Synergistic Copper–Nickel-Doped Biochar from Animal Waste as Efficient Catalyst for Hydrogen Evolution Reaction †
Abstract
1. Introduction
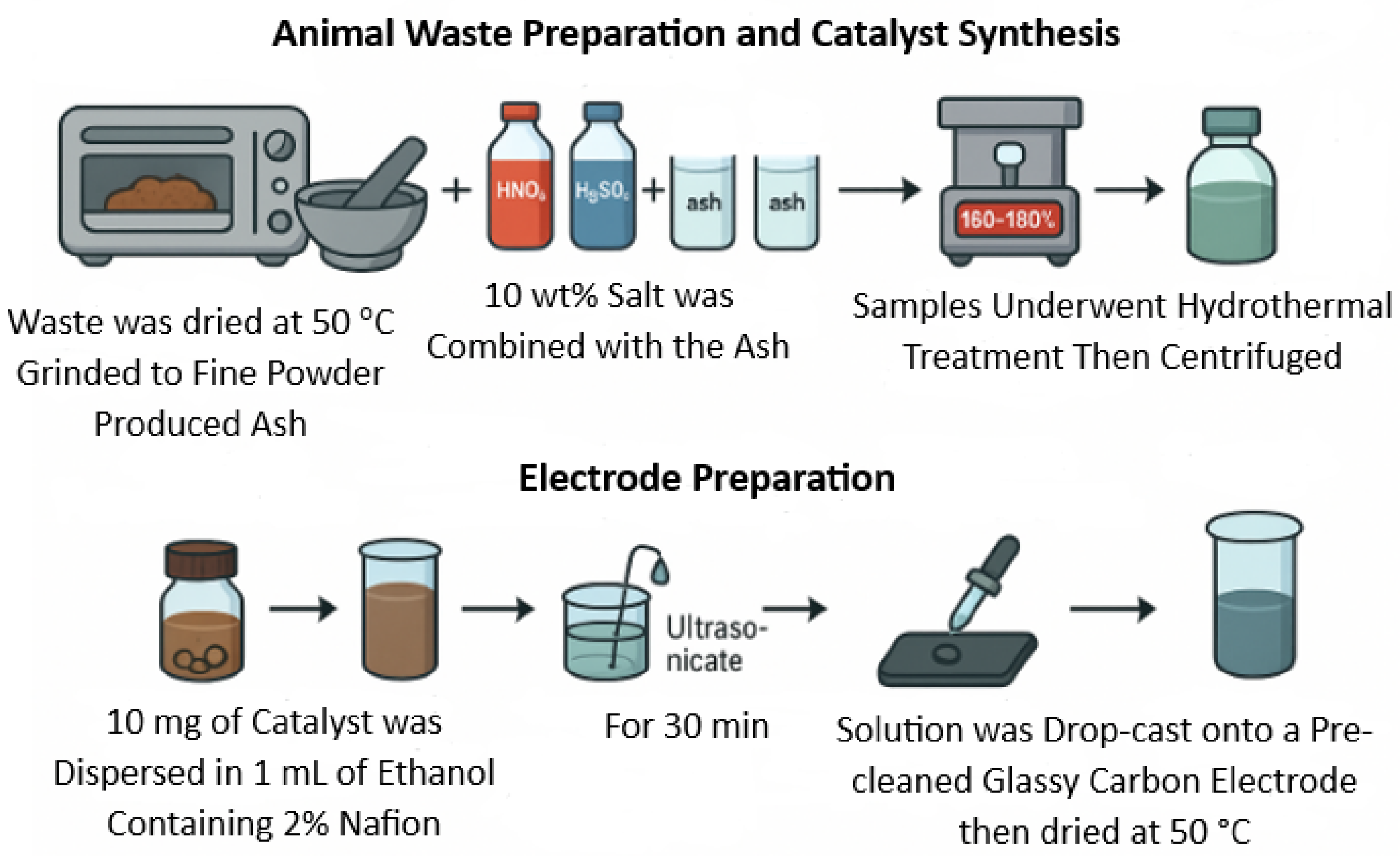
2. Materials and Methods
3. Results and Discussion
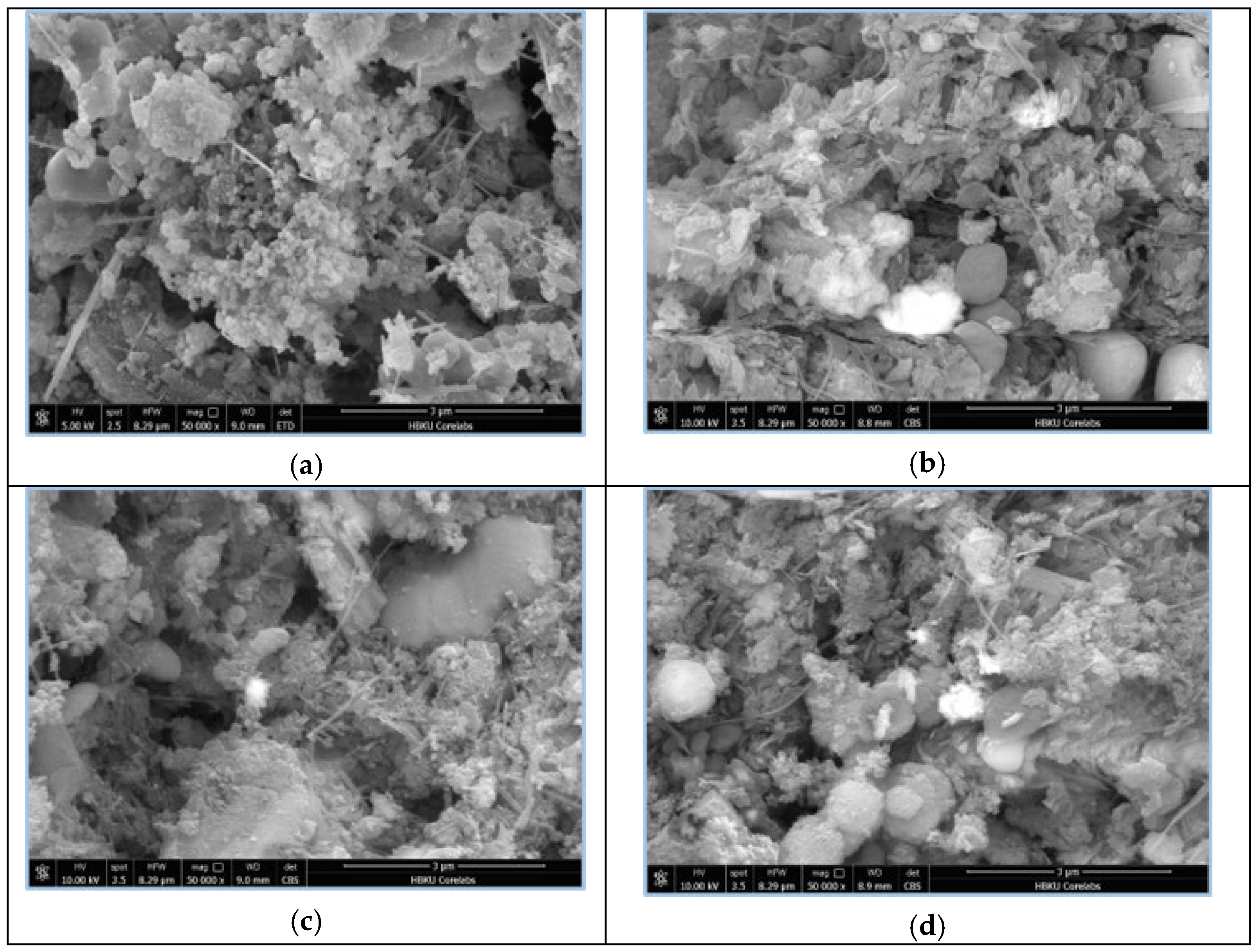
3.1. Scanning Electron Microscopy (SEM)
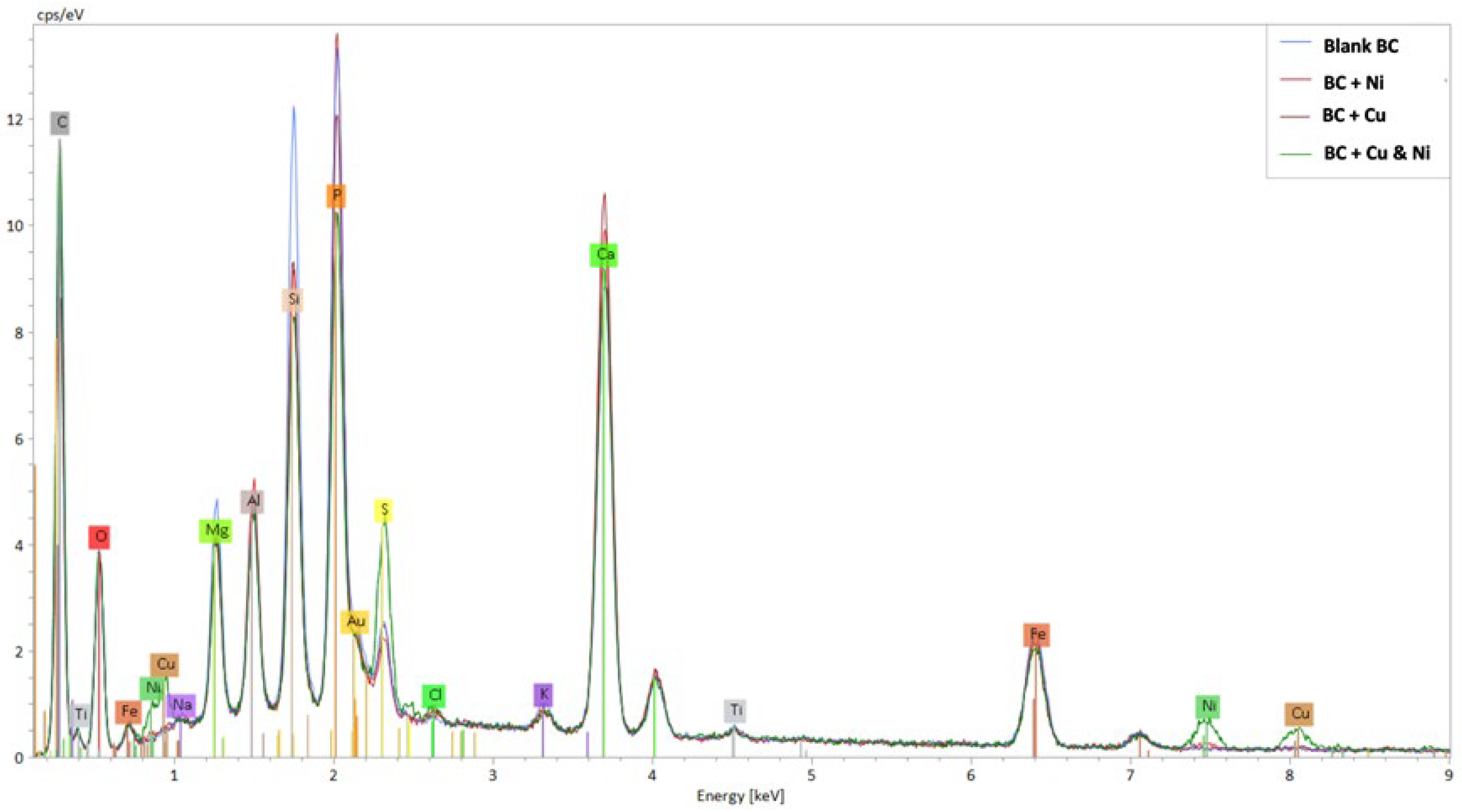
3.2. Energy-Dispersive X-Ray Spectroscopy (EDX)
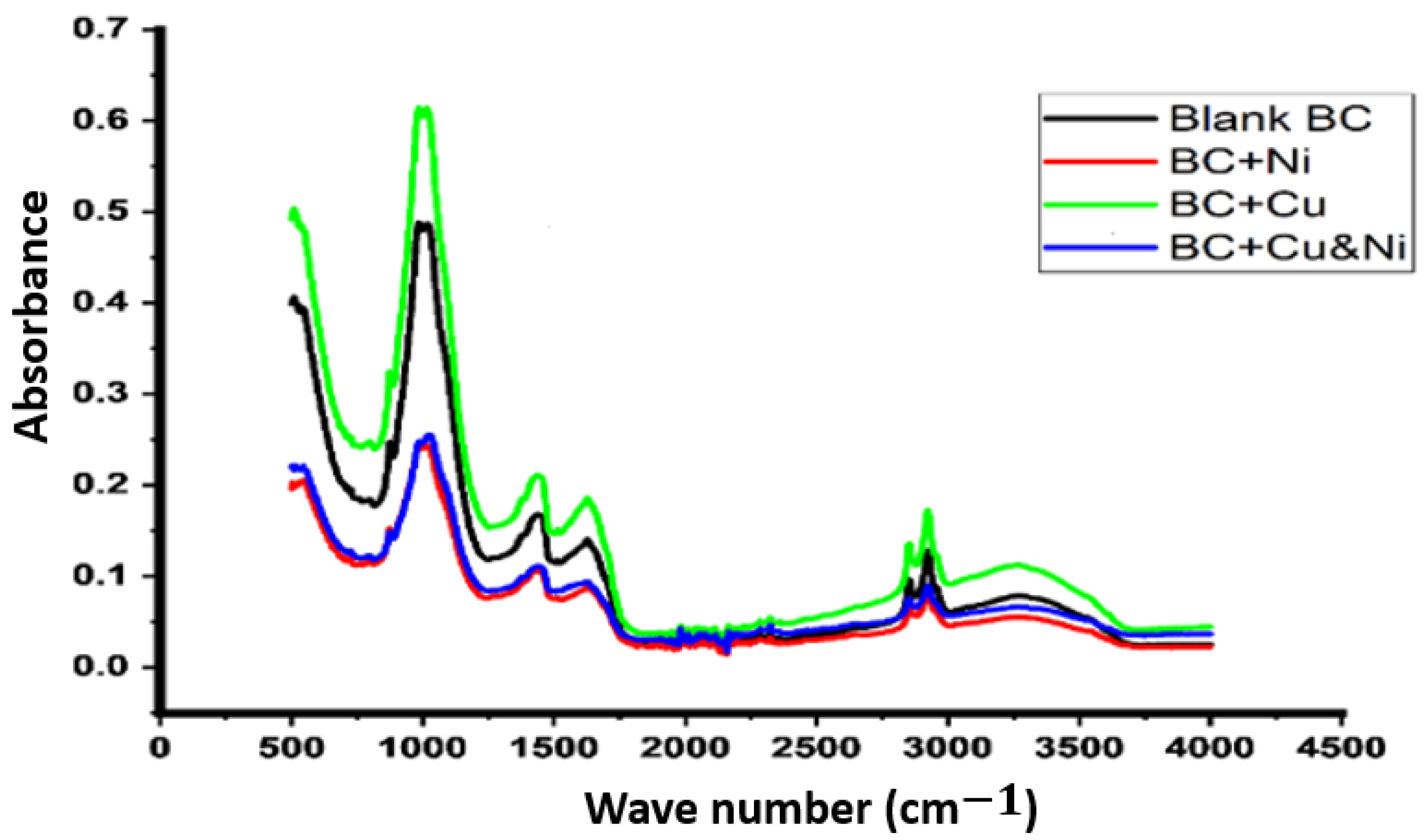
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Status, prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.Z.; Delucchi, M.A. Providing all global energy with wind, water, and solar power, Part I: Technologies, energy resources, quantities and areas of infrastructure, and materials. Energy Policy 2011, 39, 1154–1169. [Google Scholar] [CrossRef]
- Spath, P.L.; Mann, M.K. Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming; National Renewable Energy Lab: Golden, CO, USA, 2001.
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; De Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; United Nations Food and Agriculture: Rome, Italy, 2006. [Google Scholar]
- Chen, Z.; Yun, S.; Wu, L.; Zhang, J.; Shi, X.; Wei, W.; Liu, Y.; Zheng, R.; Han, N.; Ni, B.J. Waste-derived catalysts for water electrolysis: Circular economy-driven sustainable green hydrogen energy. Nano-Micro Lett. 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Park, Y.K.; Lee, J. Biochar catalysts from animal manure: Production and application. Energy Environ. 2023, 0958305X231219787. [Google Scholar] [CrossRef]
- Bao, D.; Li, Z.; Tang, R.; Wan, C.; Zhang, C.; Tan, X.; Liu, X. Metal-modified sludge-based biochar enhance catalytic capacity: Characteristics and mechanism. J. Environ. Management. 2021, 284, 112113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ardah, A.; Baloochi, Z.; Kamal, Y.; Al-Neama, M.; Suwaidan, H.; Selim, M.; Al-Qahtani, N. Synergistic Copper–Nickel-Doped Biochar from Animal Waste as Efficient Catalyst for Hydrogen Evolution Reaction. Mater. Proc. 2025, 22, 7. https://doi.org/10.3390/materproc2025022007
Al-Ardah A, Baloochi Z, Kamal Y, Al-Neama M, Suwaidan H, Selim M, Al-Qahtani N. Synergistic Copper–Nickel-Doped Biochar from Animal Waste as Efficient Catalyst for Hydrogen Evolution Reaction. Materials Proceedings. 2025; 22(1):7. https://doi.org/10.3390/materproc2025022007
Chicago/Turabian StyleAl-Ardah, Ala, Zainab Baloochi, Yousra Kamal, Moza Al-Neama, Haya Suwaidan, Mostafa Selim, and Noora Al-Qahtani. 2025. "Synergistic Copper–Nickel-Doped Biochar from Animal Waste as Efficient Catalyst for Hydrogen Evolution Reaction" Materials Proceedings 22, no. 1: 7. https://doi.org/10.3390/materproc2025022007
APA StyleAl-Ardah, A., Baloochi, Z., Kamal, Y., Al-Neama, M., Suwaidan, H., Selim, M., & Al-Qahtani, N. (2025). Synergistic Copper–Nickel-Doped Biochar from Animal Waste as Efficient Catalyst for Hydrogen Evolution Reaction. Materials Proceedings, 22(1), 7. https://doi.org/10.3390/materproc2025022007




