Abstract
This study presents experimental results for the development of a process for the recovery of indium and gallium from EoL CIGS (CuGa1−xInxSe2) panels. The process consists of a thermal treatment of the panels, followed by a hydrometallurgical treatment, where quantitative leaching of In, Ga, Mo, Cu and Zn is achieved. The elements are subsequently separated and recovered from the leachate by solvent extraction. For the development of the process, samples of EoL CIGS PV panels were used, which contained a thin film of Mo (metal base electrode), sputtered on the supporting soda-lime glass and covered by the thin film containing In, Ga, Cu and Se (1 μm). These films were detected by SEM-EDS in polished sections. The thermal treatment at 550 °C for 15 min, in excess of air, led to the successful disintegration of ethyl vinyl acetate (EVA) and delamination of the thin film-coated glass from the front protective glass. The glass fragments coated by the thin film contained the following: Se: 0.03–0.05%; In: 0.02%; Cu: 0.05%; Ga: 0.004–0.006%; and Mo: 0.04%. Following thermal treatment, thin film-coated glass fragments of about 1.5 cm × 1.5 cm were used in acid leaching experiments using HNO3, HCl and H2SO4. Quantitative leaching of Cu, Ga, In, Mo, Zn and Cu was achieved by HNO3 at ambient temperature. The effects of pulp density and acid concentration on the efficiency of metal leaching were investigated. Part of Se volatilized during the thermal treatment, whereas the rest was insoluble and separated from the solution by filtration. Finally, the separation of the elements was achieved via solvent extraction by D2EHPA.
1. Introduction
In the “2050 long-term strategy on adaptation to climate change”, the EU adopted measures to achieve the transformation into a low-carbon economy, such as the replacement of fossil fuels by renewable energy resources by 30% by 2030. The use of solar energy, which represents a significant percentage of renewable resources, will increase over the next two decades by more than 40 GW/year [1]. Europe holds the highest installed capacity, 70% of the total worldwide. Since 2012, photovoltaic (PV) panels have been classified as waste electrical and electronic equipment (WEEE), which require dedicated treatment at their end of life (EoL). Based on the quantity of the already installed PV modules and the expected PV capacity, global PV waste is projected to reach 1.7–8 million tonnes by 2030 and 60–78 million tonnes by 2050 [2].
The main reasons for the development of sustainable and environmentally friendly management practices and technologies are the significant amounts of EoL panels foreseen in the coming years; the potential environmental impact, mainly related to the leaching of heavy metals (e.g., Pb) at landfills; and the potential benefit from the recovery of precious (Ag), rare (In, Ge, Ga and Te) and energy-intensive elements (Si) [1,3].
Photovoltaic cells with semiconductors based on chalcopyrite structure, such as CuInSe2, CuGa1−xInxSe2 and CuInS2, combine the advantages of thin films (lower manufacturing costs) with the efficiency and stability of Si-based PV cells. All of these PV cells are referred to by the general name CI(G)S [4]. The recovery of In and Ga from EoL CIGS is a priority due to both their scarcity and increasing demand.
The scope of this study is the development of a hydrometallurgical process for the recovery of In and Ga from EoL CIGS. The process to be developed involves two stages: acid leaching for the extraction of the metals of interest in soluble form and solvent extraction for the separation of the leached metals [5,6]. This study reports experimental results on the selection of an efficient leaching medium and the effect of selected key factors, i.e., pulp density and acid concentration, on leaching. The separation of indium from gallium was experimentally tested by solvent extraction using D2EHPA [6].
2. Materials and Methods
2.1. Photovoltaic (PV) Materials
The raw materials used originated from EoL CIGS thin film photovoltaic panels made by Q-Smart. After dismantling, they were thermally treated for the removal of EVA and PVF layers. Following thermal treatment, thin film-coated glass fragments of about 1.5 cm × 1.5 cm were used for the acid leaching experiments.
2.2. Characterization of the EoL Thin Film PV Panels
Analysis of the microstructure of the thin film PV panels was conducted using a Jeol 6380 LV scanning electron microscope (SEM). Microanalysis was performed using an Oxford INCA energy-dispersive spectrometer (EDS) connected to the SEM.
2.3. Thermal Treatment of EoL Thin Film PV Panels and Separation of Main Parts
The decomposition of the adhesive resins and the separation of the individual elements of the thin film PV panels were carried out in a Carbolite laboratory furnace (1500 °C), where air can be blown inside the chamber to accelerate the oxidation of the organic components. The complete decomposition of the EVA and PVF (Tedlar®) membranes takes place in the temperature range of 540–550 °C [7]. The remaining material after the thermal treatment was manually separated into three streams: clear glass fragments, thin film-coated glass fragments and ash. The metal ribbon electrodes and polymer films were also manually collected and removed. Glass can be recovered and recycled. The separated thin film-coated glass fragments were used at the subsequent stages for the leaching tests.
2.4. Characterization of the Thin Film-Coated Glass Fragments
The chemical composition of the resulting thin film-coated glass fragments was determined by wavelength-dispersive X-ray fluorescence (WD-XRF, Rigaku Primus IV) as well as by ICP-OES and AAS upon acid digestion.
2.5. Acid Leahing for Metal Extraction
The separated thin film-coated glass fragments were further treated for the recovery of In, Ga, Cu, Zn and Se from the CIGS thin film coating; Zn from the ZnO layer; and Mo from the Mo substrate, which acts as back contact/cathode. Leaching tests were carried out with the following:
- HNO3, H2SO4 and HCl (6 N) on thin film-coated glass fragments with a solid/liquid (S/L) ratio of 30% at ambient temperature. A leaching test with HCl was also carried out at 80 °C.
- HNO3 (1 N, 3 N and 6 N) on thin film-coated glass fragments (S/L ratio: 50% and 70%) at ambient temperature.
2.6. Separation and Recovery of Metals
The solubility diagrams obtained by simulating the composition of the leachates with Visual Minteq at the concentration ranges determined in the leachates for In, Ga, Mo, Zn and Cu show that In and Ga cannot be separated via selective precipitation (pH regulation) (Figure 1). Thus, solvent extraction was applied as a promising alternative, using D2EHPA (di-2-ethylhexyl phosphoric acid) diluted in kerosene [5].
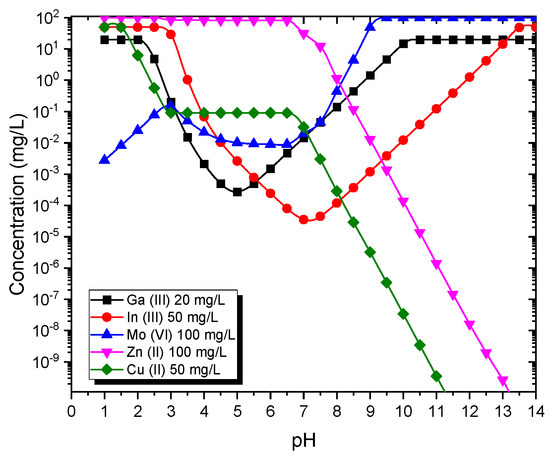
Figure 1.
Solubility of Ga, In, Mo, Zn and Cu vs. pH obtained by Visual Minteq.
Preliminary experiments were carried out using synthetic solutions of In (100 mg/L), Ga (100 mg/L) and Mo (200 mg/L). All metal leaching and recovery experiments were repeated twice. For In extraction recovery, the solvent extraction parameters were the following:
- pH = 1.5;
- Extraction: 0.02 M D2EHPA, aqueous/organic (A/O) phase volume ratio = 2:1;
- Stripping: 1 N HCl, organic/aqueous (O/A) phase volume ratio = 1:1.
For Ga extraction recovery, pH was adjusted to 3.3 with the addition of 1 M NaOH. For Ga extraction recovery, the solvent extraction parameters were the following:
- pH = 3.3;
- Extraction: 0.015 M D2EHPA, A/O = 3:1;
- Stripping: 2 N HCl, O/A = 3:1.
3. Results and Discussion
3.1. Characterization of the Structure and Composition of the EoL Thin Film PV Panels
The microstructural and morphological characteristics of the thin film PV panels were investigated by scanning electron microscopy in polished sections (Figure 2). The identified layers were the outer glass layer, the EVA membrane, the ZnO layer, the thin film semiconductor (CIGS layer), the Mo back contact/backsheet and the glass substrate.
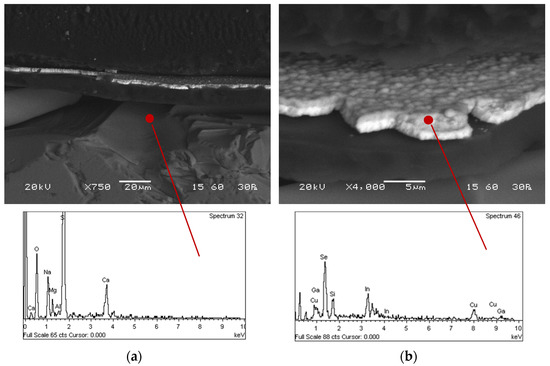
Figure 2.
Internal structure of the thin film photovoltaic panel: (a) glass and (b) thin film.
3.2. Acid Leaching for Metal Extraction
Table 1 shows the composition of the thin film-coated glass fragments. The glass content corresponds to 99.1% of the mass of the fragments, while the thin film containing In and Ga is only 0.05% of the mass. Zn and Mo are also present at higher quantities than those of In and Ga.

Table 1.
Composition of the thin film-coated glass fragments.
Figure 3a shows the leaching results for the thin film-coated glass fragments with a S/L ratio of 30% for the different leaching media tested. Higher concentrations were obtained for all metals when HNO3 (25 °C) and HCl (80 °C) were used. Moreover, leaching with HNO3 led to faster delamination of the thin film (30 min vs. 4 days in the case of HCl), so HNO3 was selected for further leaching experiments.
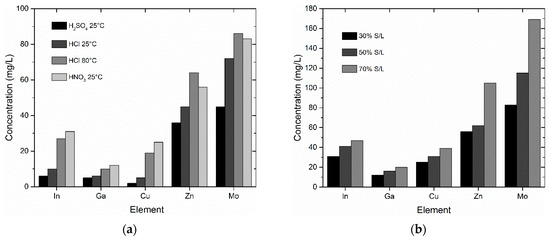
Figure 3.
Effect of (a) different acids and (b) different S/L ratios on the extraction of metals.
For the metals of interest, i.e., In and Ga, it is necessary to develop a process where their concentrations in the leachate shall reach the highest possible levels. To achieve this, leaching experiments were carried out at high S/L ratios of 50% and 70% with 1 N, 3 N and 6 N HNO3. Figure 3b shows that higher S/L values led to higher metal concentrations. Table 2 shows that the quantitative extraction of In, Ga, Mo, Zn and Cu was achieved with 6 N HNO3. The solid residue upon solid/liquid separation consisted mainly of selenium (Figure 4).

Table 2.
Thin film-coated glass fragment leachate.
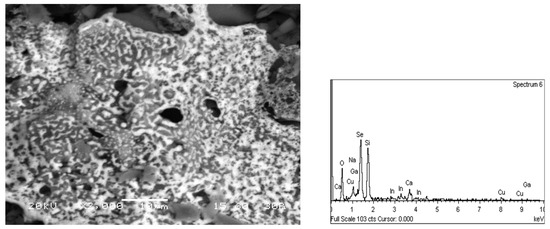
Figure 4.
Solid residue upon solid/liquid separation after leaching with 6 N HNO3.
3.3. Separation and Recovery of Metals
The preliminary solvent extraction results showed that the selective separation of In was achieved at a percentage of 97% in the first stage without Ga co-extraction (Table 3). Ga was received in the second-stage raffinate solution, where the extraction efficiency reached 72% (Table 4).

Table 3.
Solvent extraction results for indium.

Table 4.
Solvent extraction results for gallium.
Indium and gallium are stripped by HCl and subsequently precipitated as metal oxides [6].
4. Conclusions
This study proposes a process for In and Ga recovery from CIGS PV wastes. The PV panels were dismantled and thermally treated for the delamination of EVA and PVF. CIGS waste characterization indicated the simultaneous presence of Mo and Zn with In, Ga, Cu and Se. The thermally treated panels were leached in one step with HNO3, where the thin film metallic content (In, Ga and Cu) was quantitatively extracted together with Mo and Zn. After acid leaching, Se remained mostly in the solid residue, whereas part of it volatilized during the thermal treatment. The separation of In and Ga was achieved by solvent extraction with D2EHPA diluted in kerosene. Further research is being carried out to optimize the stripping parameters (i.e., acid concentration and A/O ratio) for the recovery of In and Ga.
Funding
This research was funded by the European Union and Greek national funds through the Operational Program ‘’Competitiveness, Entrepreneurship and Innovation’’, under call ‘’RESEARCH-CREATE-INNOVATE’’, and it was carried out in the frame of the PHOTOREC Project (T1EDK-04249).
Acknowledgments
The authors acknowledge the analytical support provided by E. Mylona and A.D. Harokopou (School of Mining and Metallurgical Engineering, NTUA). The materials used for the experiments in this study were provided by Polyeco S.A.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Yang, E.H.; Lee, J.K.; Lee, J.S.; Ahn, Y.S.; Kang, G.H.; Cho, C.H. Environmentally friendly recovery of Ag from end-of-life c-Si solar cell using organic acid and its electrochemical purification. Hydrometallurgy 2017, 167, 129–133. [Google Scholar] [CrossRef]
- Weckend, S.; Wade, A.; Heath, G. End-of Life Management: Solar Photovoltaic Panels; International Renewable Energy Agency (IRENA) and International Energy Agency-Photovoltaic Power Systems (IEA-PVPS): Masdar, United Arab Emirates, 2016. [Google Scholar]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H. Recycling WEEE: Extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Manag. 2017, 57, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Marwede, M.; Berger, W.; Schlummer, M.; Mäurer, A.; Reller, A. Recycling paths for thin-film chalcogenide photovoltaic waste—Current feasible processes. Renew. Energy 2013, 55, 220–229. [Google Scholar] [CrossRef]
- Drinkard, W.F.; Long, M.O.; Goozner, R.E. Recycling of CIS Photovoltaic Waste. U.S. Patent 5779877, 14 July 1998. [Google Scholar]
- Chen, W.-S.; Wang, Y.-C.; Chiu, K.-L. The separation and recovery of indium, gallium and zinc from spent GZO (IGZO) targets. J. Environ. Chem. Eng. 2017, 5, 381–390. [Google Scholar] [CrossRef]
- Theocharis, M.; Pavlopoulos, C.; Kousi, P.; Hatzikioseyian, A.; Zarkadas, I.; Tsakiridis, P.E.; Remoundaki, E.; Zoumboulakis, L.; Lyberatos, G. An integrated thermal and hydrometallurgical process for the recovery of Silicon and Silver from end-of-life crystalline Si photovoltaic panels. Waste Biomass Valorization 2021. submitted. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).