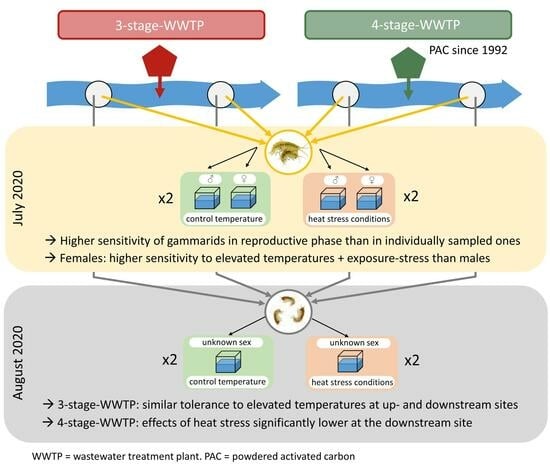
Heat Tolerance of Gammarus fossarum (Crustacea, Amphipoda) Is Influenced by the Level of Stress Associated with Reproduction and the Water Quality of Their Habitat
Abstract
1. Introduction
2. Materials and Methods
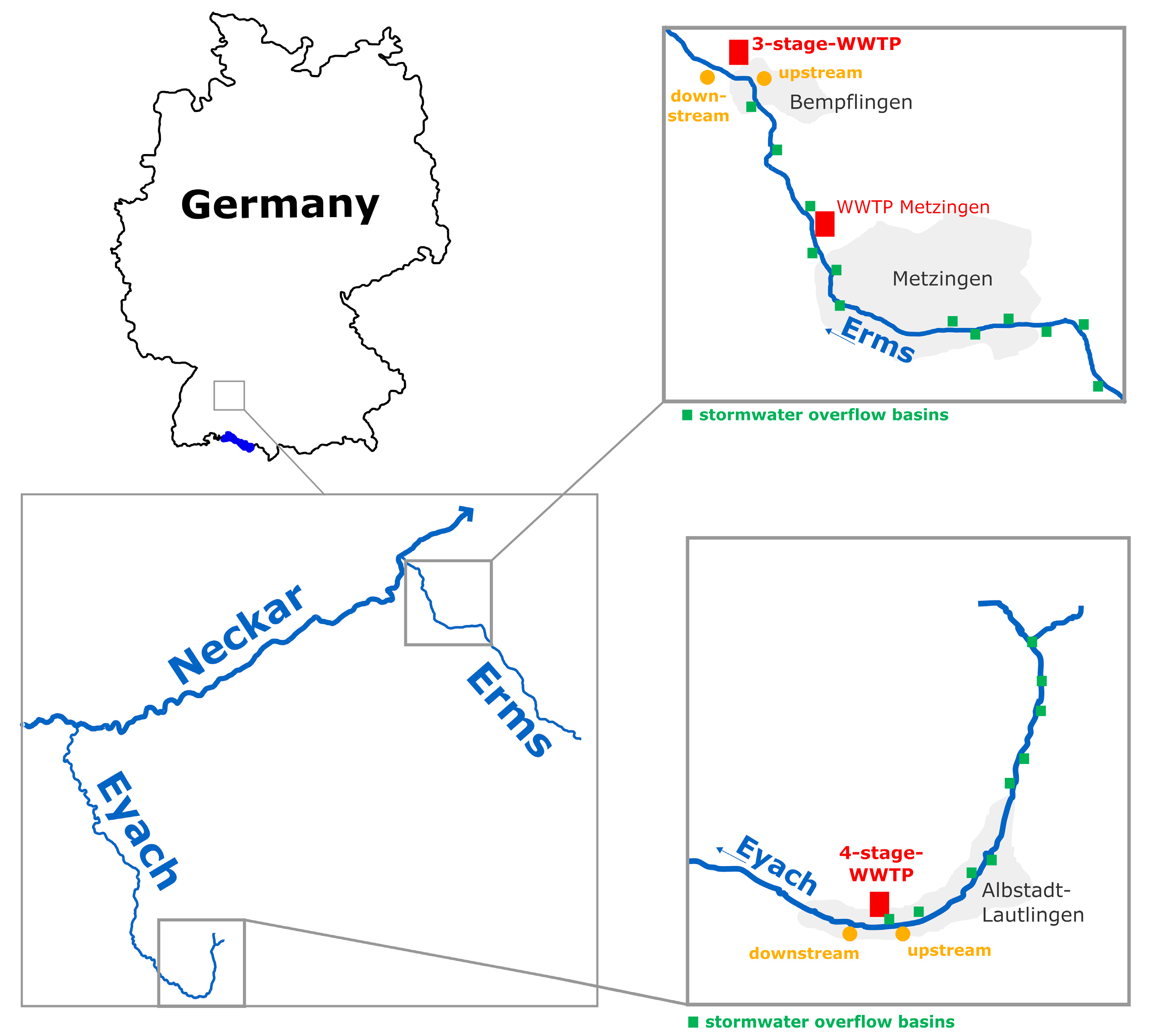
2.1. Sampling Sites
2.2. Exposure of Gammarids
2.2.1. Exposure of Male and Female Gammarids Separated from Precopula Pairs
2.2.2. Exposure of Gammarids of Unknown Sex
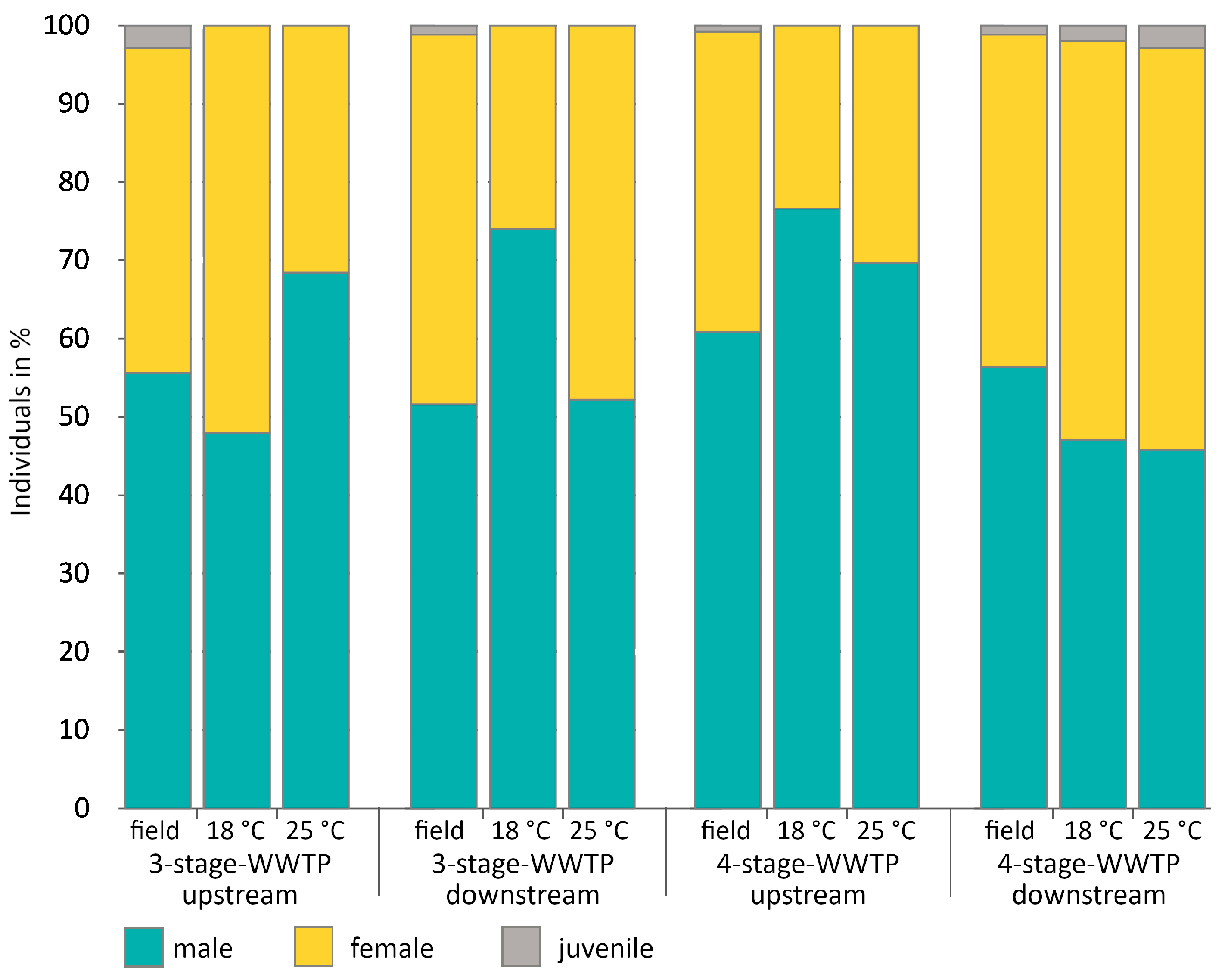
2.3. Sex Ratio in Field Samples
2.4. Physicochemical Parameters
2.5. Data Analyses and Statistics
3. Results
3.1. Exposure of Gammarids
3.1.1. Exposure of Male and Female Gammarids Separated from Precopula Pairs
- (a).
- Exposure to the water temperature prevailing at the time of sampling, 15 °C
- (b).
- Exposure to temperature extremes at 25 °C
3.1.2. Exposure of Gammarids of Unknown Sex
- (a).
- Exposure to the water temperature prevailing at the time of sampling, 18 °C
- (b).
- Exposure to temperature extremes at 25 °C
3.2. Determination of Sex Ratio (Random Field Samples vs. Exposed Gammarids in August 2020)
3.3. Physicochemical Parameters
4. Discussion
4.1. Effects of Temperature and Life Stage
4.2. Differences between Males and Females
4.3. Differences between the Two Rivers and Sites—Influence of the WWTPs
4.4. Relevance of Our Results in the Context of Climate Change
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nõges, P.; Argillier, C.; Borja, Á.; Garmendia, J.M.; Hanganu, J.; Kodeš, V.; Pletterbauer, F.; Sagouis, A.; Birk, S. Quantified biotic and abiotic responses to multiple stress in freshwater, marine and ground waters. Sci. Total Environ. 2016, 540, 43–52. [Google Scholar] [CrossRef]
- WMO. State of the Global Climate 2020; WMO: Geneva, Switzerland, 2021; Volume 52. [Google Scholar]
- Drewes, J.; Karakurt, S.; Schmid, L.; Bachmaier, M.; Hübner, U.; Clausnitzer, V.; Timmermann, R.; Schätzl, P.; McCurdy, S. Dynamik der Klarwasseranteile in Oberflächengewässern und mögliche Herausforderungen für die Trinkwassergewinnung in Deutschland; Umweltbundesamt: Dessau-Roßlau, Germany, 2018. [Google Scholar]
- Häder, D.-P.; Barnes, P.W. Comparing the impacts of climate change on the responses and linkages between terrestrial and aquatic ecosystems. Sci. Total Environ. 2019, 682, 239–246. [Google Scholar] [CrossRef]
- Wrona, F.; Prowse, T.; Reist, J.; Hobbie, J.; Lévesque, L.; Vincent, W. Climate Change Effects on Aquatic Biota, Ecosystem Structure and Function. Ambio 2006, 35, 359–369. [Google Scholar] [CrossRef]
- Anufriieva, E.; Shadrin, N. Extreme hydrological events destabilize aquatic ecosystems and open doors for alien species. Quat. Int. 2018, 475, 11–15. [Google Scholar] [CrossRef]
- Hitchcock, J.N. Storm events as key moments of microplastic contamination in aquatic ecosystems. Sci. Total Environ. 2020, 734, 139436. [Google Scholar] [CrossRef] [PubMed]
- Reoyo-Prats, B.; Aubert, D.; Sellier, A.; Roig, B.; Palacios, C. Dynamics and sources of pharmaceutically active compounds in a coastal Mediterranean river during heavy rains. Environ. Sci. Pollut. Res. 2018, 25, 6107–6121. [Google Scholar] [CrossRef]
- Daufresne, M.; Roger, M.C.; Capra, H.; Lamouroux, N. Long-term changes within the invertebrate and fish communities of the Upper Rhône River: Effects of climatic factors. Glob. Chang. Biol. 2004, 10, 124–140. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Z.; Liu, B.; Wang, Y.; Gao, J.; Zeng, Y.; Xie, J.; Xie, Z.; Jia, B.; Qin, P.; et al. Global river water warming due to climate change and anthropogenic heat emission. Glob. Planet. Change 2020, 193, 103289. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Füreder, L. Real-time warming of Alpine streams: (re)defining invertebrates’ temperature preferences. River Res. Appl. 2021, 37, 283–293. [Google Scholar] [CrossRef]
- O’Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; McIntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 710–773+781. [Google Scholar] [CrossRef]
- Wanders, N.; van Vliet, M.T.H.; Wada, Y.; Bierkens, M.F.P.; van Beek, L.P.H. High-Resolution Global Water Temperature Modeling. Water Resour. Res. 2019, 55, 2760–2778. [Google Scholar] [CrossRef]
- Arle, J.; Mohaupt, V.; Kirst, I. Monitoring of Surface Waters in Germany under the Water Framework Directive—A Review of Approaches, Methods and Results. Water 2016, 8, 217. [Google Scholar] [CrossRef]
- Cooper, R.J.; Hiscock, K.M. Two decades of the EU Water Framework Directive: Evidence of success and failure from a lowland arable catchment (River Wensum, UK). Sci. Total Environ. 2023, 869, 161837. [Google Scholar] [CrossRef]
- van Kats, N.; Dieperink, C.; van Rijswick, M.; de Senerpont Domis, L. Towards a Good Ecological Status? The Prospects for the Third Implementation Cycle of the EU Water Framework Directive in The Netherlands. Water 2022, 14, 486. [Google Scholar] [CrossRef]
- Wuijts, S.; Van Rijswick, H.F.M.W.; Driessen, P.P.J.; Runhaar, H.A.C. Moving forward to achieve the ambitions of the European Water Framework Directive: Lessons learned from the Netherlands. J. Environ. Manag. 2023, 333, 117424. [Google Scholar] [CrossRef]
- Zacharias, I.; Liakou, P.; Biliani, I. A Review of the Status of Surface European Waters Twenty Years after WFD Introduction. Environ. Process. 2020, 7, 1023–1039. [Google Scholar] [CrossRef]
- Bassem, S.M. Water pollution and aquatic biodiversity. Biodivers. Int. J. 2020, 4, 10–16. [Google Scholar]
- Grooten, M.; Almond, R.E.A. Living Planet Report 2018: Aiming Higher; World Wildlife Fund: Gland, Switzerland, 2018. [Google Scholar]
- He, F.; Zarfl, C.; Bremerich, V.; David, J.N.W.; Hogan, Z.; Kalinkat, G.; Tockner, K.; Jähnig, S.C. The global decline of freshwater megafauna. Glob. Chang. Biol. 2019, 25, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Marques, L. Collapse of Biodiversity in the Aquatic Environment. In Capitalism and Environmental Collapse; Springer International Publishing: Cham, Switzerland, 2020; pp. 275–301. [Google Scholar]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. Bioscience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.; vom Berg, C.; Schirmer, K.; Tlili, A. Anthropogenic Chemicals as Underestimated Drivers of Biodiversity Loss: Scientific and Societal Implications. Environ. Sci. Technol. 2022, 56, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, G.; Ågerstrand, M.; Antonelli, A.; Backhaus, T.; Brodin, T.; Diamond, M.L.; Erdelen, W.R.; Evers, D.C.; Hofmann, T.; Hueffer, T.; et al. Addressing chemical pollution in biodiversity research. Glob. Chang. Biol. 2023, 29, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, K.G.; Gleich, A.; Große-Ophoff, M.; Körner, W. The Necessity of a Global Binding Framework for Sustainable Management of Chemicals and Materials—Interactions with Climate and Biodiversity. Sustain. Chem. 2022, 3, 205–237. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Brückner, I.; Kirchner, K.; Müller, Y.; Schiwy, S.; Klaer, K.; Dolny, R.; Wendt, L.; Könemann, S.; Pinnekamp, J.; Hollert, H.; et al. Status quo report on wastewater treatment plant, receiving water’s biocoenosis and quality as basis for evaluation of large-scale ozonation process. Water Sci. Technol. 2017, 77, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Schulz, R. Ozonation of secondary treated wastewater reduces ecotoxicity to Gammarus fossarum (Crustacea; Amphipoda): Are loads of (micro)pollutants responsible? Water Res. 2011, 45, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Peschke, K.; Capowiez, Y.; Köhler, H.-R.; Wurm, K.; Triebskorn, R. Impact of a Wastewater Treatment Plant Upgrade on Amphipods and Other Macroinvertebrates: Individual and Community Responses. Front. Environ. Sci. 2019, 7, 64. [Google Scholar] [CrossRef]
- Pistocchi, A.; Alygizakis, N.A.; Brack, W.; Boxall, A.; Cousins, I.T.; Drewes, J.E.; Finckh, S.; Gallé, T.; Launay, M.A.; McLachlan, M.S.; et al. European scale assessment of the potential of ozonation and activated carbon treatment to reduce micropollutant emissions with wastewater. Sci. Total Environ. 2022, 848, 157124. [Google Scholar] [CrossRef]
- Triebskorn, R.; Blaha, L.; Gallert, C.; Giebner, S.; Hetzenauer, H.; Köhler, H.-R.; Kuch, B.; Lüddeke, F.; Oehlmann, J.; Peschke, K.; et al. Freshwater ecosystems profit from activated carbon-based wastewater treatment across various levels of biological organisation in a short timeframe. Environ. Sci. Eur. 2019, 31, 85. [Google Scholar] [CrossRef]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic Review of Toxicity Removal by Advanced Wastewater Treatment Technologies via Ozonation and Activated Carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef]
- Wolf, Y.; Oster, S.; Shuliakevich, A.; Brückner, I.; Dolny, R.; Linnemann, V.; Pinnekamp, J.; Hollert, H.; Schiwy, S. Improvement of wastewater and water quality via a full-scale ozonation plant?—A comprehensive analysis of the endocrine potential using effect-based methods. Sci. Total Environ. 2022, 803, 149756. [Google Scholar] [CrossRef]
- Huang, A.; Mangold-Döring, A.; Guan, H.; Boerwinkel, M.-C.; Belgers, D.; Focks, A.; Van den Brink, P.J. The effect of temperature on toxicokinetics and the chronic toxicity of insecticides towards Gammarus pulex. Sci. Total Environ. 2023, 856, 158886. [Google Scholar] [CrossRef] [PubMed]
- Labaude, S.; Moret, Y.; Cézilly, F.; Reuland, C.; Rigaud, T. Variation in the immune state of Gammarus pulex (Crustacea, Amphipoda) according to temperature: Are extreme temperatures a stress? Dev. Comp. Immunol. 2017, 76, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Raths, J.; Švara, V.; Lauper, B.; Fu, Q.; Hollender, J. Speed it up: How temperature drives toxicokinetics of organic contaminants in freshwater amphipods. Glob. Chang. Biol. 2023, 29, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Vellinger, C.; Felten, V.; Sornom, P.; Rousselle, P.; Beisel, J.-N.; Usseglio-Polatera, P. Behavioural and Physiological Responses of Gammarus pulex Exposed to Cadmium and Arsenate at Three Temperatures: Individual and Combined Effects. PLoS ONE 2012, 7, e39153. [Google Scholar] [CrossRef] [PubMed]
- Pöckl, M. Beitrage zur Ökologie des Bachflohkrebses (Gammarus fossarum) und Flussflohkrebses (Gammarus roeseli). Nat. Und Mus. 1993, 123, 114–125. [Google Scholar]
- Pöckl, M. Effects of temperature, age and body size on moulting and growth in the freshwater amphipods Gammarus fossarum and G. roeseli. Freshw. Biol. 1992, 27, 211–225. [Google Scholar] [CrossRef]
- Pöckl, M.; Webb, B.W.; Sutcliffe, D.W. Life history and reproductive capacity of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda) under naturally fluctuating water temperatures: A simulation study. Freshw. Biol. 2003, 48, 53–66. [Google Scholar] [CrossRef]
- Eggers, T.O.; Martens, A. Bestimmungsschlüssel der Süßwasser-Amphipoda (Crustacea) Deutschlands. Lauterbornia 2001, 42, 1–68. [Google Scholar]
- Welton, J.S. Life-history and production of the amphipod Gammarus pulex in a Dorset chalk stream. Freshw. Biol. 1979, 9, 263–275. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- OGewV. Verordnung zum Schutz der Oberflächengewässer (Oberflächengewässerverordnung-OGewV). 2016. Surface Waters Ordinance of 20 June 2016 (BGBl. I p. 1373), Which Was Last Amended by Article 2 (4) of the Law of 9 December 2020 (BGBI. I p. 2873). German Civil Code, German Federal Ministry of Justice, Berlin. Available online: https://www.gesetze-im-internet.de/ogewv_2016/OGewV.pdf (accessed on 22 January 2024).
- Kazmi, S.S.U.H.; Wang, Y.Y.L.; Cai, Y.-E.; Wang, Z. Temperature effects in single or combined with chemicals to the aquatic organisms: An overview of thermo-chemical stress. Ecol. Indic. 2022, 143, 109354. [Google Scholar] [CrossRef]
- Pander, J.; Habersetzer, L.; Casas-Mulet, R.; Geist, J. Effects of Stream Thermal Variability on Macroinvertebrate Community: Emphasis on Native Versus Non-Native Gammarid Species. Front. Environ. Sci. 2022, 10, 869396. [Google Scholar] [CrossRef]
- Blockwell, S.J.; Maund, S.J.; Pascoe, D. The acute toxicity of lindane to hyalella azteca and the development of a sublethal bioassay based on precopulatory guarding behavior. Arch. Environ. Contam. Toxicol. 1998, 35, 432–440. [Google Scholar] [CrossRef]
- Malbouisson, J.F.C.; Young, T.W.K.; Bark, A.W. Disruption of precopula in Gammarus pulex as a result of brief exposure to Gamma-hexachlorocyclohexane (Lindane). Chemosphere 1994, 28, 2011–2020. [Google Scholar] [CrossRef]
- Pascoe, D.; Kedwards, T.J.; Maund, S.J.; Muthi, E.; Taylor, E.J. Laboratory and field evaluation of a behavioural bioassay—The Gammarus pulex (L.) precopula separation (GaPPS) test. Water Res. 1994, 28, 369–372. [Google Scholar] [CrossRef]
- Love, A.C.; Crooks, N.; Ford, A.T. The effects of wastewater effluent on multiple behaviours in the amphipod, Gammarus pulex. Environ. Pollut. 2020, 267, 115386. [Google Scholar] [CrossRef]
- Poulton, M.; Pascoe, D. Disruption of precopula in Gammarus pulex (L.)—Development of a behavioural bioassay for evaluating pollutant and parasite induced stress. Chemosphere 1990, 20, 403–415. [Google Scholar] [CrossRef]
- Sheldon, B.C.; Verhulst, S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996, 11, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Buikema, A.L., Jr.; Benfield, E.F. Use of Macroinvertebrate Life History Information in Toxicity Tests. J. Fish. Res. Board Can. 1979, 36, 321–328. [Google Scholar] [CrossRef]
- Sutcliffe, D.W. Reproduction in Gammarus (Crustacea, Amphipoda): Female strategies. Freshw. Forum 1993, 3, 26–64. [Google Scholar]
- Pöckl, M. Reproductive potential and lifetime potential fecundity of the freshwater amphipods Gammarus fossarum and G. roeseli in Austrian streams and rivers. Freshw. Biol. 1993, 30, 73–91. [Google Scholar] [CrossRef]
- Schellenberg, A. Krebstiere oder Crustacea IV: Flohkrebse oder Amphipoda. Die Tierwelt Dtschl. Und Angrenzende Meeresteile 1942, 40, 1–252. [Google Scholar]
- Charron, L.; Geffard, O.; Chaumot, A.; Coulaud, R.; Jaffal, A.; Gaillet, V.; Dedourge-Geffard, O.; Geffard, A. Influence of Molting and Starvation on Digestive Enzyme Activities and Energy Storage in Gammarus fossarum. PLoS ONE 2014, 9, e96393. [Google Scholar] [CrossRef]
- Gismondi, E.; Cossu-Leguille, C.; Beisel, J.N. Do male and female gammarids defend themselves differently during chemical stress? Aquat. Toxicol. 2013, 140–141, 432–438. [Google Scholar] [CrossRef]
- Sroda, S.; Cossu-Leguille, C. Effects of sublethal copper exposure on two gammarid species: Which is the best competitor? Ecotoxicology 2011, 20, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Sornom, P.; Felten, V.; Médoc, V.; Sroda, S.; Rousselle, P.; Beisel, J.-N. Effect of gender on physiological and behavioural responses of Gammarus roeseli (Crustacea Amphipoda) to salinity and temperature. Environ. Pollut. 2010, 158, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Adam, O.; Degiorgi, F.; Crini, G.; Badot, P.M. High sensitivity of Gammarus sp. juveniles to deltamethrin: Outcomes for risk assessment. Ecotoxicol. Environ. Saf. 2010, 73, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; De Lange, H.J.; Peeters, E.T. Contrasting sensitivities to toxicants of the freshwater amphipods Gammarus pulex and G. fossarum. Ecotoxicology 2010, 19, 133–140. [Google Scholar] [CrossRef]
- Böttger, R.; Schaller, J.; Mohr, S. Closer to reality—The influence of toxicity test modifications on the sensitivity of Gammarus roeseli to the insecticide imidacloprid. Ecotoxicol. Environ. Saf. 2012, 81, 49–54. [Google Scholar] [CrossRef]
- Cold, A.; Forbes, V.E. Consequences of a short pulse of pesticide exposure for survival and reproduction of Gammarus pulex. Aquat. Toxicol. 2004, 67, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.O.; Migliaccio, K.W. Contribution of Wastewater Treatment Plant Effluents to Nutrient Dynamics in Aquatic Systems: A Review. Environ. Manag. 2009, 44, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. An Analytical Review of Different Approaches to Wastewater Discharge Standards with Particular Emphasis on Nutrients. Environ. Manag. 2020, 66, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Thellmann, P.; Köhler, H.-R.; Rößler, A.; Scheurer, M.; Schwarz, S.; Vogel, H.-J.; Triebskorn, R. Fish embryo tests with Danio rerio as a tool to evaluate surface water and sediment quality in rivers influenced by wastewater treatment plants using different treatment technologies. Environ. Sci. Pollut. Res. 2015, 22, 16405–16416. [Google Scholar] [CrossRef] [PubMed]
- Harth, F.U.R.; Arras, C.; Brettschneider, D.J.; Misovic, A.; Oehlmann, J.; Schulte-Oehlmann, U.; Oetken, M. Small but with big impact? Ecotoxicological effects of a municipal wastewater effluent on a small creek. J. Environ. Sci. Health A Toxicol. Hazard. Subst. Environ. Eng. 2018, 53, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Triebskorn, R.; Thellmann, P.; Vogel, H.-J.; Wurm, K. Die Kläranlage Albstadt-Ebingen: Aktivkohlefilterung im Vollstrom seit 1992. Ein langfristiger Erfolg für die Fischgesundheit und die Gewässerökologie? Korresp. Wasserwirtsch. 2014, 10, 587–593. [Google Scholar]
- Anguiano, O.L.; Vacca, M.; Rodriguez Araujo, M.E.; Montagna, M.; Venturino, A.; Ferrari, A. Acute toxicity and esterase response to carbaryl exposure in two different populations of amphipods Hyalella curvispina. Aquat. Toxicol. 2017, 188, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Barata, C.; Baird, D.J.; Soares, A.M.V.M.; Guilhermino, L. Biochemical Factors Contributing to Response Variation among Resistant and Sensitive Clones of Daphnia magna Straus Exposed to Ethyl parathion. Ecotoxicol. Environ. Saf. 2001, 49, 155–163. [Google Scholar] [CrossRef]
- Olima, C.; Pablo, F.; Lim, R.P. Comparative tolerance of three populations of the freshwater shrimp (Paratya australiensis) to the organophosphate pesticide, chlorpyrifos. Bull. Environ. Contam. Toxicol 1997, 59, 321–328. [Google Scholar] [CrossRef]
- Schill, R.O.; Köhler, H.R. Does the environment or the source of the population define stress status and energy supply in the freshwater amphipod, Gammarus fossarum? Ecotoxicology 2004, 13, 683–695. [Google Scholar] [CrossRef]
- Link, M.; von der Ohe, P.C.; Voß, K.; Schäfer, R.B. Comparison of dilution factors for German wastewater treatment plant effluents in receiving streams to the fixed dilution factor from chemical risk assessment. Sci. Total Environ. 2017, 598, 805–813. [Google Scholar] [CrossRef]
- Abily, M.; Acuña, V.; Gernjak, W.; Rodríguez-Roda, I.; Poch, M.; Corominas, L. Climate change impact on EU rivers’ dilution capacity and ecological status. Water Res. 2021, 199, 117166. [Google Scholar] [CrossRef] [PubMed]
- Fässler, S.; Stöckli, A. Das Fehlen von Bachflohkrebsen. In-situ Versuche in der Wyna im Kanton Aargau. Aqua Gas 2013, 5, 62–72. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peschke, K.; Sawallich, L.; Köhler, H.-R.; Triebskorn, R. Heat Tolerance of Gammarus fossarum (Crustacea, Amphipoda) Is Influenced by the Level of Stress Associated with Reproduction and the Water Quality of Their Habitat. Pollutants 2024, 4, 42-57. https://doi.org/10.3390/pollutants4010004
Peschke K, Sawallich L, Köhler H-R, Triebskorn R. Heat Tolerance of Gammarus fossarum (Crustacea, Amphipoda) Is Influenced by the Level of Stress Associated with Reproduction and the Water Quality of Their Habitat. Pollutants. 2024; 4(1):42-57. https://doi.org/10.3390/pollutants4010004
Chicago/Turabian StylePeschke, Katharina, Lilith Sawallich, Heinz-R. Köhler, and Rita Triebskorn. 2024. "Heat Tolerance of Gammarus fossarum (Crustacea, Amphipoda) Is Influenced by the Level of Stress Associated with Reproduction and the Water Quality of Their Habitat" Pollutants 4, no. 1: 42-57. https://doi.org/10.3390/pollutants4010004
APA StylePeschke, K., Sawallich, L., Köhler, H.-R., & Triebskorn, R. (2024). Heat Tolerance of Gammarus fossarum (Crustacea, Amphipoda) Is Influenced by the Level of Stress Associated with Reproduction and the Water Quality of Their Habitat. Pollutants, 4(1), 42-57. https://doi.org/10.3390/pollutants4010004