Abstract
The development of a truly circular economy necessitates the recovery and recycling of resources from secondary streams. In this work, we studied the extraction of metals from printed circuit boards (PCBs) using choline chloride: ethylene glycol deep eutectic solvents: Cu, Ni, Zn, and Sn were selectively extracted from the PCBs, with >75% extraction after 72 h for Cu, Ni, and Sn, and circa. 45% extraction for Zn. This solvometallurgical approach promises to minimize the use of water and acid/base reagents in processing. The results show a considerable ability to compete with current methods of metal extraction and therefore generate a strong potential to attain the goal of a sustainable circular economy via zero-waste green urban mining.
1. Introduction
The vast amount of electronic waste generated from our tech-heavy societies is a significant challenge currently facing the world. In fact, waste electrical and electronic equipment (WEEE) is one of the fastest growing waste streams, with more than 5% annual growth, and represents a valuable secondary resource for strategic, critical, and precious metals [1]. In fact, many WEEE streams contain higher concentrations of valuable metals than primary resources. Although metal separation and recovery from these complex matrices is a challenge, green urban mining has become significantly important worldwide [2].
Recently, a new zero-waste solvometallurgical processing route has been proposed for metal extraction and recovery. It relies on the use of green anhydrous solvents, namely, deep eutectic solvents (DESs), and promises to minimize the use of water and acid/base consumption in process circuits [3]. DESs are eco-friendly, biodegradable, low cost, and easy to prepare solvents with tunable properties [4]. Specifically, they are mixtures of salts which, due to hydrogen bonding, result in a lower melting point than their respective discrete components [4]. Thus far, DESs have been successfully used in several studies, including selective metal dissolution and electrowinning from ores and wastes [5,6,7,8,9].
In this work, we studied the solvometallurgical extraction of metals from printed circuits boards (PCBs). Due to the high association of metals with plastics, metal liberation processes often lead to fine particles, which may, in turn, result in inefficient metal recovery [10]. To reduce such metal losses, we used large pieces of PCBs instead of pulverized PCB powder [11,12]. Ethaline, a DES mixture of choline chloride (ChCl) and ethylene glycol (EG) at a 1:2 molar ratio, was used as the solvent in this study. Ethaline is a promising green solvent, as it is chemically stable in both air and moisture [13] and has low viscosity (36 cP) [14]. In addition, the use of iodine was evaluated as the oxidant in this work. Iodine is an environmentally benign strong oxidant that can be used in solvometallurgical processing since it is both highly soluble in ethaline and able to oxidize most elements [15].
2. Methodology
Choline chloride (HOC2H4N−(CH3)3+Cl−, ChCl; Sigma Aldrich, St. Louis, MO, United States, 99%) was dried under vacuum for 24 h prior to being used, while ethylene glycol (C2H6O2, Sigma Aldrich, >99%) and iodine (I2, Sigma Aldrich, >99.8%) were used as received. To prepare the DES, ChCl and EG were mixed at a 1:2 molar ratio and stirred at 80 ℃ until a colorless homogeneous liquid was formed.
Printed circuit boards (PCBs) were collected from a local scrap market. The electronic elements attached to the PCBs were manually removed prior to exposing the PCBs in a 10 M NaOH solution for 24 h to remove any chemical coating, which could prevent contact between the metals and the solvent. The PCBs were then washed with DI water, dried, and cut into 1 × 1 cm2 pieces. All leaching experiments were carried out in 250 mL beakers at 85 ℃, 150 rpm, for 72 h, with and without the presence of 0.1 mol L−1 I2. The experiments were duplicated. Liquid samples were collected at pre-determined time intervals, filtered, diluted with 5% HNO3, and analyzed using microwave plasma atomic emission spectroscopy (MP-AES, Agilent Technologies 4100+). Sodium peroxide fusion was used to completely digest the remaining PCB pieces post-leaching; metal concentrations were determined by MP-AES.
3. Results
The average metal content of the PCBs is shown in Table 1.

Table 1.
Metal content in PCBs.
Metal extraction (%) during leaching from the 1 × 1 cm2 PCB pieces was calculated using Equation (1).
where M1 is the metal weight (mg) in the pregnant leach solution (PLS), and M2 is the metal weight (mg) in the residue. M1 and M2 were calculated based on Equations (2) and (3), respectively.
where C1 (ppm) is the metal concentration in the PLS, V1 (L) is the volume of the leaching solution, C2 (ppm) is the metal concentration in the solid residue after leaching, and V2 (L) is the volume of the fusion digestion solution.
M1 = C1 · V1
M2 = C2 · V2
Figure 1 and Figure 2 show the metal extraction (%) from the PCBs in ethaline with and without I2 after 72 h. The presence of I2 significantly enhanced metal extraction, except for Al, which in both cases resulted in less than 1% extraction. The lower effectiveness of ethaline compared to ethaline:I2 may be attributed to the metallic state of the metals present on the PCBs [6,16]. As can be seen in Figure 2, in the presence of I2, the extraction of Cu, Sn, and Ni was higher than 75%, while that of Zn was circa. 45%. However, without the presence of I2 in ethaline, metal extraction was below 15%, except for Sn (circa. 100%).
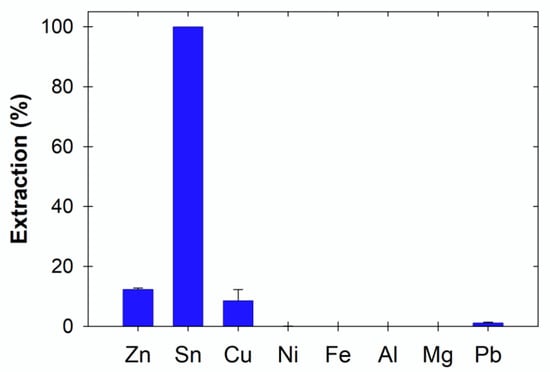
Figure 1.
Extraction of metals from 1 × 1 cm2 PCB pieces after 72 h in ChCl:EG without I2.
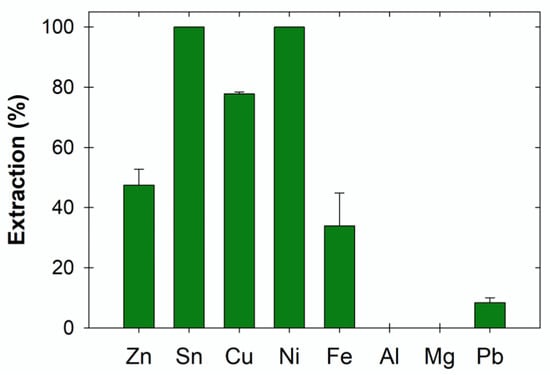
Figure 2.
Extraction of metals from 1 × 1 cm2 PCB pieces after 72 h in ChCl:EG in the presence of I2.
4. Discussion
Solvometallurgical processing shows a strong potential in the field of sustainable urban mining, especially in the recovery of metals from waste electrical and electronic equipment. This work investigated the extraction of metals from large pieces of printed circuit boards (PCBs) by leaching in a green non-aqueous choline chloride: ethylene glycol deep eutectic solvent. The results indicate that the use of an oxidant, namely, iodine, is necessary to achieve acceptable metal extraction levels from non-oxidized metal resources. Specifically, without using water or acid/base reagents in the leaching process, Cu, Ni, Zn, and Sn metals were selectively extracted from 1 × 1 cm2 PCB pieces (>75% extraction after 72 h for Cu, Ni, and Sn, and circa. 45% for Zn); Al, Mg, and Pb extraction was below 10%, and that of Fe was below 40%. These results are a strong indicator of the potential success of the solvometallurgical approach as a green zero-waste metal extraction and recycling processing route.
Author Contributions
H.A.S. designed the experimental matrix and carried out the experiments presented in this work, contributed to the interpretation of results, and participated in the writing of the manuscript. G.K. contributed to the experimental design, interpretation of the results, and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) (Discovery Grant) and Fonds de recherche du Québec—Nature et technologies (ERA-MIN 2 Grant).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schaeffer, N.; Passos, H. Recovery of metals from waste electrical and electronic equipment (WEEE) using unconventional solvents based on ionic liquids. Environ. Sci. Technol. 2018, 48, 859–922. [Google Scholar] [CrossRef] [Green Version]
- Gramatyka, P.; Nowosielski, R. Recycling of waste electrical and electronic equipment. J. Achiev. Mater. Manuf. Eng. 2007, 20, 535–538. [Google Scholar]
- Alonso, D.A.; Baeza, A. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Vigier, K. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Abbott, A.P.; Collins, J. Processing of Electric Arc Furnace Dust using Deep Eutectic Solvents. Aust. J. Chem. 2009, 62, 341–347. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G. Metal complexation in ionic liquids. Annu. Rep. Prog. Chem. Sect. A Inorg. Chem. 2008, 104, 21–45. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical Recovery of Metals from Large Printed Circuit Board Pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhav, U.; Su, C. Leaching of metals from large pieces of printed circuit boards using citric acid and hydrogen peroxide. ESPR 2016, 23, 24384–24392. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Harris, R.C. Electrocatalytic Recovery of Elements from Complex Mixtures using Deep Eutectic Solvents. Green Chem. 2015, 17, 2172–2179. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkin, G.R.T.; Al-Bassam, A.Z.M. The application of deep eutectic solvent ionic liquids for environmentally-friendly dissolution and recovery of precious metals. Miner. Eng. 2016, 87, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Rimsza, J.M.; Corrales, L.R. Adsorption complexes of copper and copper oxide in the deep eutectic solvent 2:1 urea–choline chloride. Comput. Theor. Chem. 2012, 987, 57–61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).