Surface and Morphological Features of ZrO2 Sol-Gel Coatings Obtained by Polymer Modified Solution †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermal Behavior of Precursor Solution
3.2. Phase Structure and Composition
3.3. Surface Morphology
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Izumi, K.; Minami, N.; Uchida, Y. Sol-Gel-Derived Coatings on Steel Sheets. Key Eng. Mater. 1998, 150, 77–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, G.; Shi, Z. Tribological properties of ZrO2 nanofilms coated on stainless steel in a 5% NaCl solution, distilled water and a dry environment. Surf. Coat. Technol. 2018, 350, 128–135. [Google Scholar] [CrossRef]
- Dankeaw, A.; Poungchan, G.; Panapoy, M.; Ksapabutr, B. In-situ one-step method for fabricating three-dimensional grass-like carbon-doped ZrO2 films for room temperature alcohol and acetone sensors. Sens. Actuators B Chem. 2017, 242, 202–214. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Kirubaharan, K.; Dharini, T.; Muthaiah, B. Corrosion and biocompatibility behaviour of zirconia coating by EBPVD for biomedical applications. Surf. Coat. Technol. 2018, 334, 336–343. [Google Scholar] [CrossRef]
- Berlin, I.J.; Lekshmy, S.S.; Ganesan, V.; Thomas, P.V.; Joy, K. Effect of Mn doping on the structural and optical properties of ZrO2 thin films prepared by sol–gel method. Thin Solid Film. 2014, 550, 199–205. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Y.; Wu, D.; Sun, Y. A simple sol–gel route to ZrO2 films with high optical performances. Mater. Chem. Phys. 2009, 114, 252–256. [Google Scholar] [CrossRef]
- Berlin, I.J.; Maneeshya, L.V.; Thomas, J.K.; Thomas, P.V.; Joy, K. Enhancement of photoluminescence emission intensity of zirconia thin films via aluminum doping for the application of solid state lighting in light emitting diode. J. Lumin. 2012, 132, 3077–3081. [Google Scholar] [CrossRef]
- Friis, M.; Persson, C.; Wigren, J. Influence of particle in-flight characteristics on the microstructure of atmospheric plasma sprayed yttria stabilized ZrO2. Surf. Coat. Technol. 2001, 141, 115–127. [Google Scholar] [CrossRef]
- Hobein, B.; Tietz, F.; Stöver, D.; Kreutz, E.W. Pulsed laser deposition of yttria stabilized zirconia for solid oxide fuel cell applications. J. Power Sources 2002, 105, 239–242. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, F.; Xu, K.W.; Liang, H.F. Optical properties and structural characterization of bias sputtered ZrO2 films. J. Alloy Compd. 2008, 453, 453–457. [Google Scholar] [CrossRef]
- Stefanov, P.; Stoychev, D.; Valov, I.; Kakanakova-Georgieva, A.; Marinova, T.S. Electrochemical deposition of thin zirconia films on stainless steel 316 L. Mater. Chem. Phys. 2000, 65, 222–225. [Google Scholar] [CrossRef]
- Garcı́a-Hipólito, M.; Alvarez-Fregoso, O.; Martı́nez, E.; Falcony, C.; Aguilar-Frutis, M.A. Characterization of ZrO2:Mn, Cl luminescent coatings synthesized by the Pyrosol technique. Opt. Mater. 2002, 20, 113–118. [Google Scholar] [CrossRef]
- Burleson, D.J.; Roberts, J.T.; Gladfelter, W.L.; Campbell, S.A.; Smith, R.C. A Study of CVD Growth Kinetics and Film Microstructure of Zirconium Dioxide from Zirconium Tetra-tert-Butoxide. Chem. Mater. 2002, 14, 1269–1276. [Google Scholar] [CrossRef]
- Lee, J.S.; Matsubara, T.; Sei, T.; Tsuchiya, T. Preparation and properties of Y2O3-doped ZrO2 thin films by the sol–gel process. J. Mater. Sci. 1997, 32, 5249–5256. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Zhou, B.; Shen, J. Effect of crystal structure on optical properties of sol–gel derived zirconia thin films. J. Alloy Compd. 2013, 556, 182–187. [Google Scholar] [CrossRef]
- Ehrhart, G.; Capoen, B.; Robbe, O.; Boy, P.; Turrell, S.; Bouazaoui, M. Structural and optical properties of n-propoxide sol–gel derived ZrO2 thin films. Thin Solid Film. 2006, 496, 227–233. [Google Scholar] [CrossRef]
- Mehner, A.; Datchary, W.; Bleil, N.; Zoch, H.W.; Klopfstein, M.J.; Lucca, D.A. The Influence of Processing on Crack Formation, Microstructure, Density and Hardness of Sol-Gel Derived Zirconia Films. J. Sol-Gel Sci. Technol. 2005, 36, 25–32. [Google Scholar] [CrossRef]
- Rifki, S.; Ahmad, N.; Eneng, M.; Suhanda, S.; Sunendar, P.B. Synthesis of Zirconia 1-D Nanomaterials from Local Zircon-Based Zr(OH)4 mediated by PEG-6000. Res. J. Chem. Environ. 2018, 22, 163–171. [Google Scholar]
- Lazarova, K.; Vasileva, M.; Marinov, G.; Babeva, T. Optical characterization of sol–gel derived Nb2O5 thin films. Opt. Laser Technol. 2014, 58, 114–118. [Google Scholar] [CrossRef]
- Li, W.; Gao, L. Nano ZrO2 (Y2O3) particles processing by heating of ethanol–aqueous salt solutions. Ceram. Int. 2001, 27, 543–546. [Google Scholar] [CrossRef]
- Gorodylova, N.; Šulcová, P.; Bosacka, M.; Filipek, E. DTA-TG and XRD study on the reaction between ZrOCl2·8H2O and (NH4)2 HPO4 for synthesis of ZrP2O7. J. Therm. Anal. Calorim. 2014, 118, 1095–1100. [Google Scholar] [CrossRef]
- Beden, B.; Guillaume, I. Thermal decomposition in air of zirconium chloride octahydrate. Compt. Rend. Ser. 1969, 269C, 1629–1632. [Google Scholar]
- Berlin, I.J.; Lakshmi, J.S.; Lekshmy, S.S.; Daniel, G.P.; Thomas, P.V.; Joy, K. Effect of sol temperature on the structure, morphology, optical and photoluminescence properties of nanocrystalline zirconia thin films. J. Sol-Gel Sci. Technol. 2011, 58, 669–676. [Google Scholar] [CrossRef]
- Garvie, R.C. The Occurrence of Metastable Tetragonal Zirconia as a Crystallite Size Effect. J. Phys. Chem. 1965, 69, 1238–1243. [Google Scholar] [CrossRef]
- Quan, Z.W.; Wang, L.S.; Lin, J. Synthesis and characterization of spherical ZrO2:Eu3+ phosphors by spray pyrolysis process. Mater. Res. 2005, 40, 810–820. [Google Scholar] [CrossRef]
- Elvira, M.R.; Mazo, M.A.; Tamayo, A.; Rubio, F.; Rubio, J.; Oteo, J.L. Study and characterization of organically modified silica–zirconia anti-Graffiti coatings obtained bysol–gel. J. Chem. Chem. Eng. 2013, 7, 120–131. [Google Scholar]
- Georgieva, I.; Danchova, N.; Gutsov, S.; Trendafilova, N. DFT modeling, UV-Vis and IR spectroscopic study of acetylacetone-modified zirconia sol-gel materials. J. Mol. Model. 2012, 18, 2409–2422. [Google Scholar] [CrossRef] [PubMed]
- Cueto, L.F.; Sanchez, E.; Torres-Martınez, L.M.; Hirata, G.A. On the optical, structural, and morphological properties of ZrO2 and TiO2 dip-coated thin films supported on glass substrates. Mater. Charact. 2005, 55, 263–271. [Google Scholar] [CrossRef]
- Kassim, A.; Ekarmul Mahmud, H.N.M.; Yee, L.M.; Hanipah, N. Electrochemical Preparation and Characterization of Polypyrrole-Polyethylene Glycol Conducting Polymer Composite Films. Pac. J. Sci. Technol. 2006, 7, 103–107. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Catauro, M.; Bollino, F.; Papale, F.; Mozzati, M.C.; Ferrara, C.; Mustarelli, P. ZrO2/PEG hybrid nanocomposites synthesized via sol–gel: Characterization and evaluation of the magnetic properties. J. Non-Cryst. Solids 2015, 413, 1–7. [Google Scholar] [CrossRef]
- Torralvo, M.J.; Alario, M.A.; Soria, J. Crystallization behaviour of zirconium oxide gels. J. Catal. 1984, 86, 473–476. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Giamello, E.; Burgess, R.; Valentin, C.D.; Pacchioni, G. Paramagnetic defects in polycrystalline zirconia: An EPR and DFT study. Chem. Mater. 2013, 25, 2243–2253. [Google Scholar] [CrossRef]
- Kostova, N.; Fabian, M.; Mladenova, R.; Shopska, M.; Eliyas, A. Structure characterization and photodegradation properties of mechanochemically synthesized nanosized N-doped ZrO2. Nanosci. Nanotechnol. Nanostructured Mater. Appl. Innov. Transf. 2019, 2, 5–10. [Google Scholar]
- Lin, C.; Zhang, C.; Lin, J. Phase Transformation and Photoluminescence Properties of Nanocrystalline ZrO2 Powders Prepared via the Pechini-type Sol−Gel Process. J. Phys. Chem. C 2007, 111, 3300–3307. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Georgiev, R.; Georgieva, B.; Vasileva, M.; Ivanov, P.; Babeva, T. Optical properties of sol-Gel Nb2O5 films with tunable porosity for sensing applications. Adv. Condens. Matter Phys. 2015, 2015, 403196. [Google Scholar] [CrossRef]
- Dimitrov, O.; Stambolova, I.; Lazarova, K.; Babeva, T.; Vassilev, S.; Shipochka, M. The effect of different acid stabilizers on the morphology and optical properties of ZrO2 sol-gel films. Bulg. Chem. Commun. 2018, 50, 167–172. [Google Scholar]
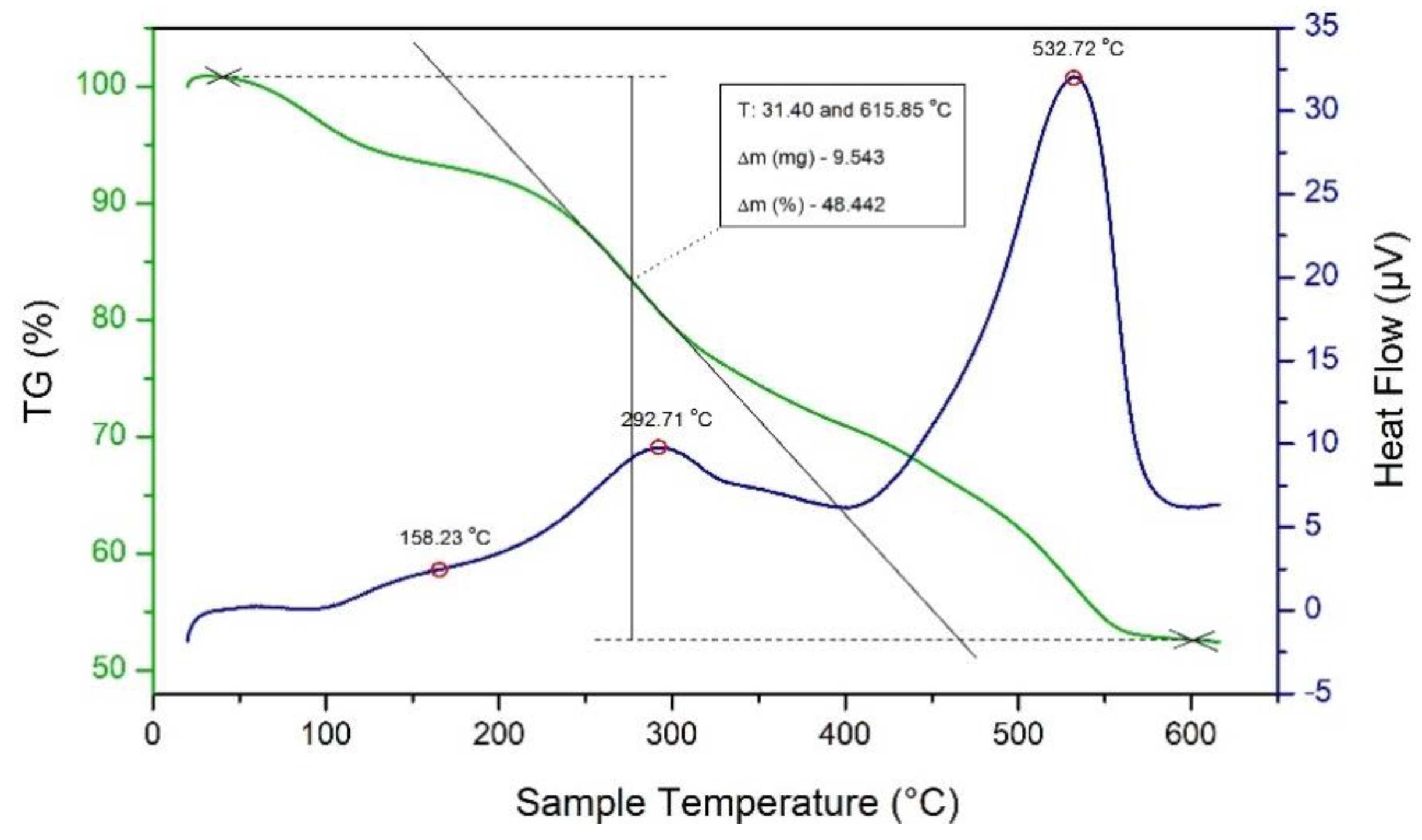
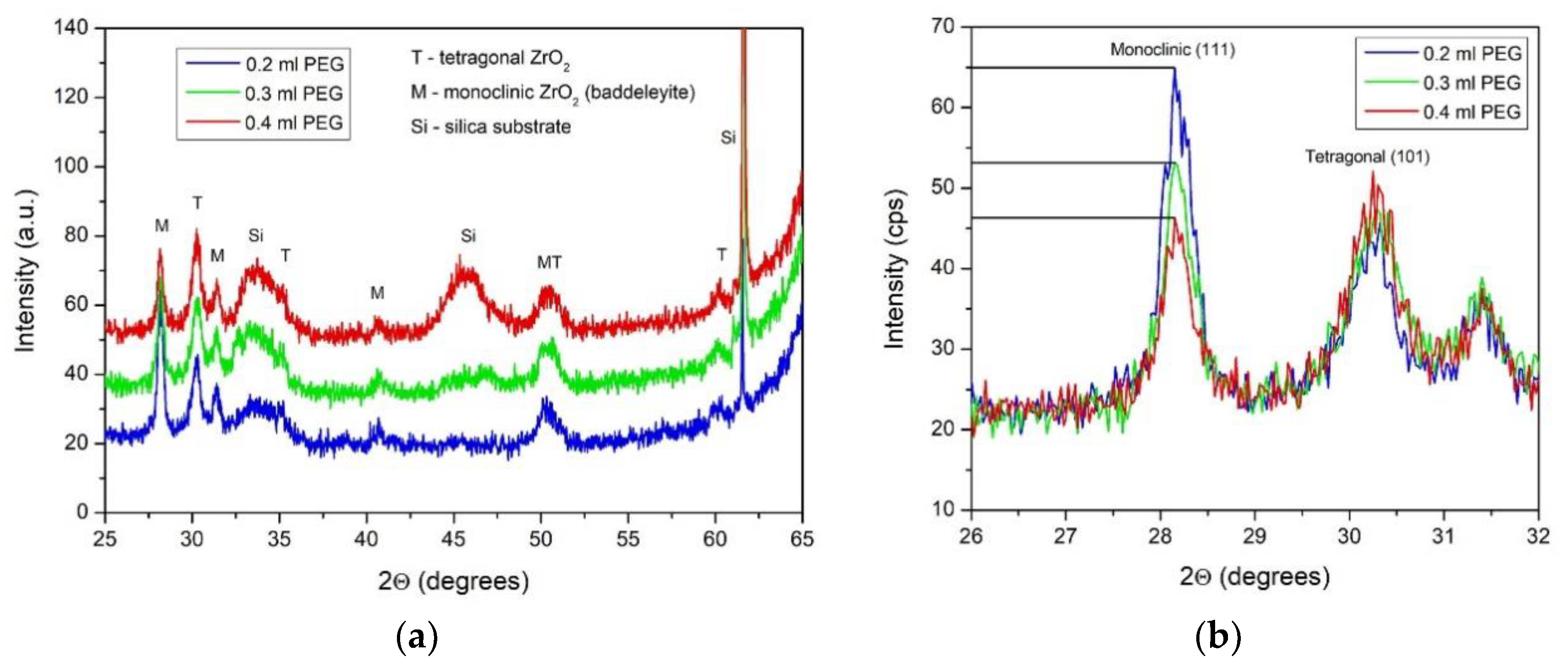
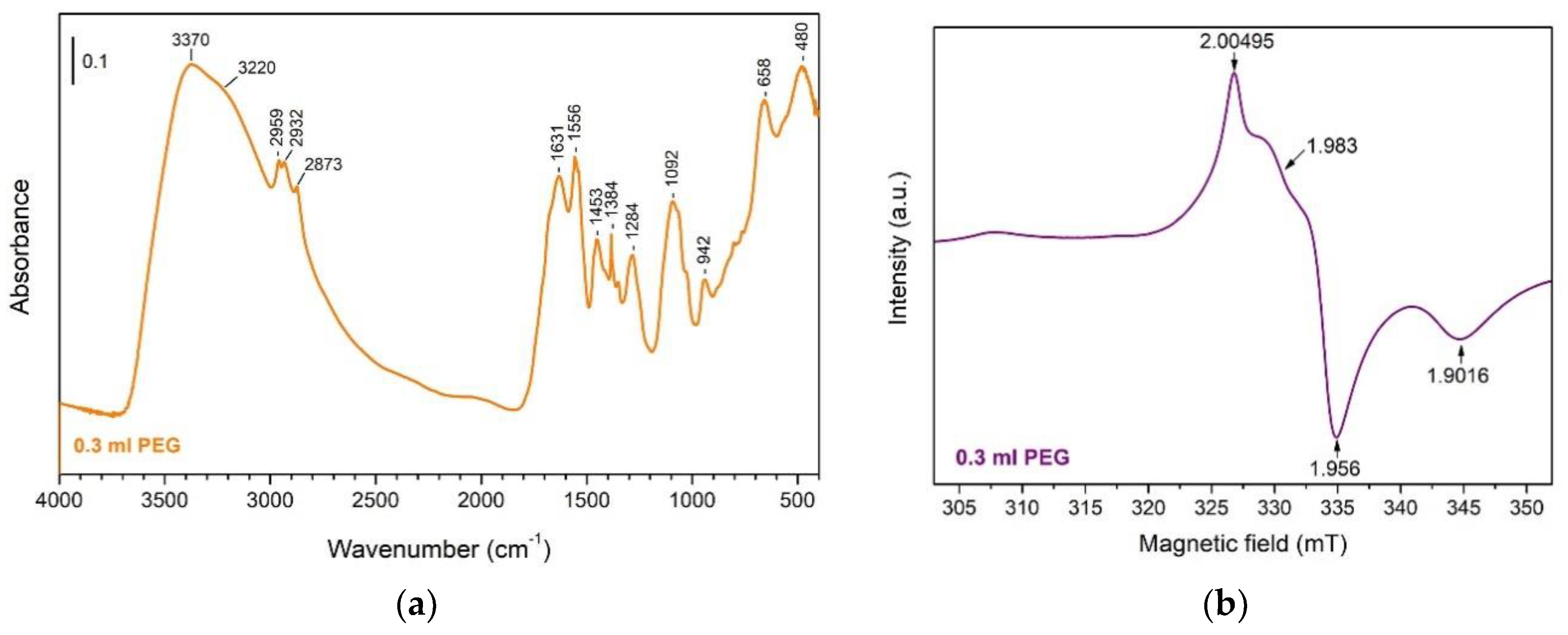

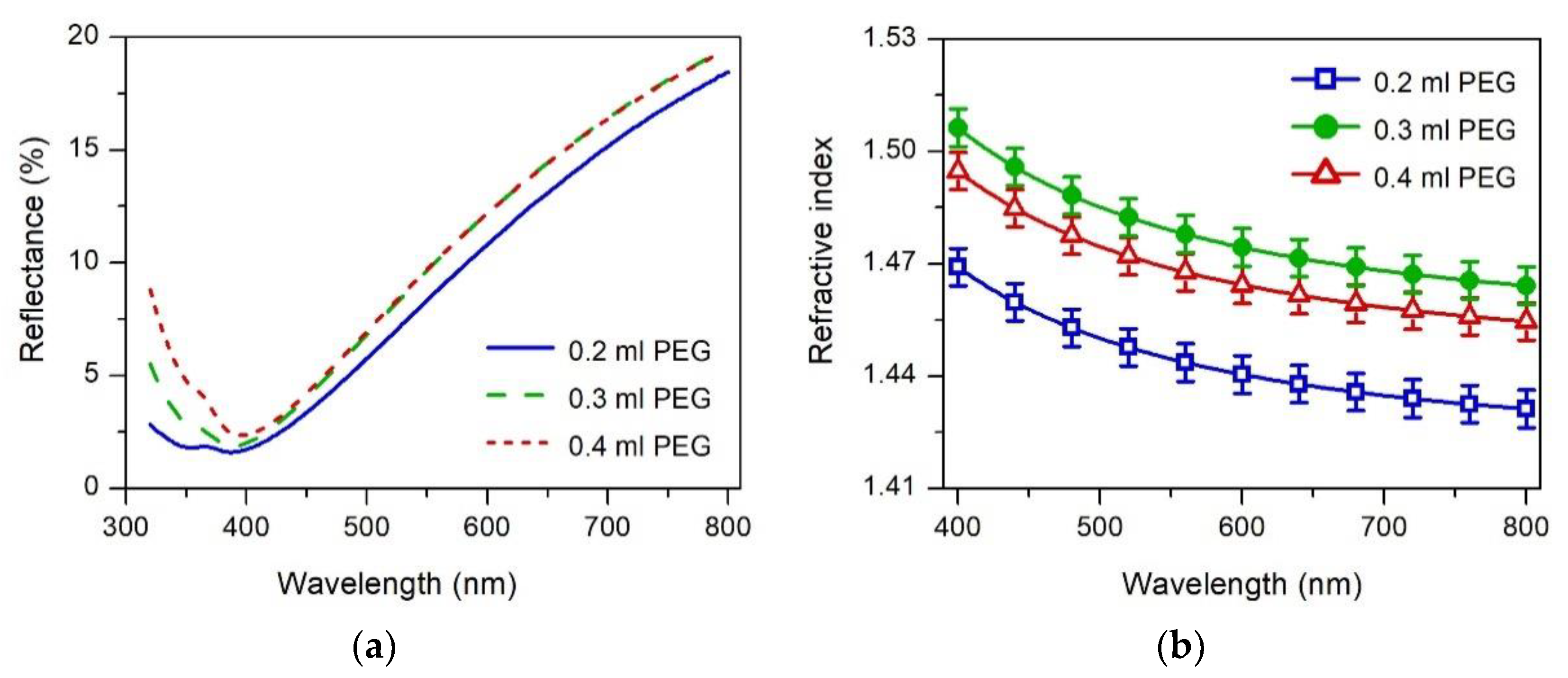
| Samples | Monoclinic (111) Peak | Tetragonal (101) Peak |
|---|---|---|
| 0.2 mL PEG | 23 nm | 15 nm |
| 0.3 mL PEG | 24 nm | 13 nm |
| 0.4 mL PEG | 24 nm | 14 nm |
| Samples | Thickness (nm) | Refractive Index 1 | Extinction Coefficient | Free Volume (%) |
|---|---|---|---|---|
| 0.2 mL PEG | 81 ± 1 | 1.440 ± 0.005 | 0.080 ± 0.005 | 21 ± 1 |
| 0.3 mL PEG | 79 ± 1 | 1.474 ± 0.005 | 0.075 ± 0.005 | 15 ± 1 |
| 0.4 mL PEG | 80 ± 1 | 1.464 ± 0.005 | 0.074 ± 0.005 | 16 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, O.; Stambolova, I.; Vassilev, S.; Lazarova, K.; Babeva, T.; Mladenova, R. Surface and Morphological Features of ZrO2 Sol-Gel Coatings Obtained by Polymer Modified Solution. Mater. Proc. 2020, 2, 6. https://doi.org/10.3390/CIWC2020-06810
Dimitrov O, Stambolova I, Vassilev S, Lazarova K, Babeva T, Mladenova R. Surface and Morphological Features of ZrO2 Sol-Gel Coatings Obtained by Polymer Modified Solution. Materials Proceedings. 2020; 2(1):6. https://doi.org/10.3390/CIWC2020-06810
Chicago/Turabian StyleDimitrov, Ognian, Irina Stambolova, Sasho Vassilev, Katerina Lazarova, Tsvetanka Babeva, and Ralitsa Mladenova. 2020. "Surface and Morphological Features of ZrO2 Sol-Gel Coatings Obtained by Polymer Modified Solution" Materials Proceedings 2, no. 1: 6. https://doi.org/10.3390/CIWC2020-06810
APA StyleDimitrov, O., Stambolova, I., Vassilev, S., Lazarova, K., Babeva, T., & Mladenova, R. (2020). Surface and Morphological Features of ZrO2 Sol-Gel Coatings Obtained by Polymer Modified Solution. Materials Proceedings, 2(1), 6. https://doi.org/10.3390/CIWC2020-06810






