Abstract
Lake Victoria (L. Victoria) is the largest African tropical and freshwater lake, with one of the highest pollution levels, globally. It is shared among Uganda, Kenya and Tanzania, but it is drained only by the river Nile, the longest river in Africa. Though environmental studies have been conducted in the lake, investigations of the heavy metals (HMs) contamination of sediments from fish landing sites and ports on the Ugandan portion of L. Victoria are limited. In this study, sediments of an urban, industrial and fish landing site (Port Bell) on L. Victoria, Uganda was investigated to establish its HMs pollution levels and potential health risks to humans and ecosystems. Sediment samples were collected in triplicate (n = 9) from three different points of Port Bell, digested and analyzed using atomic absorption spectrometry for the presence of these HMs: copper (Cu), lead (Pb), cadmium (Cd) and chromium (Cr). The average daily dose through dermal contact and hazard quotient (HQ) were calculated to assess the health risk that is associated with dredging works (lake sand mining). Four geochemical enrichment indices: contamination factor (CF), geo-accumulation index (Igeo), pollution load index (PLI) and potential ecological risk (PERI) were used to quantify the contamination of the HMs in the sediments. The results showed that the mean HM content of the samples ranged from: 6.111 ± 0.01 to 7.111 ± 0.002 mg/kg for Cu; from 40.222 ± 0.003 to 44.212 ± 0.002 mg/kg for Pb; from 0.352 ± 0.007 to 0.522 ± 0.010 mg/kg for Cr; from 3.002 ± 0.002 to 3.453 ± 0.003 mg/kg for Cd. Health risk assessments indicated that there are no discernible non-carcinogenic health risks that could arise from the dredging works that are conducted in the study area as the indices were all below one. The contamination factors that were obtained suggest that Cd has reached a state of severe enrichment in the sediments (CF > 6). An assessment using Igeo established that the sediments were not contaminated with regards to Cu and Cr, but they exhibited low-to-median and median contamination with respect to Pb and Cd, respectively. Though the pollution load indices show that the contamination levels raise no serious concerns, the potential ecological risk indices show that there is considerable pollution of the Port Bell sediments, particularly with regard to Cd. Upon examination using multivariate statistical analyses, Cd and Cr showed a strong correlation which alluded to their introduction from anthropogenic sources. Based on the sedimentary HMs concentrations and the environmental indices that are employed in this study, it is recommended that the spatial variations in the concentrations of the HMs in water, sediments and biota should be monitored.
1. Introduction
Many challenges that are currently faced by humanity are due to environmental pollution, for example, disease outbreaks, climate change, the scarcity of safe drinking water, biodiversity, forest and wetland losses [1,2,3,4,5]. These are fueled by the ever-increasing human population which has in turn caused great pressure on the pristine environment with their need for habitats, resources and waste assimilation [6]. Consequently, the direct and indirect introduction of contaminants such as heavy metals (HMs), plastics, agrochemicals (such as fertilizers and pesticides), preservatives, endocrine-disrupting compounds, personal care products and pharmaceuticals into the environment has raised concerns [7]. This is arguably because they pose threats to the pursuit and realization of some Sustainable Development Goals and thus, need to be continuously monitored [8]. Of immediate concern is the pollution of water, which is an indispensable necessity for life on earth.
HMs are chemical elements with high molecular weights and a specific gravity (that is at least five times greater than that of water) and are toxic at concentrations that exceed their threshold values [9]. Their high densities and toxicities are believed to be inter-related, and these HMs include metals such as cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), mercury (Hg), molybdenum (Mo), nickel (Ni), strontium (Sr), titanium (Ti), vanadium (V) and zinc (Zn), and metalloids such as lead (Pb), arsenic (As) and tin (Sn) [10]. HMs are used in domestic, industrial, agricultural, medical and technological applications which have led to their uncontrollable distribution in the environment. The physicochemical nature of HMs makes them persistent, toxic and bio-accumulative. Owing to their high degree of toxicity, As, Cd, Cr, Pb and Hg are listed as priority HMs that are of public health significance [11]. HMs can react with biological systems by losing one or more electrons and forming cations that can ably bind with the nucleophilic sites of the vital macromolecules. Their toxicity is caused through the disruption of cellular activities such as growth, differentiation, damage-repairing processes and apoptosis. These may be mediated through the generation of reactive oxygen species (thus, causing oxidative stress), weakening the organism’s antioxidant defense system, complexation or ligand-formation with organic compounds and the active sites of enzymes [9,11]. Their toxicity is contingent on the exposure route, dose, chemical form and the age, gender and nutritional status of the individual that is in question.
Upon entry into aquatic ecosystems, HMs equilibrate between aqueous and solid phases, and can bioaccumulate depending on their solubility and the toxicokinetics in the organism. Due to the ability of HMs to exist in two forms i.e., dissolved and accumulated, a large proportion of HMs tend to accumulate in sediments [7,12,13]. Thus, sediments and biota form a useful portion of the passive samples that can be used to reflect the exposures and developments of the detection of compounds that are otherwise undetectable in the aqueous phase [14,15,16,17]. Human exposure to HMs is routinely in utero, through inhalation or by contact with contaminated matrices (occupational exposure), or by the ingestion of contaminated foods or water [18,19]. HMs exert various health effects in humans once their permissible levels are exceeded. For example, Pb is a toxic, non-essential metal that is known to result in kidney failure, anaemia, weakness and brain damage upon exposure to it in high doses [20,21]. Long-term exposure may result in Pb poisoning, an increased risk of hypertension and the toxicity of the hematopoietic and nervous systems [22,23]. In addition, inorganic Pb compounds are cited as probable carcinogens to humans (Group 2A) according to the International Agency for Research on Cancer [24].
Whereas HM pollution is systematically monitored in developed countries, this is not the case in developing countries. On the African continent, one of the regions with marked pollution challenges is the East African community (EAC), which is constituted by countries: Burundi, Democratic Republic of Congo, Uganda, Kenya, Tanzania, Rwanda and South Sudan. It is a region that is rich in water resources, such as Lake Turkana, Lake Tanganyika, Lake Kivu, Lake Malawi, the Western Indian Ocean and L. Victoria. Of great interest is L. Victoria, the world’s largest tropical lake and Africa’s largest freshwater lake which is shared among Tanzania (49%), Uganda (45%) and Kenya (6%) [25]. It is an exoreic African Great Lake that is primarily drained by the longest river in the continent (the river Nile) into the Mediterranean Sea [26]. The size of L. Victoria (surface area: 68,800 km2) [27], its complex shorelines with iconic island clusters and it having a rich fish species diversity has positioned it as the largest freshwater inland fishery in the world, which is largely based on Nile perch and Nile Tilapia [28,29]. Thus, it is a source of food (in the form of fish and other edible freshwater animals and plants), employment (livelihood), foreign exchange, water and other ecosystem services to at least 42 million people in the riparian EAC countries [30].
The high levels of urbanization, industrialization and the large number of human settlements on the shores of L. Victoria has led to its inevitable pollution by both legacy and contaminants of emerging concern [30,31]. With a long retention time (23 years) and a flushing time of 123 years [29], various studies have found contaminants such as microplastics [30,32,33,34,35], polycyclic aromatic hydrocarbons [36,37,38], per- and poly-fluoroalkyl substances [39,40,41], active pharmaceutical ingredients and personal care products [42], agrochemicals [43], HMs, polybrominated diphenyl ethers, alternative flame retardants [44,45] and cyanotoxins [46,47,48] in the water and fish from L. Victoria. Despite the foregoing studies, there is paucity of reports on the HM contamination of sediments from fish landing sites and ports on the Ugandan portion of L. Victoria. Some of the fish landing sites on the Ugandan Portion of L. Victoria include Ripon, Wairaka and Masese in Jinja, Katosi in Mukono, and Port Bell in Kampala. From an industrial perspective, Port Bell was considered for this study because it has one of the first instant tea factories in Uganda. Currently, the major industries in its vicinity are the Uganda Breweries (a subsidiary of the East African Breweries and maker of Uganda Waragi and Bell beer), Afroplastics Enterprises Limited (manufacturers of plastic items), and Cipla Quality Chemical Industries Limited which manufactures antiretroviral drugs [49]. These factories are industrially located in the Luzira Industrial and Business Park. In addition to these, L. Victoria is endowed with alluvial depositions that contain sand, which is mined at Port Bell and sold to construction industries by the indigenous communities. Though previous reports have raised concerns that the mining activities might disfigure the lake shores, bed and fish breeding grounds [50], no study has assessed the health risks that are associated with such dredging works. The main objective of the present study was, therefore, to assess the levels of HMs in the sediments from Port Bell, Northern L. Victoria, Uganda and the associated health risks that they could pose to humans and the lake’s ecosystem.
2. Materials and Methods
2.1. Description of Study Area
Port Bell is a 0.79-km-long port and fish landing site (Figure 1) that is situated in Luzira, Nakawa-East, in the greater metropolitan Kampala area, Central Uganda (0°17′20.0″ N, 32°39′13.0″ E). The port takes its name from the British commissioner (Henry Hesketh Bell) who was an administrator who executed the interest of Britain in Uganda from 1906 [51]. The port is positioned at the end of a narrow inlet of L. Victoria (shores of Murchison Bay), southeast of the central business district and the capital city of Uganda, Kampala (Figure 2). Ferries operating from Port Bell provide a linkage between Kampala and other ports on L. Victoria, e.g., Mwanza and Musoma in Tanzania, Jinja in Uganda and Kisumu in Kenya [49].

Figure 1.
Port Bell, L. Victoria, Uganda. Engine-operated boats and plastic pollution are visible in the fore ground while the ferries appear in the background.
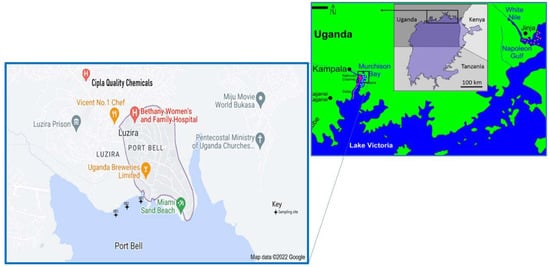
Figure 2.
Map of Port Bell, L. Victoria, Uganda showing the location of the sampling sites (SP1 to SP3).
2.2. Collection and Preparation of Samples
The sediments were selected to determine the concentration of HMs that were being retained in the solid phase. The samples were obtained on Thursday 24 February 2022 in triplicate (n = 9; 3 for each site) using a grab sampler at 0–5 cm. Within each sampling station, the samples were collected at distances of at least 500 m from one another. The three sampling points (SP1, SP2 and SP3) were chosen as follows:
- (i)
- SP1 is at the end of the terminal where Nakivubo channel pours its water into Port Bell.
- (ii)
- SP2 is situated near the shores of Nakivubo channel.
- (iii)
- SP3 is at the extreme end of the port towards the mainland.
The samples that were obtained were transferred into sterilized plastic polypropylene bottles, tightly sealed, labelled and submitted within 4 h to the Chemistry Laboratory, Uganda Industrial Research Institute (UIRI), plot 42A, Mukabya road, Nakawa industrial area, Kampala, Uganda. The samples were oven-dried at 80 °C to 95 °C for 16 h and then homogenized. They were crushed in a stone mortar and passed through a 0.63 μm nylon mesh sieve. The powdered sediment samples were preserved at 4 °C in an ice block.
2.3. Analysis of Physicochemical Parameters
The pH of the samples was determined at a sediment-to-water ratio of 1:2.5 using a precalibrated Hanna 211 digital microprocessor-based bench top pH/mV/°C meter (Hanna instruments, Italy) [7]. A measurement of 20 g of the sample was transferred into a 100 mL beaker and 50 mL of distilled water was added to it. The mixture was shaken using a magnetic stirrer for 15 min. After 30 min, the suspension was shaken another 2 min and the pH of the suspension was directly recorded. The probe of the pH meter was rinsed with distilled water in between measurements. All of the measurements were performed in triplicate.
The moisture content of the samples was measured using the oven method at 60 °C. Briefly, 1.0 g of the samples were weighed into moisture dishes and transferred to the oven for 2 h, followed by their cooling in a desiccator [52]. Thereafter, their weights were determined, and the moisture content was determined as a percentage of the differences between the wet and dry weights divided by the wet weight. The moisture analyses were done in triplicate.
2.4. Heavy Metal Analysis
Measurements of 0.25 g of the samples were digested on a hot plate in 20 mL of aqua regia (3 HNO3:1 HCl v/v) until the solution became colourless. The digestates were thereafter heated to near dryness and then they were cooled. A measurement of 20 mL of 1% nitric acid was added, mixed, and heated. After, filtration of the sample was done and then transferred into 50 mL sample vials. The solutions were then analysed for the presence of Cu, Pb, Cr and Cd (at wavelengths of 324.8 nm, 283.3 nm, 357.9 nm and 228.8 nm, respectively) using an Atomic Absorption Spectrometer (Perkin Elmer 3030) with a graphite furnace. All of the instrumental analyses were replicated twice.
The results that were obtained from the instrument were converted to mg/kg dry weight. A quality control was performed through an analysis of the blank and spiked samples according to the same procedure. Recoveries that were obtained ranged from 94% to 101%. Analytical precision (expressed as Relative Standard Deviation) varied between 3% and 5%. The method detection limit (LOD) of each metal was computed as Blank + 3 × Standard deviations of four samples analyzed in triplicate.
2.5. Human Health Risk Assessment
The United States Environment Protection Agency (US EPA) suggested that the human health risk assessment model should estimate the potential health risks of contaminants based on exposure and toxicity assessments [53]. This study appreciated that lake sand mining occurs at Port Bell and inside L. Victoria, and miners come into contact with dredged HMs-contaminated sediments. Accordingly, the average daily dose (ADDtherm) in mg/kg/day was calculated (Equation (1)) to discern if there is any potential HM intoxication through skin contact [54,55,56,57].
where Cm = heavy metal concentration (mg/kg), SA is the exposed surface area = 4350 cm2 for adults [56], DAF is the dermal absorption factor = 0.001, AF is the skin adherence factor in mg/cm2/day = 0.7 for adults [12], Ef = exposure frequency (365 days/year), Ed = exposure duration, the average lifetime (58.65 years for an adult Ugandan) [14,58], Wab = average body weight (considered to be 60 kg for adults) and Taet is the average exposure time for non-carcinogens = Ef × Ed [59].
The hazard quotient (HQ) was calculated using Equation (2). On the whole, a HQ ≤ 1 implies that the exposure is very unlikely to have adverse effects while a HQ > 1 represents a possibility of non-carcinogenic effects, with its probability increasing as the value of the HQ increases [12].
The dermal reference doses (RfD) for Cu, Pb, Cr and Cd through dermal contact are 4.0 × 10−4, 5.4 × 10−4, 3.0 × 10−3 and 1.0 × 10−3 mg/kg/day, respectively [12]. The reference dose is the maximum daily dose of a metal from a specific exposure pathway that is believed not to lead to an appreciable risk of deleterious effects to sensitive individuals during their lifetime [60]. For this study, the HQ was computed through a single pathway (dermal contact) with the assertion that such contact of lake sand miners with periodically dredged sediments are inevitable [12,14].
Since exposure to multiple HMs can increase the non-carcinogenic health risks due to instances of dermal contact with contaminated sediments, the cumulative risk (hazard index, HI) was estimated using Equation (3) [61].
2.6. Sediment Quality Assessment
To evaluate the level of contamination of the sediments from Port Bell, four pollution indices were computed namely; contamination factor (CF), geo-accumulation index (Igeo), pollution load index (PLI) and the potential ecological risk index (PERI) [62,63,64]. The CF was calculated using Equation (4), which was suggested by Hakanson [62].
where Cm is the metal concentration in the sediment sample and CBn is the geochemical background/preindustrial concentration of the same metal.
Geo-accumulation index (Igeo) for the sediments was obtained from computations utilizing Equation (5), which was suggested by Müller [65].
where Cm and CBn follow from Equation (4), whereas, in this equation, 1.5 is the background matrix correction factor which was introduced to cater for lithological variability [66,67].
The pollution load index (PLI) was calculated using Equation (6) as follows:
where CF is the contamination factor (Equation (4)) and n = 4 is the number of HMs that were studied.
Lastly, the potential ecological risk () and potential ecological risk index (PERI) were computed employing Equations (7) and (8) in tandem:
where is the biological toxic factor for the HMs = 5, 5, 2 and 30 for Cu, Pb, Cr and Cd, respectively [68,69]. The degrees of ecological risk for single elements and PERI for all factors combined are outlined in Table 1.

Table 1.
Classification of potential ecological risk for a single regulator and PERI.
2.7. Statistical Analysis
All experiments were performed in triplicate, and the data that were obtained were checked for normality and averaged prior to statistical evaluation. One-way analysis of variance was used to examine significant differences between the means, followed by Tukey post hoc test. Pearson’s bivariate correlation and principal component analysis (PCA) were used to explore the inter-relationships between metal concentrations and aquatic environmental parameters (pH and moisture content). All statistical analyses were executed at 95% confidence interval using GraphPad Prism for Windows (v9.3.1, GraphPad Software, San Diego, CA, USA).
3. Results and Discussion
3.1. Physicochemical Parameters and HMs Concentration of the Sediment Samples
The average pH values that were recorded were 5.52, 8.22 and 5.66 from the sampling points SP1, SP2 and SP3, respectively. The acidic pH values that were obtained at points SP1 and SP3 could be attributed to them having high concentrations of organic matter [7,70]. At sampling point SP1, the lowest mean pH could be due to the loading of industrial effluents that pour from the Nakivubo channel into the port, while that of SP2 may be attributed to dilution effects that occur with distance from SP1. On the other hand, the moisture content of the samples ranged from 7.04% at SP1 to 7.52% and 7.66% at SP2 and SP3.
The mean concentrations of the analyzed HMs are depicted in Figure 3. The concentrations of the HMs were 6.177 ± 0.003 mg/kg, 7.111 ± 0.002 mg/kg and 6.111 ± 0.01 mg/kg for Cu; 42.118 ± 0.008 mg/kg, 44.212 ± 0.002 mg/kg, and 40.222 ± 0.003 mg/kg for Pb; 0.494 ± 0.003 mg/kg, 0.522 ± 0.010 mg/kg and 0.352 ± 0.007 mg/kg for Cr; 3.393 ± 0.005 mg/kg, 3.453 ± 0.003 mg/kg, and 3.002 ± 0.002 mg/kg for Cd. Thus, the concentration of the HMs in the samples followed the sequence Pb > Cu > Cd > Cr. In addition, there were significant (p <0.05) fluctuations in the concentration of the HMs between the sampled sites (SP1 to SP3), as per the one-way ANOVA results.
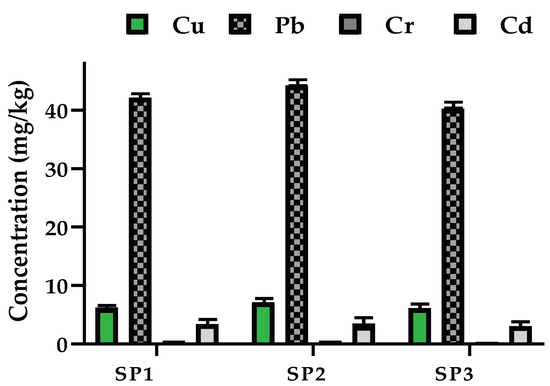
Figure 3.
Mean concentration of HMs in sediments from Port Bell, Northern L. Victoria, Uganda. Error bars represent standard deviations of analyses that were performed in triplicate (n = 9).
Sampling point SP2 recorded the highest concentration of the HMs because the sample was drawn from the shores where the HMs form suspended particles that later settle as sediments by leaching. Moreover, the untreated industrial effluents tend to contain higher quantities of HMs like Cu, Pb and Cd [71,72]. Other significant sources of the HMs are fertilizers and domestic sewage that are produced due to settlements, the use of leaded gasoline in outboard boat engines and automobiles, lead-based paints, lead-acid and nickel-cadmium batteries (in the context of Pb and Cd) [73,74]. Sampling points SP1 and SP3, on the other hand, recorded slightly lower concentration of the HMs because the sediment samples were collected from non-point sources. The concentrations of Cu, Pb and Cd were high, and only that of Cr fell within the average shale and toxicity reference values and threshold effect concentration (Table 2). Technically, consensus-based sediment quality guidelines contain two effect values: the threshold effect concentration (TEC) and the probable effect concentration (PEC). If the HMs content of the sediment are below the TEC, such HMs are not expected to have any adverse effects on organisms. Otherwise, HMs that are in concentrations above the PEC implies that toxic effects are likely to occur [75]. Thus, adverse effects are likely to be observed on organisms in the studied areas of Port Bell, which is similar to an observation from Winam Gulf of L. Victoria, Kenya [76].
In order to mitigate HMs pollution in the study area, the establishment of the current levels only is inadequate, and therefore, it is important to predict and identify the pollution sources [74,77]. The Pearson’s correlative analysis showed that were positive correlations between the concentrations of the HMs, though these were not significant except for that between Cd and Cr (Table 3). The principal component analysis showed that the first principal component was dominated by Cd and Cr, while the second principal component was dominated by Cu and pH. Moisture content in the third principal component was not associated with any of the HMs, and Pb lay between the first and second principal components (Figure 4). These results agree with the results of the Pearson’s correlation analysis. The strong correlation between Cr and Cd indicates that they should have entered into the environment through anthropogenic contributions, or may be due to the similarities in their retention phenomena in solid matrices [78]. This association is in agreement with Mothersill [79] who made a similar observation for sediments from the Ugandan portion of L. Victoria. The association between Cu and pH could be explained by the fact that the solubility of Cu (II) ions in solution, soils and sediments is pH-dependent [80]. However, an assessment of more factors that is ensued by factor and hierarchical cluster analyses would better allow for the discernment of the sources behind the individual HM contamination values of Port Bell, L. Victoria, Uganda.

Table 3.
Pearson’s correlation matrix for the HMs in the sediments from Port Bell, L. Victoria, Uganda.
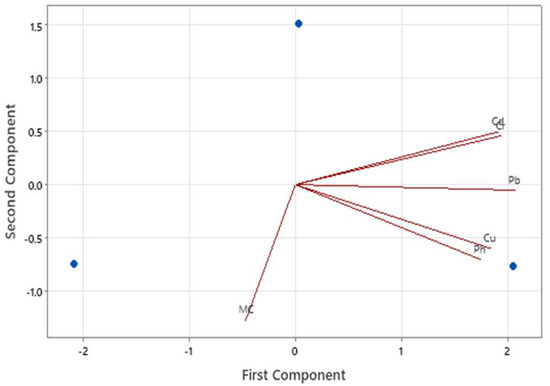
Figure 4.
Principal component analysis plots showing the effect of two components influencing the variation of HMs in sediments from Port Bell, L. Victoria, Uganda.

Table 2.
Comparison of trace metals in sediments (mg/kg) from Port Bell, L. Victoria, Uganda with international sediment guidelines, previous and other global reports.
Table 2.
Comparison of trace metals in sediments (mg/kg) from Port Bell, L. Victoria, Uganda with international sediment guidelines, previous and other global reports.
| Lake (Country) | Cu | Pb | Cr | Cd | References |
|---|---|---|---|---|---|
| L. Victoria (Uganda) | 6.467 | 42.184 | 0.456 | 3.283 | This study |
| L. Victoria (Uganda) | 41.0 | ND | 67.0 | ND 4 | Mothersill [79] |
| L. Victoria (Tanzania) 1 | 26.1 | 29.6 | 11.0 | 2.5 | Kishe and Machiwa [81] |
| L. Victoria (Tanzania) 1 | BDL–147 3 | 17–1922 | ND | ND | Makundi [82] |
| L. Victoria (Kenya) 2 | 39.8 | 37.7 | ND | 0.5 | Onyari [83] |
| L. Victoria (Kenya) 2 | 14–259 | 195 | 12–84 | 12–84 | Outa et al. [76] |
| Lake Manzala (Egypt) | 0.11 | 0.50 | ND | 0.002 | Redwan and Elhaddad [84] |
| Northern Delta Lakes: Edku, Borollus and Manzala (Egypt) | 12.71–412.00 | BDL–193.25 | ND | BDL–110.00 | Saeed and Shaker [85] |
| Lake of Ahémé (Benin) | ND | 2.78–92.6 | ND | 0.33–3.50 | Hounkpè et al. [86] |
| Lake Bafa (Turkey) | 19.55–25.28 | 10.12–13.75 | 59.2–80.97 | 0.40–1.02 | Algül and Beyhan [74] |
| East Dongting Lake (China) | 0.7262–0.7720 | 55.54–61.13 | 109.4–121.63 | 0.92–1.03 | Yan et al. [87] |
| 46.35 | 35.15 | 33.06 | 2.74 | Makokha et al. [88] | |
| Honghu Lake (China) | 78.0 | 20.66 | 25.0 | 0.14 | Makokha et al. [88] |
| Yilong Lake (China) | 31.4 | 53.19 | 0.76 | 86.73 | Bai et al. [89] |
| Veeranam Lake (India) | 94.12 | 30.06 | 88.2 | 0.81 | Suresh et al. [90] |
| Hussain Sagar Lake (India) | 90.108 | 79.885 | 90.0 | 19.89 | Ayyanar et al. [91] |
| Zariwar Lake (Iran) | 16.97 | ND | 74.41 | 0.25 | Kachoosangi et al. [92] |
| Lake Van (Turkey) | 20.0 | 5.0 | 46.0 | ND | Erenturk et al. [93] |
| Dongting Lake (China) | 47.48 | 60.99 | 4.65 | 88.29 | Li et al. [94] |
| Sediment quality guidelines | |||||
| Average shale value | 45.0 | 20.0 | 90.0 | 0.3 | Turekian and Wedepohl [95] |
| Toxicity reference value | 16.0 | 31.0 | 26.0 | 0.6 | US EPA [96] |
| Threshold effect concentration (TEC) | 31.6 | 35.8 | 43.4 | 0.99 | MacDonald et al. [75] |
| 149.0 | 128.0 | 111.0 | 4.99 | ||
1 Studies conducted on Mwanza Gulf, Tanzanian portion of L. Victoria; 2 Studies conducted on Winam Gulf of L. Victoria; 3 BDL: Below detection limit, 4 ND: Not determined.
In reference to previous studies that have been conducted on L. Victoria, the concentration of Pb that is reported in this study are comparable to the 30.7 mg/kg that was reported by Kishe and Machiwa [81] in Mwanza, Tanzania, but higher than the 0.283 to 0.330 mg/kg for Pb that was reported by Ogoyi et al. [97] in Winam Gulf, Kenya. The comparison of the HM content in the sediments from Port Bell, L. Victoria with those that have been reported in other lakes globally showed that high concentrations of Cd, Cr and Pb have been found in some lakes, which could be attributed to local anthropogenic activities (Table 2). For example, Li et al. [94] reported a mean concentration of Cd which is twenty seven-fold the values that are reported in this study. Yan et al. [87] found Cr at concentrations over 240-fold than those that are reported in this study. However, a recent report by Redwan and Elhaddad [84] found very low concentrations of Cu, Pb and Cd in sediments from Lake Manzala (Egypt) in comparison to those that were detected in this study. The differences in the concentrations of the HMs that have been reported in sediments across the globe and the current study could also be due to the differences in the geological formation of the lakes and their physicochemical conditions (such as sediment grain size, pH, and organic matter content of the sediment) [81].
The results of this study indicate potential negative health effects that the HMs could induce in the people who reside and work in the studied port. For example, Cu, though it is an essential trace metal in the human body, results in damaging of the liver, kidney, heart, brain and subsequently, death upon exposure to it at high concentrations [98]. Similarly, exposure to Pb is detrimental and is associated with renal failure, anaemia, neuromuscular weakness, cancer, brain damage, hypertension and death [99].
3.2. Human Health Risk Assessment Results
The ADDtherm, HQ and hazard indices were computed and are presented in Table 4. The values range from 1.79 × 10−8 mg/kg/day for Cr to 3.6088 × 10−7 mg/kg/day for Cu for the ADDtherm. The corresponding HQ and hazard index values are from 6.0 × 10−6 to 9.02 × 10−4 and 4.7134 × 10−3 to 5.2240 × 10−3, respectively. These results showed that the values are all less than one, thus indicating that there are no potential non-carcinogenic health risks that could arise from dermal contact with the HMs-contaminated sediments.

Table 4.
Average daily dose, hazard quotient and indices through dermal adsorption of HMs from dredging works in sediments from Port Bell, L. Victoria, Uganda.
3.3. Sediment Quality Assessment Results
With regards to the contamination factors, the values range from 0.004 to 11.510 (Table 5). According to the classification that was advanced by Hakanson [62], four contamination categories are distinguished; CF< 1: low contamination; 1 CF < 3: moderate contamination, 3 CF < 6: considerable contamination and CF 6: very high contamination. Thus, there is very high contamination of sediments in the studied parts of Port Bell, particularly with regard to Cd.

Table 5.
Contamination assessment indices for sediments from Port Bell, L. Victoria, Uganda.
On the other hand, the geoaccumulation index (Igeo) was used to assess the degree of contamination by comparing the current levels of the HMs to the previous status of Port Bell. The index is made up of seven grades that can be used to gauge the sediment quality levels according to the degree of HM pollution, i.e., class 0 (Igeo < 0): uncontaminated; class 1 (0 < Igeo < 1): low to median contamination; class 2 (1 < Igeo < 2): median contamination; class 3 (2 < Igeo < 3): median to strong contamination; class 4 (3 < Igeo < 4): serious contamination; class 5 (4 < Igeo < 5): serious to extreme contamination; class 6 (Igeo > 5): extreme contamination [7,12]. In this study, the geoaccumulation indices are from −8.015 to 2.940 (Table 5). This implies that the sediments were uncontaminated with regards to Cu and Cr, low-to-medially contamination with respect to Pb and medially contamination in the case of Cd.
For the PLI, the criteria are as follows: a PLI < 1 corresponds to a perfect sediment quality, a PLI = 1 shows that only baseline levels of HMs are present, while a PLI > 1 indicates deterioration of the sample site’s quality. In this study, the PLI never surpassed one, indicating that there is perfect sediment quality.
The potential ecological risk factors and the PERI showed that there is considerable pollution, particularly with regard to Cd (Table 6). The potential ecological risk for single HMs in the sediments followed the sequence: Cd > Pb > Cu > Cr. The pollution degrees of Cd were the highest in Port Bell due to both natural and anthropogenic inputs. Effluents from numerous industries that are discharged into Nakivubo channel including electronic-wastes could explain the high concentration of Cd in this port. Moreover, high levels of Cd and Pb in water resources (and hence, in the sediments) may also be due to use of leaded petrol and lead-based paints in outboard boat engines and automobiles, the thoughtless disposal of dead nickel-cadmium batteries and lead-acid accumulators, and the indiscriminate use of phosphate fertilizers [73,74]. The results of this assessment reiterate that the ecological biodiversity of the studied portion of Port Bell is at great risk.

Table 6.
Potential ecological risk factor () and PERI of trace metals in sediments from Port Bell, L. Victoria, Uganda.
4. Conclusions
This study assessed the HM contamination of sediments from Port Bell, L. Victoria, Uganda. The results showed that Cu (from 6.111 to 7.111 mg/kg) and Pb (from 40.222 to 44.212 mg/kg) occurred in the highest concentrations. The health risk assessments suggested that there are no discernible non-carcinogenic health risks that could arise from dredging works in the study area, but the sediment quality assessment indices showed that Cd has reached severe enrichment in the sediments; the sediments are not contaminated with regards to Cu and Cr, but exhibited low-to-median and median levels of contamination with respect to Pb and Cd, respectively. Thus, the spatial variations in the concentrations of the HMs and other micropollutants in water, biota and sediments should be monitored.
Author Contributions
Conceptualization, G.B., A.M. and H.T.; methodology, G.B., A.M., T.O. (Thomas Otema), A.O. and T.O. (Timothy Omara); validation, H.T., A.G., P.O., C.A., A.O., B.O., T.O. (Thomas Otema) and T.O. (Timothy Omara); formal analysis, C.K.N., C.A. and T.O. (Timothy Omara); writing—original draft preparation, G.B., A.M., P.O. and T.O. (Timothy Omara); writing—review and editing, H.T., C.K.N., A.G., A.O., P.O., D.O., I.B., E.N. and T.O. (Thomas Otema); supervision, H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data supporting the conclusions of this study are available on request from the authors.
Acknowledgments
The authors are grateful to Wilber Waibale of Uganda Industrial Research Institute (UIRI) for the spectroscopic analysis of the samples. Timothy Omara acknowledges Austrian Partnership Programme in Higher Education and Research (APPEAR) for the doctoral fellowship awarded to him through its academic partnership project 249 ‘Environmental Chemistry for Sustainable Development (ECSDevelop)’ hosted at Makerere University, Uganda which made this collaborative research possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santhakumari, M.; Sagar, N. The Environmental Threats Our World Is Facing Today. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–20. [Google Scholar]
- King, E.A. Here, There, and Everywhere: How the SDGs Must Include Noise Pollution in Their Development Challenges. Environ. Sci. Policy Sustain. Dev. 2022, 64, 17–32. [Google Scholar] [CrossRef]
- Shezi, B.; Street, R.A.; Webster, C.; Kunene, Z.; Mathee, A. Heavy Metal Contamination of Soil in Preschool Facilities around Industrial Operations, Kuils River, Cape Town (South Africa). Int. J. Environ. Res. Public Health 2022, 19, 4380. [Google Scholar] [CrossRef]
- Angom, J.; Angiro, C.; Omara, T. Air Quality Improvement from COVID-19 Lockdown in the East African Community: Evidences from Kampala and Nairobi Cities. OALib 2021, 08, 1107389. [Google Scholar] [CrossRef]
- Jenny, J.; Anneville, O.; Arnaud, F.; Baulaz, Y.; Bouffard, D.; Domaizon, I.; Bocaniov, S.A.; Chèvre, N.; Dittrich, M.; Dorioz, J.-M.; et al. Scientists’ Warning to Humanity: Rapid degradation of the world’s large lakes. J. Great Lakes Res. 2020, 46, 686–702. [Google Scholar] [CrossRef]
- Ochola, G.O. Natural Resource Use Dilemma: A Review of Effects of Population Growth on Natural Resources in Kenya. Int. J. Environ. Sci. Nat. Resour. 2018, 13, 555867. [Google Scholar] [CrossRef]
- E Alam, N.; Salam, M.A.; Dewanjee, S.; Hasan, F.; Rahman, H.; Rak, A.E.; Islam, A.R.M.T.; Miah, Y. Distribution, Concentration, and Ecological Risk Assessment of Trace Metals in Surface Sediment of a Tropical Bangladeshi Urban River. Sustainability 2022, 14, 5033. [Google Scholar] [CrossRef]
- Twinomucunguzi, F.R.B.; Nyenje, P.M.; Kulabako, R.N.; Semiyaga, S.; Foppen, J.W.; Kansiime, F. Emerging organic contaminants in shallow groundwater underlying two contrasting peri-urban areas in Uganda. Environ. Monit. Assess. 2021, 193, 228. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Wojciechowska, E.; Nawrot, N.; Walkusz-Miotk, J.; Matej-Łukowicz, K.; Pazdro, K. Heavy Metals in Sediments of Urban Streams: Contamination and Health Risk Assessment of Influencing Factors. Sustainability 2019, 11, 563. [Google Scholar] [CrossRef]
- Nargis, A.; Harun-Or-Rashid; Jhumur, A.K.; Haque, M.E.; Islam, M.N.; Habib, A.; Cai, M. Human health risk assessment of toxic elements in fish species collected from the river Buriganga, Bangladesh. Hum. Ecol. Risk Assess 2020, 26, 120–146. [Google Scholar] [CrossRef]
- Omara, T.; Karungi, S.; Kalukusu, R.; Nakabuye, B.V.; Kagoya, S.; Musau, B. Mercuric pollution of surface water, superficial sediments, Nile tilapia (Oreochromis nilotica Linnaeus 1758 [Cichlidae]) and yams (Dioscorea alata) in auriferous areas of Namukombe stream, Syanyonja, Busia, Uganda. PeerJ. 2019, 7, 7919. [Google Scholar] [CrossRef] [PubMed]
- Mayoma, B.S.; Sørensen, C.; Shashoua, Y.; Khan, F.R. Microplastics in beach sediments and cockles (Anadara antiquata) along the Tanzanian coastline. Bull. Environ. Contam. Toxicol. 2020, 105, 513–521. [Google Scholar] [CrossRef]
- Kandie, F.J.; Krauss, M.; Massei, R.; Ganatra, A.; Fillinger, U.; Becker, J.; Liess, M.; Torto, B.; Brack, W. Multi-compartment chemical characterization and risk assessment of chemicals of emerging concern in freshwater systems of western Kenya. Environ. Sci. Eur. 2020, 32, 115. [Google Scholar] [CrossRef]
- Nawrot, N.; Wojciechowska, E.; Mohsin, M.; Kuittinen, S.; Pappinen, A.; Rezania, S. Trace Metal Contamination of Bottom Sediments: A Review of Assessment Measures and Geochemical Background Determination Methods. Minerals 2021, 11, 872. [Google Scholar] [CrossRef]
- Shah-Kulkarni, S.; Lee, S.; Jeong, K.S.; Hong, Y.-C.; Park, H.; Ha, M.; Kim, Y.; Ha, E.-H. Prenatal exposure to mixtures of heavy metals and neurodevelopment in infants at 6 months. Environ. Res. 2020, 182, 109122. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Zheng, X.; Reponen, T.; Chen, A.; Huo, X. Heavy metals in PM2.5 and in blood, and children’s respiratory symptoms and asthma from an e-waste recycling area. Environ. Pollut. 2016, 210, 346–353. [Google Scholar] [CrossRef]
- Salem, H.M.; Eweida, E.A.; Farag, A. Heavy metals in drinking water and their environmental impact on human health. In Proceedings of the International Conference for Environmental Hazard Mitigation ICEHM 2000, Cairo University, Cairo, Egypt, 9–12 September 2000. [Google Scholar]
- Manna, K.; Debnath, B.; Singh, W.S. Sources and toxicological effects of lead on human health. Indian J. Med Spéc. 2019, 10, 66. [Google Scholar] [CrossRef]
- WHO. Exposure to Lead: A Major Public Health Concern, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240037656 (accessed on 30 July 2022).
- Saeed, S.; Hasan, S.; Kuldeep; Choudhury, P. Lead Poisoning: A Persistent Health Hazard-General and Oral Aspects. Biomed. Pharmacol. J. 2017, 10, 439–445. [Google Scholar] [CrossRef]
- Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Hong, Y.-S. Evaluation and management of lead exposure. Ann. Occup. Environ. Med. 2015, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Njiru, J.; van der Knaap, M.; Kundu, R.; Nyamweya, C. Lake Victoria fisheries: Outlook and management. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2018, 23, 152–162. [Google Scholar] [CrossRef]
- Moon, W.; Hannachi, A. River Nile discharge, the Pacific Ocean and world climate—A seasonal synchronization perspective. Tellus A 2021, 73, 1–12. [Google Scholar] [CrossRef]
- Ndegwa, D.M.; Nyamweya, C.S.; Obuba, E. Ecotroph: A simple model to assess fishing and trophic interactions in Lake Victoria. Int. J. Fish Aquat. Stud. 2019, 7, 210–215. [Google Scholar]
- Evans, H. Setting Sail on Lake Victoria to Beat Plastic Pollution. Available online: https://s.the-star.co.ke/-setting-sail-on-lake-victoria-to-beat-plastic-pollution-/index.html?&external (accessed on 20 March 2022).
- Kayombo, S.; Jorgenson, S. Lake Victoria: Experiences and Lessons Learned Brief. 2006. Available online: https://www.ilec.or.jp/wp-content/uploads/pub/27_Lake_Victoria_27February2006.pdf (accessed on 1 September 2022).
- Nicholson, H. Investigation of Microplastics in the Tilapia found in Lake Victoria, Kenya. Master’s Thesis, University of Nottingham, Nottingham, UK, 2021. [Google Scholar]
- Howell, B. The 11 Most Polluted Bodies of Water Around the World. 2021. Available online: https://www.theecoexperts.co.uk/blog/the-most-polluted-bodies-of-water (accessed on 15 March 2022).
- Ngupula, G.; Kayanda, R.; Mashafi, C. Abundance, composition and distribution of solid wastes in the Tanzanian waters of Lake Victoria. Afr. J. Aquat. Sci. 2014, 39, 229–232. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Basooma, R.; Nabwire, R. Occurrence, distribution and size relationships of plastic debris along shores and sediment of northern Lake Victoria. Environ. Pollut. 2019, 257, 113442. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Ocaya, H.; Pabire, W.G. Microplastic pollution in surface water of Lake Victoria. Sci. Total Environ. 2020, 741, 140201. [Google Scholar] [CrossRef]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First evidence of microplastics in the African Great Lakes: Recovery from Lake Victoria Nile perch and Nile tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Kerebba, N.; Ssebugere, P.; Kwetegyeka, J.; Arinaitwe, K.; Wasswa, J. Concentrations and sources apportionment of polycyclic aromatic hydrocarbons in sediments from the Uganda side of Lake Victoria. Environ. Sci. Process. Impacts 2017, 19, 570–577. [Google Scholar] [CrossRef]
- Kwach, B.O.; Lalah, J.O.; Shem, W.O. Spartial and Seasonal Variations in Concentrations of Polycyclic Aromatic Hydrocarbons in Water and Sediment of Kisumu City Bay of Winam Gulf, Lake Victoria-Kenya. Bull. Environ. Contam. Toxicol. 2009, 83, 734–741. [Google Scholar] [CrossRef]
- Mahugija, J.A.M.; Njale, E. Levels of polycyclic aromatic hydrocarbons (PAHs) in smoked and sun-dried fish samples from areas in Lake Victoria in Mwanza, Tanzania. J. Food Compos. Anal. 2018, 73, 39–46. [Google Scholar] [CrossRef]
- Orata, F.; Quinete, N.; Maes, A.; Werres, F.; Wilken, R. Perfluorooctanoic acid and perfluorooctane sulfonate in Nile Perch and tilapia from gulf of Lake Victoria. Afr. J. Pure Appl. Chem. 2008, 2, 75–79. [Google Scholar]
- Orata, F.; Quinete, N.; Werres, F.; Wilken, R.-D. Determination of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Lake Victoria Gulf Water. Bull. Environ. Contam. Toxicol. 2008, 82, 218–222. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per- and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Sci. Total Environ. 2018, 631–632, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Nantaba, F.; Wasswa, J.; Kylin, H.; Palm, W.-U.; Bouwman, H.; Kümmerer, K. Occurrence, distribution, and ecotoxicological risk assessment of selected pharmaceutical compounds in water from Lake Victoria, Uganda. Chemosphere 2019, 239, 124642. [Google Scholar] [CrossRef] [PubMed]
- Arinaitwe, K.; Kiremire, B.T.; Muir, D.C.; Fellin, P.; Li, H.; Teixeira, C.; Mubiru, D.N. Legacy and currently used pesticides in the atmospheric environment of Lake Victoria, East Africa. Sci. Total Environ. 2015, 543, 9–18. [Google Scholar] [CrossRef]
- Arinaitwe, K.; Muir, D.C.G.; Kiremire, B.T.; Fellin, P.; Li, H.; Teixeira, C. Polybrominated Diphenyl Ethers and Alternative Flame Retardants in Air and Precipitation Samples from the Northern Lake Victoria Region, East Africa. Environ. Sci. Technol. 2014, 48, 1458–1466. [Google Scholar] [CrossRef]
- Wang, S.; Steiniche, T.; Romanak, K.A.; Johnson, E.; Quirós, R.; Mutegeki, R.; Wasserman, M.D.; Venier, M. Atmospheric Occurrence of Legacy Pesticides, Current Use Pesticides, and Flame Retardants in and around Protected Areas in Costa Rica and Uganda. Environ. Sci. Technol. 2019, 53, 6171–6181. [Google Scholar] [CrossRef]
- Mchau, G.J.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.Y.; Mpolya, E.; Meneely, J.P.; Elliott, C.T.; Greer, B. First Report of the Co-occurrence of Cylindrospermopsin, Nodularin and Microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo. Health 2020, 13, 185–194. [Google Scholar] [CrossRef]
- Roegner, A.; Sitoki, L.; Weirich, C.; Corman, J.; Owage, D.; Umami, M.; Odada, E.; Miruka, J.; Ogari, Z.; Smith, W.; et al. Harmful Algal Blooms Threaten the Health of Peri-Urban Fisher Communities: A Case Study in Kisumu Bay, Lake Victoria, Kenya. Expo. Health 2020, 12, 835–848. [Google Scholar] [CrossRef]
- Githukia, C.; Onyango, D.; Lusweti, D.; Ramkat, R.; Kowenje, C.; Miruka, J.; Lung’Ayia, H.; Orina, P. An Analysis of Knowledge, Attitudes and Practices of Communities in Lake Victoria, Kenya on Microcystin Toxicity. Open J. Ecol. 2022, 12, 198–210. [Google Scholar] [CrossRef]
- Musisi, F. Port Bell Set for Major Revamp in 108 Years. 2016. Available online: http://www.monitor.co.ug/artsculture/Reviews/Port-Bell-set-for-major-revamp-in-108-years/691232-3358428-item-00-10ss3p2/index.html (accessed on 12 July 2022).
- Tenywa, G.; Balagadde, S. Sand Mining Ruining Lake Victoria Fish Breeding Areas (Uganda). 2013. Available online: https://www.business-humanrights.org/en/latest-news/sand-mining-ruining-lake-victoria-fish-breeding-areas-uganda/ (accessed on 15 June 2022).
- Kampala City Guide, Port Bell. 2022. Available online: http://www.kampalacityguide.com/kampala-city/nakawa-division/port-bell.html (accessed on 15 July 2022).
- Wörner, S.; Pester, M. Microbial Succession of Anaerobic Chitin Degradation in Freshwater Sediments. Appl. Environ. Microbiol. 2019, 85, e00963-19. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Dermal Exposure Assessment: A Summary of EPA Approaches; USEPA: Washington, DC, USA, 2007; p. 20460.
- Omara, T.; Ogwang, R.; Ndyamuhaki, S.; Kagoya, S.; Kigenyi, E.; Musau, B.; Adupa, E. Spectroscopic analysis of selected priority trace metals in the extant East African gilled lungfish (Protopterus amphibius) in Lira municipal lagoon and its edibility health risk. Sci. J. Anal. Chem. 2018, 6, 38–45. [Google Scholar] [CrossRef]
- Nowell, L.H.; Moran, P.W.; Gilliom, R.J.; Calhoun, D.L.; Ingersoll, C.G.; Kemble, N.E.; Kuivila, K.M.; Phillips, P.J. Contaminants in Stream Sediments from Seven United States Metropolitan Areas: Part I: Distribution in Relation to Urbanization. Arch. Environ. Contam. Toxicol. 2012, 64, 32–51. [Google Scholar] [CrossRef]
- Ordóñez, A.; Álvarez, R.; Charlesworth, S.; De Miguel, E.; Loredo, J. Risk assessment of soils contaminated by mercury mining, Northern Spain. J. Environ. Monit. 2010, 13, 128–136. [Google Scholar] [CrossRef]
- Omara, T.; Nteziyaremye, P.; Akaganyira, S.; Opio, D.W.; Karanja, L.N.; Nyangena, D.M.; Kiptui, B.J.; Ogwang, R.; Epiaka, S.M.; Jepchirchir, A.; et al. Physicochemical quality of water and health risks associated with consumption of African lung fish (Protopterus annectens) from Nyabarongo and Nyabugogo rivers, Rwanda. BMC Res. Notes 2020, 13, 66. [Google Scholar] [CrossRef]
- Bamuwamye, M.; Ogwok, P.; Tumuhairwe, V.; Eragu, R.; Nakisozi, H.; Ogwang, P.E. Dietary Content and Potential Health Risks of Metals in Commercial Black Tea in Kampala (Uganda). J. Food Res. 2017, 6, 1. [Google Scholar] [CrossRef]
- Saha, N.; Zaman, M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2012, 185, 3867–3878. [Google Scholar] [CrossRef]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef]
- S Sharma, S.D. Risk assessment via oral and dermal pathways from heavy metal polluted water of Kolleru lake—A Ramsar wetland in Andhra Pradesh, India. Env. Anal. Health Toxicol. 2020, 35, 2020019. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, W.; Yan, X.; Shu, T.; Xiong, Q.; Chen, F. Pollution Characteristics and Health Risk Assessment of Airborne Heavy Metals Collected from Beijing Bus Stations. Int. J. Environ. Res. Public Health 2015, 12, 9658–9671. [Google Scholar] [CrossRef]
- Müller, G. Die Schwermetallbelastung der Sedimenten des Neckars und Seiner Nebenflüsse. Chem.-Ztg. 1981, 6, 157–164. [Google Scholar]
- Chen, C.; Kao, C.; Chen, C.; Dong, C. Distribution and accumulation of heavy metals in the sediments of Kaohsiung harbor, Taiwan. Chemosphere 2007, 66, 1431–1440. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, H.; Chang, J.; Qu, J.; Xie, H.; Yu, L. Heavy metal contamination in surface sediments of Yangtze River intertidal zone: An assessment from different indexes. Environ. Pollut. 2009, 157, 1533–1543. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, Q.; Liang, Z.; Zheng, D. Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China. Environ. Pollut. 2008, 154, 135–142. [Google Scholar] [CrossRef]
- Kahal, A.; El-Sorogy, A.S.; Qaysi, S.; Almadani, S.; Kassem, O.M.; Al-Dossari, A. Contamination and ecological risk assessment of the Red Sea coastal sediments, southwest Saudi Arabia. Mar. Pollut. Bull. 2020, 154, 111125. [Google Scholar] [CrossRef]
- Lim, W.Y.; Aris, A.Z.; Ismail, T.H.T. Spatial Geochemical Distribution and Sources of Heavy Metals in the Sediment of Langat River, Western Peninsular Malaysia. Environ. Forensics 2013, 14, 133–145. [Google Scholar] [CrossRef]
- Bahiru, D.B. Determination of Heavy Metals in Wastewater and Their Toxicological Implications around Eastern Industrial Zone, Central Ethiopia. J. Environ. Chem. Ecotoxicol. 2020, 12, 72–79. [Google Scholar]
- Das, M.; Ahmed, M.K.; Islam, M.S.; Islam, M.M.; Akter, M.S. Heavy Metals in Industrial Effluents (Tannery and Textile) and Adjacent Rivers of Dhaka City, Bangladesh. Terr. Aquat. Environ. Toxicol. 2014, 5, 8–13. [Google Scholar]
- Huang, X.; Luo, D.; Zhao, D.; Li, N.; Xiao, T.; Liu, J.; Wei, L.; Liu, Y.; Liu, L.; Liu, G. Distribution, Source and Risk Assessment of Heavy Metal (oid)s inWater, Sediments, and Corbicula Fluminea of Xijiang River, China. Int. J. Environ. Res. Public Health 2019, 16, 1823. [Google Scholar] [CrossRef] [PubMed]
- Algül, F.; Beyhan, M. Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Sci. Rep. 2020, 10, 11782. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- Outa, J.O.; Kowenje, C.O.; Plessl, C.; Jirsa, F. Distribution of arsenic, silver, cadmium, lead and other trace elements in water, sediment and macrophytes in the Kenyan part of Lake Victoria: Spatial, temporal and bioindicative aspects. Environ. Sci. Pollut. Res. 2019, 27, 1485–1498. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, G.H.; Guo, H.; Zhang, W.; Hao, Z. Spatio-temporal patterns and source apportionment of coastal water pollution in eastern Hong Kong. Water Res. 2007, 41, 3429–3439. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Mohamed, L.A.; Mahmoud, M.G.; Shaban, K.S.; Fahmy, M.A.; Ebeid, M.H. Potential ecological hazards assessment and prediction of sediment heavy metals pollution along the Gulf of Suez, Egypt. Egypt. J. Aquat. Res. 2019, 45, 329–335. [Google Scholar] [CrossRef]
- Mothersill, J.S. The mineralogy and geochemistry of the sediments of northwestern Lake Victoria. Sedimentology 1976, 23, 553–565. [Google Scholar] [CrossRef]
- Rader, K.J.; Carbonaro, R.F.; van Hullebusch, E.D.; Baken, S.; Delbeke, K. The Fate of Copper Added to Surface Water: Field, Laboratory, and Modeling Studies. Environ. Toxicol. Chem. 2019, 38, 1386–1399. [Google Scholar] [CrossRef]
- A Kishe, M.; Machiwa, J.F. Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environ. Int. 2003, 28, 619–625. [Google Scholar] [CrossRef]
- Makundi, I.N. A Study of Heavy Metal Pollution in Lake Victoria Sediments by Energy Dispersive X-Ray Fluorescence. J. Environ. Sci. Health Part A 2001, 36, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Onyari, J. The concentration of Mn, Fe, Cu, Zn, Cd and Pb in Sediments and Fish from the Winam Gulf of Lake Victoria Bought in Mombasa Town Markets. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 1985. [Google Scholar]
- Redwan, M.; Elhaddad, E. Heavy metal pollution in Manzala Lake sediments, Egypt: Sources, variability, and assessment. Environ. Monit. Assess. 2022, 194, 436. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.M.; Shaker, I.M. Assessment of heavy metals pollution in water and sediments and their effect on Oreochromis niloticus in the Northern Delta Lakes, Egypt. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 475–489. [Google Scholar]
- Hounkpè, J.; Kélomè, N.; Adèchina, R.; Lawani, R. Assessment of heavy metals contamination in sediments at the lake of Ahémé in southern of Benin (West Africa). J. Mater. Environ. Sci. 2017, 8, 4369–4377. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, B.; Liu, H. Spatial distribution and source identification for heavy metals in surface sediments of East Dongting Lake, China. Sci. Rep. 2022, 12, 7940. [Google Scholar] [CrossRef]
- Makokha, V.A.; Qi, Y.; Shen, Y.; Wang, J. Concentrations, Distribution, and Ecological Risk Assessment of Heavy Metals in the East Dongting and Honghu Lake, China. Expo. Health 2015, 8, 31–41. [Google Scholar] [CrossRef]
- Bai, J.; Cui, B.; Chen, B.; Zhang, K.; Deng, W.; Gao, H.; Xiao, R. Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol. Model. 2011, 222, 301–306. [Google Scholar] [CrossRef]
- Suresh, G.; Sutharsan, P.; Ramasamy, V.; Venkatachalapathy, R. Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol. Environ. Saf. 2012, 84, 117–124. [Google Scholar] [CrossRef]
- Ayyanar, A.; Thatikonda, S. Distribution and ecological risks of heavy metals in Lake Hussain Sagar, India. Acta Geochim. 2019, 39, 255–270. [Google Scholar] [CrossRef]
- Kachoosangi, F.T.; Karbassi, A.; Sarang, A.; Noori, R. Sedimentation rate determination and heavy metal pollution assessment in Zariwar Lake, Iran. SN Appl. Sci. 2020, 2, 1483. [Google Scholar] [CrossRef]
- Erenturk, S.; Yusan, S.; Turkozu, D.A.; Camtakan, Z.; Olgen, M.K.; Aslani, M.A.A.; Aytas, S.; Isik, M.A. Spatial distribution and risk assessment of radioactivity and heavy metal levels of sediment, surface water and fish samples from Lake Van, Turkey. J. Radioanal. Nucl. Chem. Artic. 2014, 300, 919–931. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Zeng, G.; Yuan, X.; Li, X.; Liang, J.; Wang, X.; Tang, X.; Bai, B. Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J. Geochem. Explor. 2013, 132, 75–83. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (US EPA). Sediment Quality Guidelines; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- Ogoyi, D.O.; Mwita, C.J.; Nguu, E.K.; Shiundu, P.M. Determination of Heavy Metal Content in Water, Sediment and Microalgae from Lake Victoria, East Africa. Open Environ. Eng. J. 2011, 4, 156–161. [Google Scholar]
- Araya, M.; Olivares, M.; Pizarro, F. Copper in human health. Int. J. Environ. Health 2007, 1, 608–620. [Google Scholar] [CrossRef]
- Assi, M.A.; Hezmee, M.N.M.; Haron, A.W.; Sabri, M.Y.M.; Rajion, M.A. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660–671. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).