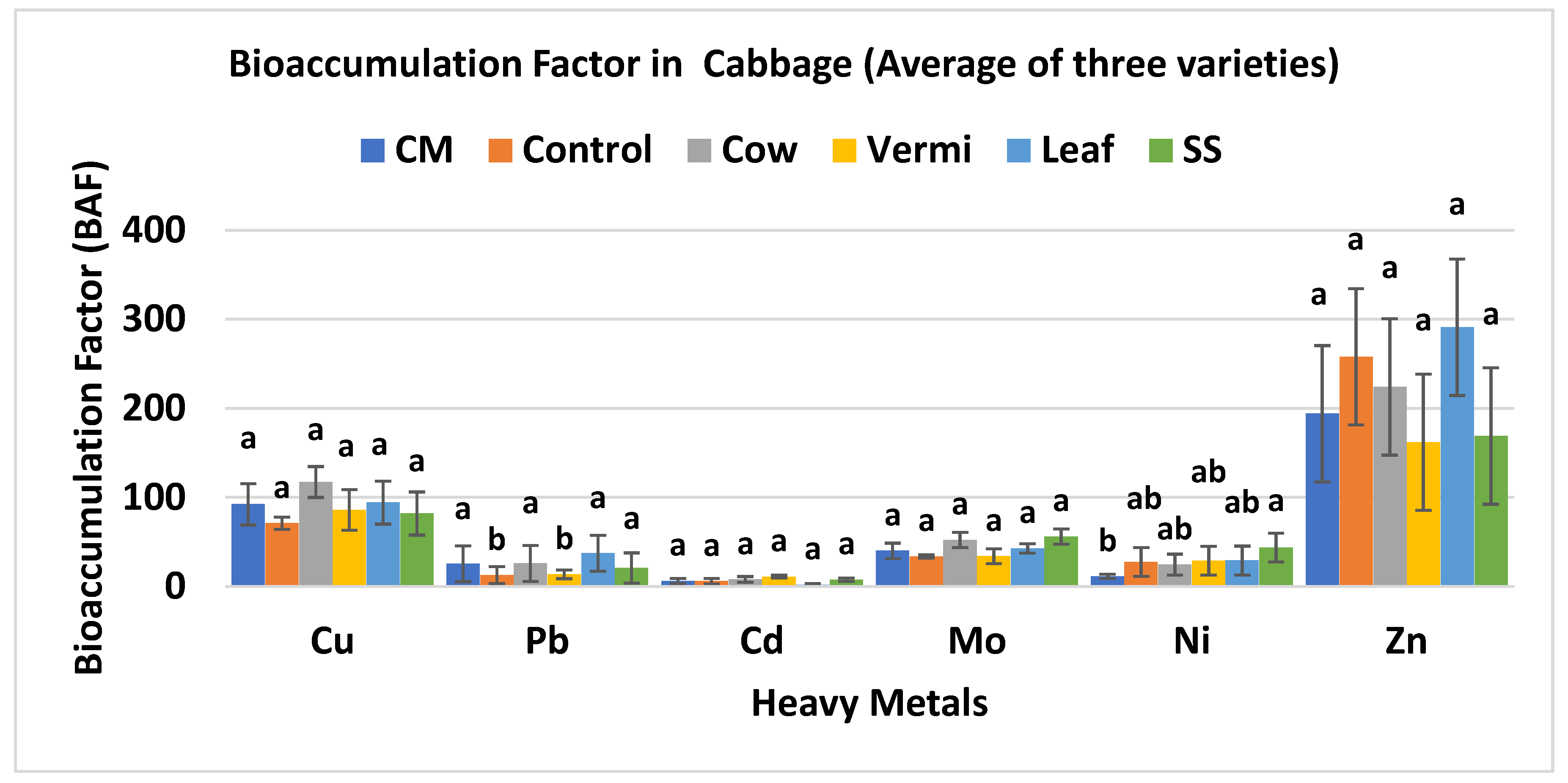
3.6. Overall BAF Values of Three Varieties of Cabbage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.G.; Lin, Q.; Gao, Y.P. Metals in exposed-lawn soils from 18 urban parks and its human health implications in southern China’s largest city, Guangzhou. J. Clean. Prod. 2016, 115, 122–129. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Pelka, R.; Bik-Malodzinska, M.; Zukowska, G.; Glen-Karolczyk, K. The effect of cellulose production waste and municipal sewage sludge on biomass and heavy metal uptake by a plant mixture. Environ. Sci. Pollut. Res. Int. 2018, 25, 31101–31112. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, N.; Ratn, A.; Awasthi, Y.; Prasad, R.; Trivedi, A.; Trivedi, S.P. Biomonitoring of heavy metals in river ganga water, sediments, plant, and fishes of different trophic levels. Biol. Trace Elem. Res. 2020, 193, 536–547. [Google Scholar] [CrossRef]
- Khan, Z.I.; Safdar, H.; Ahmad, K.; Wajid, K.; Bashir, H.; Ugulu, I.; Dogan, Y. Health risk assessment through determining bioaccumulation of iron in forages grown in soil irrigated with city effluent. Environ. Sci. Pollut. Res. 2019, 26, 14277–14286. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Qureshi, T.M.; Ugulu, I.; Riaz, M.N.; An, Q.U.; Khan, Z.I.; Ahmad, K.; Ashfaq, A.; Bashir, H.; Dogan, Y. Mineral, vitamin and phenolic contents and sugar profiles of some prominent date palm (Phoenix dactylifera) varieties of Pakistan. Pak. J. Bot. 2019, 51, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yorek, N.; Ugulu, I.; Aydin, H. Using self-organizing neural network map combined with ward’s clustering algorithm for visualization of students’ cognitive structural models about aliveness concept. Comput. Intell. Neurosci. 2016, 2016, 2476256. [Google Scholar] [CrossRef] [PubMed]
- Rezig, F.A.M.; Mubarak, A.R.; Ehadi, E.A. Impact of organic residues and mineral fertilizer application on soil–crop system: II soil attributes. Arch. Agron. Soil Sci. 2013, 59, 1245–1261. [Google Scholar] [CrossRef]
- Dogan, Y.; Unver, M.C.; Ugulu, I.; Calis, M.; Durkan, N. Heavy metal accumulation in the bark and leaves of Juglans regia planted in Artvin City, Turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 643–649. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmad, K.; Safdar, H.; Ugulu, I.; Wajid, K.; Bashir, H.; Dogan, Y. Manganese bioaccumulation and translocation of in forages grown in soil irrigated with city effluent: An evaluation on health risk. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 759–770. [Google Scholar]
- Khan, Z.I.; Ugulu, I.; Umar, S.; Ahmad, K.; Mehmood, N.; Ashfaq, A.; Bashir, H.; Sohail, M. Potential toxic metal accumulation in soil, forage and blood plasma of buffaloes sampled from Jhang, Pakistan. Bull. Environ. Contam. Toxicol. 2018, 101, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.I.; Ugulu, I.; Ahmad, K.; Yasmeen, S.; Noorka, I.R.; Mehmood, N.; Sher, M. Assessment of trace metal and metalloid accumulation and human health risk from vegetables consumption through spinach and coriander specimens irrigated with wastewater. Bull. Environ. Contam. Toxicol. 2018, 101, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Premi, O.P.; Kandpal, B.K.; Rathore, S.S.; Shekhawat, K.; Chauhan, J.S. Green manuring, mustard residue recycling and fertilizer application affects productivity and sustainability of Indian mustard (Brassica juncea L.) in Indian semi-arid tropics. Ind. Crop. Prod. 2013, 41, 423–429. [Google Scholar] [CrossRef]
- Sakizadeh, M.; Faraji, F.; Pouraghniyayi, M.J. Quality of groundwater in an area with intensive agricultural activity. Expo. Health 2016, 8, 93–105. [Google Scholar] [CrossRef]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2010, 160, 83–89. [Google Scholar] [CrossRef]
- Ugulu, I.; Unver, M.C.; Dogan, Y. Potentially toxic metal accumulation and human health risk from consuming wild Urtica urens sold on the open markets of Izmir. Euro-Mediterr. J. Environ. Integr. 2019, 4, 36. [Google Scholar] [CrossRef]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, Y.; Shen, C.; He, C.; Yuan, J.; Yang, Z. Comparative analysis between low and high cadmium accumulating cultivars of Brassica parachinensis to identify difference of cadmium-induced microRNA and their targets. Plant Soil 2017, 420, 223–237. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Kellerc, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Ahmad, A.; Hadi, F.; Ali, N. Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int. J. Phytoremediation 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Baczek-Kwinta, R.; Juzo, K.; Borek, M.; Antonkiewicz, J. Photosynthetic response of cabbage in cadmium-spiked soil. Photosynthetica 2019, 57, 731–739. [Google Scholar] [CrossRef]
- Śmiechowska, M.; Florek, A. Content of heavy metals in selected vegetables from conventional, organic and allotment cultivation. J. Res. Appl. Agric. Eng. 2011, 56, 152–156. [Google Scholar]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Rathore, S.S.; Shekhawat, K.; Dass, A.; Kandpal, B.K.; Singh, V.K. Phytoremediation mechanism in Indian mustard (Brassica juncea) and its enhancement through agronomic interventions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 419–427. [Google Scholar] [CrossRef]
- Witters, N.; Mendelsohn, R.O.; van Slycken, S.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenergy 2012, 39, 454–469. [Google Scholar] [CrossRef]
- Pfeufer, E.; Bessin, R.; Wright, S.; Strang, J. Vegetable Production Guide for Commercial Growers; Cooperative Extension Service, University of Kentucky College of Agriculture, Food and Environment: Lexington, KY, USA, 2017; pp. 44–48. [Google Scholar]
- USDA. United States Standards for Grades of Cabbage; United States Department of Agriculture, Agricultural Marketing Service: Washington, DC, USA, 2016. Available online: https://www.ams.usda.gov/grades-standards/cabbage-grades-and-standards (accessed on 2 March 2022).
- Antonious, G.F.; Kochhar, T.S.; Coolong, T. Yield, quality, and concentration of seven heavy metals in cabbage and broccoli grown in sewage sludge and chicken manure amended soil. J. Environ. Sci. Health Part A 2012, 47, 1955–1965. [Google Scholar] [CrossRef]
- Matejovic, I.; Durackova, A. Comparison of microwave digestion, wet and dry mineralization, and solubilization of plant samples for determination of calcium, magnesium, potassium, phosphorus, sodium, iron, zinc, copper, and manganese. Commun. Soil Sci. Plant Anal. 1994, 25, 1277–1288. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.S.; Lee, D.Y. Fast and green microwave-assisted digestion with diluted nitric acid and hydrogen peroxide and subsequent determination of elemental composition in brown and white rice by ICP-MS and ICP-OES. LWT 2023, 173, 11435. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Method 6010b Inductively Coupled Plasma Atomic Emission Spectrometry. Revision 2. 1996. Available online: https://www.epa.gov/sites/default/files/documents/6010b.pdf (accessed on 1 March 2023).
- McBride, M.B.; Richards, B.K.; Steenhuis, T. Bioavailability and crop uptake of trace elements in soil columns amended with sewage sludge products. Plant Soil 2004, 262, 71–84. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Kochhar, T.S. Testing bioaccumulation of Cd, Pb, and Ni in plants grown in soil amended with municipal sewage sludge at three Kentucky locations. JSM Environ. Sci. Ecol. 2017, 5, 1039. [Google Scholar]
- Khan, S.; Rehman, S.; Khan, A.Z.; Khan, M.A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef]
- Mirecki, N.; Agic, R.; Sunic, L.; Milenkovic, L.; Ilic, Z.S. Transfer factor as indicator of heavy metals content in plants. Fresenius Environ. Bull. 2015, 24, 4212–4219. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 January 2023).
- Ravindran, B.; Mupambwa, H.A.; Silwana, S.; Mnkeni, P.N. Assessment of nutrient quality, heavy metals and phytotoxic properties of chicken manure on selected commercial vegetable crops. Heliyon 2017, 3, e00493. [Google Scholar] [CrossRef] [PubMed]
- Celestina, C.; Hunt, J.R.; Sale, P.W.; Franks, A.E. Attribution of crop yield responses to application of organic amendments: A critical review. Soil Tillage Res. 2019, 186, 135–145. [Google Scholar] [CrossRef]
- Antonious, G.F.; Chiluwal, A.; Nepal, A. Chitin, Biochar, and Animal Manures Impact on Eggplant and Green Pepper Yield and Quality. Agric. Sci. 2023, 14, 368–383. [Google Scholar] [CrossRef]
- FAO & WHO. Food Additives and Contaminants—Joint Codex Alimentarius Commission, FAO/WHO Food standards Program. ALINORM 01/12A; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2014; p. 1289. [Google Scholar]
- Danjuma, M.S.; Abdulkadir, B. Bioaccumulation of heavy metals by leafy vegetables grown with industrial effluents: A review. Bayero J. Pure Appl. Sci. 2018, 11, 180–185. [Google Scholar] [CrossRef]
- Radulescu, C.; Stihi, C.; Popescu, I.V.; Dulama, I.D.; Chelarescu, E.D.; Chilian, A. Heavy metal accumulation and translocation in different parts of Brassica oleracea L. Rom. J. Phys. 2013, 58, 1337–1354. [Google Scholar]
- Smical, A.I.; Hotea, V.; Oros, V.; Juhasz, J.; Pop, E. Studies on transfer and bioaccumulation of heavy metals from soil into lettuce. Environ. Eng. Manag. J. 2008, 7, 609–615. [Google Scholar] [CrossRef]
- Adriano, D.C.; Adriano, D.C. Other trace elements. In Trace Elements in the Terrestrial Environment; Springer: New York, NY, USA, 1986; pp. 470–501. [Google Scholar] [CrossRef]
- Pajević, S.; Arsenov, D.; Nikolić, N.; Borišev, M.; Orčić, D.; Župunski, M.; Mimica-Dukić, N. Heavy metal accumulation in vegetable species and health risk assessment in Serbia. Environ. Monit. Assess. 2018, 190, 459. [Google Scholar] [CrossRef]
- Ametepey, S.T.; Cobbina, S.J.; Akpabey, F.J.; Duwiejuah, A.B.; Abuntori, Z.N. Health risk assessment and heavy metal contamination levels in vegetables from Tamale Metropolis, Ghana. Int. J. Food Contam. 2018, 5, 5. [Google Scholar] [CrossRef]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Riaz, U.; Aslam, A.; uz Zaman, Q.; Javeid, S.; Gul, R.; Iqbal, S.; Javid, S.; Murtaza, G.; Jamil, M. Cadmium contamination, bioavailability, uptake mechanism and remediation strategies in soil-plant-environment system: A critical review. Curr. Anal. Chem. 2021, 17, 49–60. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Pikuła, D. Effect of the Degree of Soil Contamination with Cd, Zn, Cu i Zn on Its Content in the Forder Crops and Mobility in the Soil Profile. In Soil Contamination-Recent Advances and Future Perspectives; IntechOpen: London, UK, 2023. [Google Scholar]


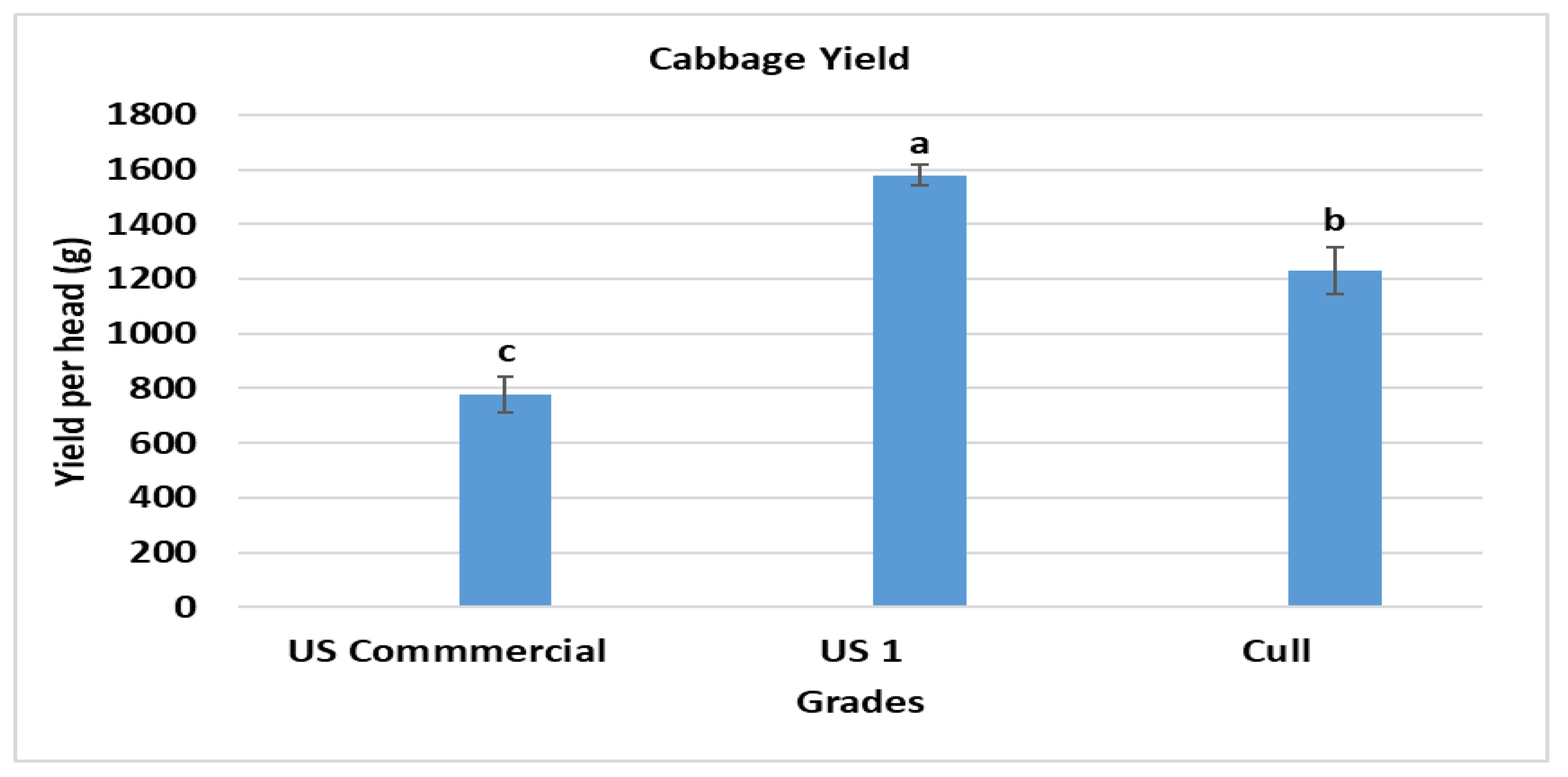
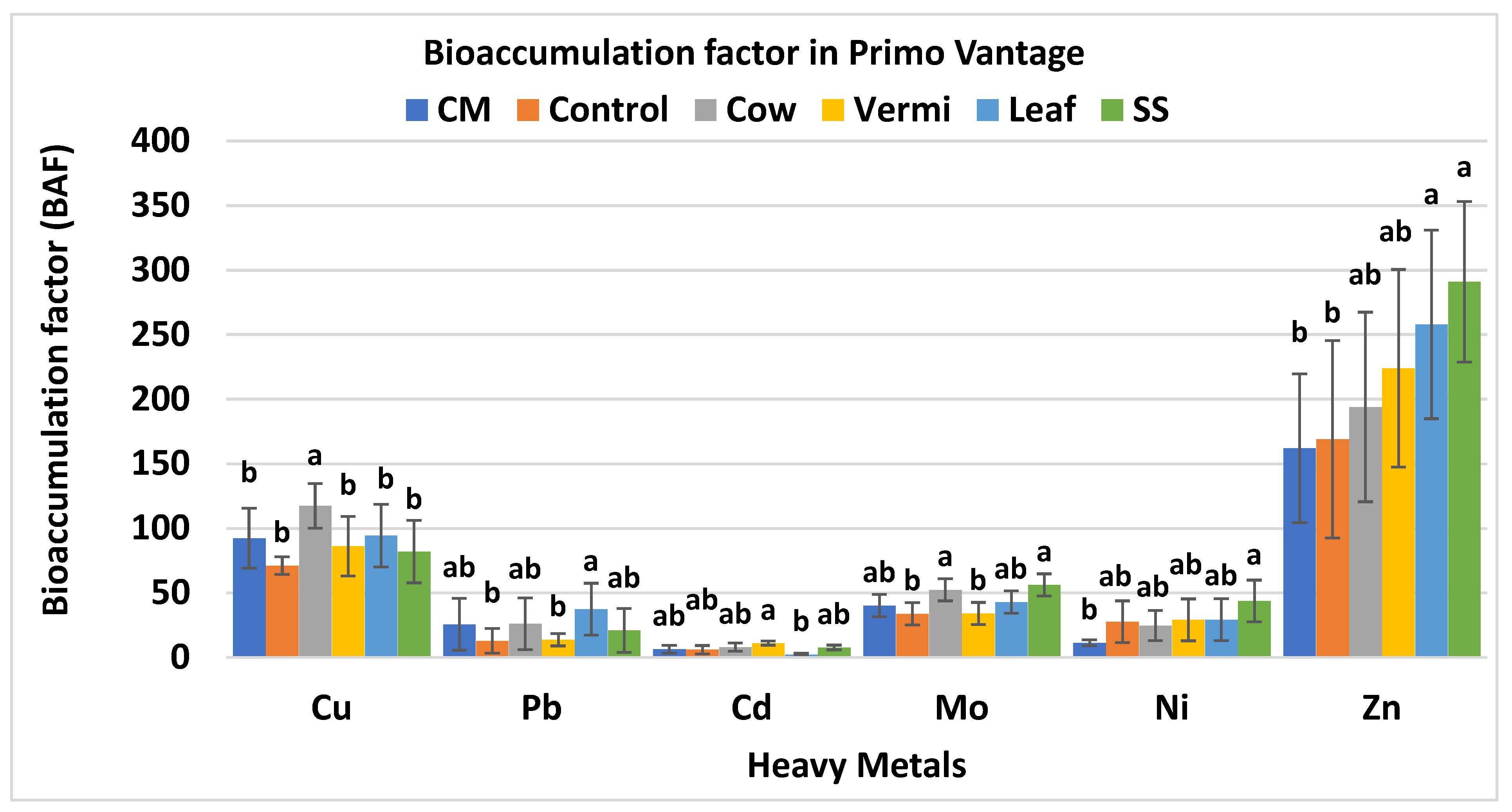
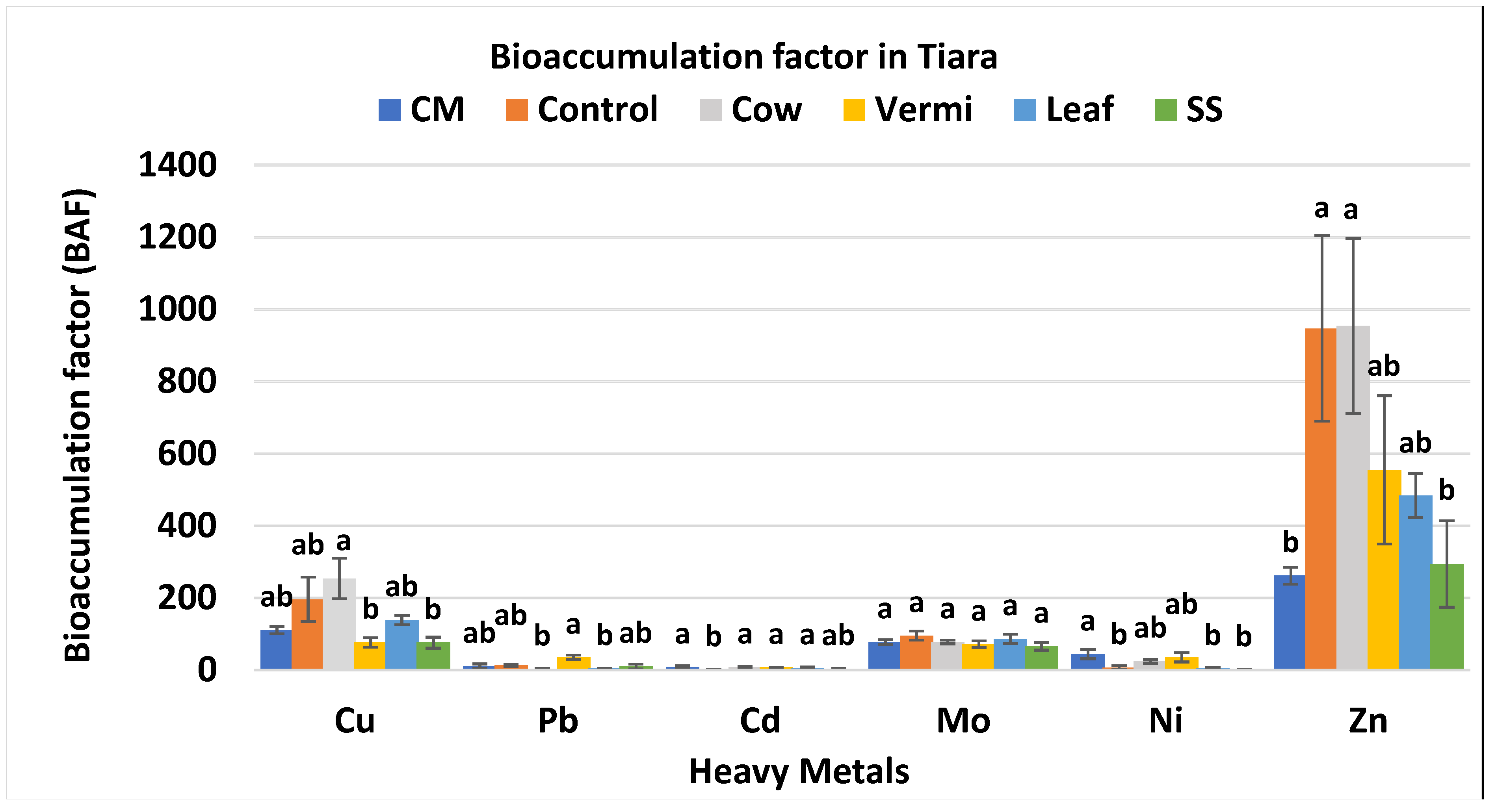
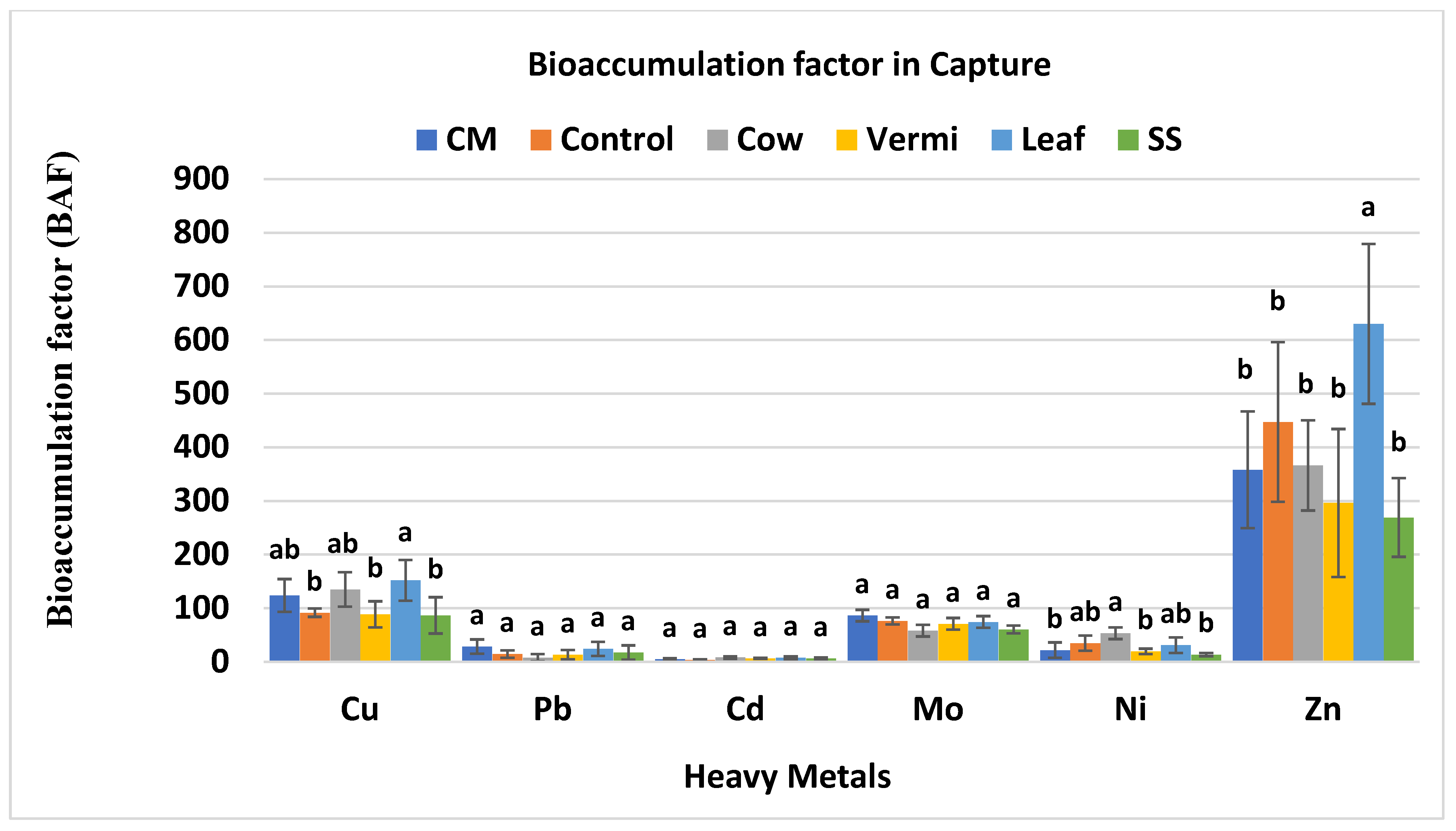
| Soil Amendments | Rate (g/m2) |
|---|---|
| Vermicompost (Vermi.) | 1120.52 |
| Sewage sludge (SS) | 224.54 |
| Chicken manure (CM) | 1022.57 |
| Cow manure (Cow) | 1937.5 |
| Leaf compost (Leaf) | 322.92 |
| Harvest 1 | Harvest 2 | ||||
|---|---|---|---|---|---|
| Amendments | Metals | Total Metal Content (mg/kg) | Soluble Metal Content (mg/kg) | Total Metal Content (mg/kg) | Soluble Metal Content (mg/kg) |
| SS | Cd | 0.237 ± 0.02 | 0.015 ± 0.00 | 0.22 ± 0.26 | 0.015 ± 0.00 |
| Cu | 9.63 ± 0.09 | 0.05 ± 0.009 | 9.52 ± 0.16 | 0.05 ± 0.009 | |
| Mo | 0.657 ± 0.08 | 0.015 ± 0.00 | 0.640 ± 0.07 | 0.015 ± 0.00 | |
| Ni | 17.2 ± 0.93 | 0.015 ± 0.001 | 17.1 ± 0.23 | 0.015 ± 0.001 | |
| Pb | 30.4 ± 1.08 | 0.036 ± 0.01 | 29.1 ± 0.3 | 0.036 ± 0.01 | |
| Zn | 57.4 ± 3.17 | 0.156 ± 0.05 | 57.2 ± 1.67 | 0.156 ± 0.05 | |
| Cow manure | Cd | 0.253 ± 0.05 | 0.015 ± 0.00 | 0.243 ± 0.01 | 0.015 ± 0.00 |
| Cu | 9.55 ± 0.45 | 0.03 ± 0.009 | 9.45 ± 0.56 | 0.03 ± 0.009 | |
| Mo | 0.673 ± 0.07 | 0.015 ± 0.00 | 0.660 ± 0.05 | 0.015 ± 0.00 | |
| Ni | 16.4 ± 0.73 | 0.016 ± 0.001 | 16.1 ± 0.53 | 0.016 ± 0.001 | |
| Pb | 28.9 ± 1.30 | 0.02 ± 0.01 | 28.7 ± 1.53 | 0.022 ± 0.01 | |
| Zn | 56.7 ± 5.21 | 0.125 ± 0.05 | 53.5 ± 3.38 | 0.125 ± 0.05 | |
| Vermicompost | Cd | 0.317 ± 0.01 | 0.015 ± 0.00 | 0.24 ± 0.02 | 0.015 ± 0.00 |
| Cu | 9.86 ± 0.77 | 0.04 ± 0.009 | 9.7 ± 0.16 | 0.04 ± 0.009 | |
| Mo | 0.847 ± 0.06 | 0.015 ± 0.00 | 0.740 ± 0.03 | 0.015 ± 0.00 | |
| Ni | 17.1 ± 0.88 | 0.015 ± 0.001 | 16.8 ± 0.50 | 0.015 ± 0.001 | |
| Pb | 30.9 ± 1.29 | 0.03 ± 0.01 | 29.7 ± 0.65 | 0.03 ± 0.01 | |
| Zn | 56.4 ± 3.9 | 0.055 ± 0.05 | 55.6 ± 1.38 | 0.055 ± 0.05 | |
| Leaf compost | Cd | 0.327 ± 0.01 | 0.015 ± 0.00 | 0.263 ± 0.02 | 0.015 ± 0.00 |
| Cu | 9.66 ± 0.80 | 0.02 ± 0.009 | 9.46 ± 0.76 | 0.024 ± 0.009 | |
| Mo | 0.830 ± 0.05 | 0.015 ± 0.00 | 0.747 ± 0.18 | 0.015 ± 0.00 | |
| Ni | 17.4 ± 1.43 | 0.02 ± 0.001 | 17.2 ± 1.37 | 0.02 ± 0.001 | |
| Pb | 29.9 ± 1.85 | 0.03 ± 0.01 | 29.0 ± 2.05 | 0.03 ± 0.01 | |
| Zn | 55.0 ± 3.95 | 0.06 ± 0.05 | 54.2 ± 3.78 | 0.06 ± 0.05 | |
| Chicken manure | Cd | 0.240 ± 0.02 | 0.015 ± 0.00 | 0.21 ± 0.02 | 0.015 ± 0.00 |
| Cu | 10.23 ± 0.69 | 0.03 ± 0.009 | 10.10 ± 0.79 | 0.04 ± 0.009 | |
| Mo | 0.693 ± 0.13 | 0.015 ± 0.00 | 0.594 ± 0.11 | 0.015 ± 0.00 | |
| Ni | 17.3 ± 1.30 | 0.016 ± 0.001 | 17.0 ± 0.90 | 0.016 ± 0.001 | |
| Pb | 31.7 ± 1.97 | 0.03 ± 0.01 | 30.7 ± 0.72 | 0.036 ± 0.01 | |
| Zn | 60.3 ± 4.56 | 0.11 ± 0.05 | 58.7 ± 4.84 | 0.11 ± 0.05 | |
| Control | Cd | 0.230 ± 0.00 | 0.015 ± 0.00 | 0.20 ± 0.02 | 0.015 ± 0.00 |
| Cu | 10.18 ± 0.56 | 0.03 ± 0.009 | 10.06 ± 0.67 | 0.032 ± 0.009 | |
| Mo | 0.76 ± 0.06 | 0.015 ± 0.00 | 0.673 ± 0.11 | 0.015 ± 0.00 | |
| Ni | 17.3 ± 1.15 | 0.017 ± 0.001 | 17.2 ± 0.52 | 0.017 ± 0.001 | |
| Pb | 30.0 ± 1.56 | 0.03 ± 0.01 | 29.0 ± 0.76 | 0.037 ± 0.01 | |
| Zn | 55.4 ± 3.34 | 0.108 ± 0.05 | 54.6 ± 3.58 | 0.1 ± 0.05 | |
| Heavy Metals | Permissible Limit in Unpolluted Soil (mg/kg) | Permissible Limit in Vegetables (mg/kg) | Total Metal Concentration in Soil from the Current Study (mg/kg) | Soluble Metal Concentration in Cabbage from Current Study (mg/kg) |
|---|---|---|---|---|
| Cd | 3 | 0.1 | 0.250 | 0.093 |
| Cu | 100 | 73 | 9.5 | 3.008 |
| Mo | NA | NA | 0.650 | 0.015 |
| Ni | 50 | 67 | 16.5 | 0.392 |
| Pb | 100 | 0.3 | 30 | 0.424 |
| Zn | 300 | 100 | 56 | 26.308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepal, A.; Antonious, G.F.; Gyawali, B.R.; Webster, T.C.; Bebe, F. Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments. Pollutants 2024, 4, 58-71. https://doi.org/10.3390/pollutants4010005
Nepal A, Antonious GF, Gyawali BR, Webster TC, Bebe F. Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments. Pollutants. 2024; 4(1):58-71. https://doi.org/10.3390/pollutants4010005
Chicago/Turabian StyleNepal, Anjan, George F. Antonious, Buddhi R. Gyawali, Thomas C. Webster, and Frederick Bebe. 2024. "Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments" Pollutants 4, no. 1: 58-71. https://doi.org/10.3390/pollutants4010005
APA StyleNepal, A., Antonious, G. F., Gyawali, B. R., Webster, T. C., & Bebe, F. (2024). Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments. Pollutants, 4(1), 58-71. https://doi.org/10.3390/pollutants4010005










