1. Introduction
Water is a necessary component of life. Freshwater scarcity and demand are increasing due to expanding population and urbanization. It was found that the earth’s hydrosphere contains only 2.5% fresh water, while the remaining 97.5% is saline water. However, only 0.26% of the aforementioned freshwater content is accessible for human needs [
1,
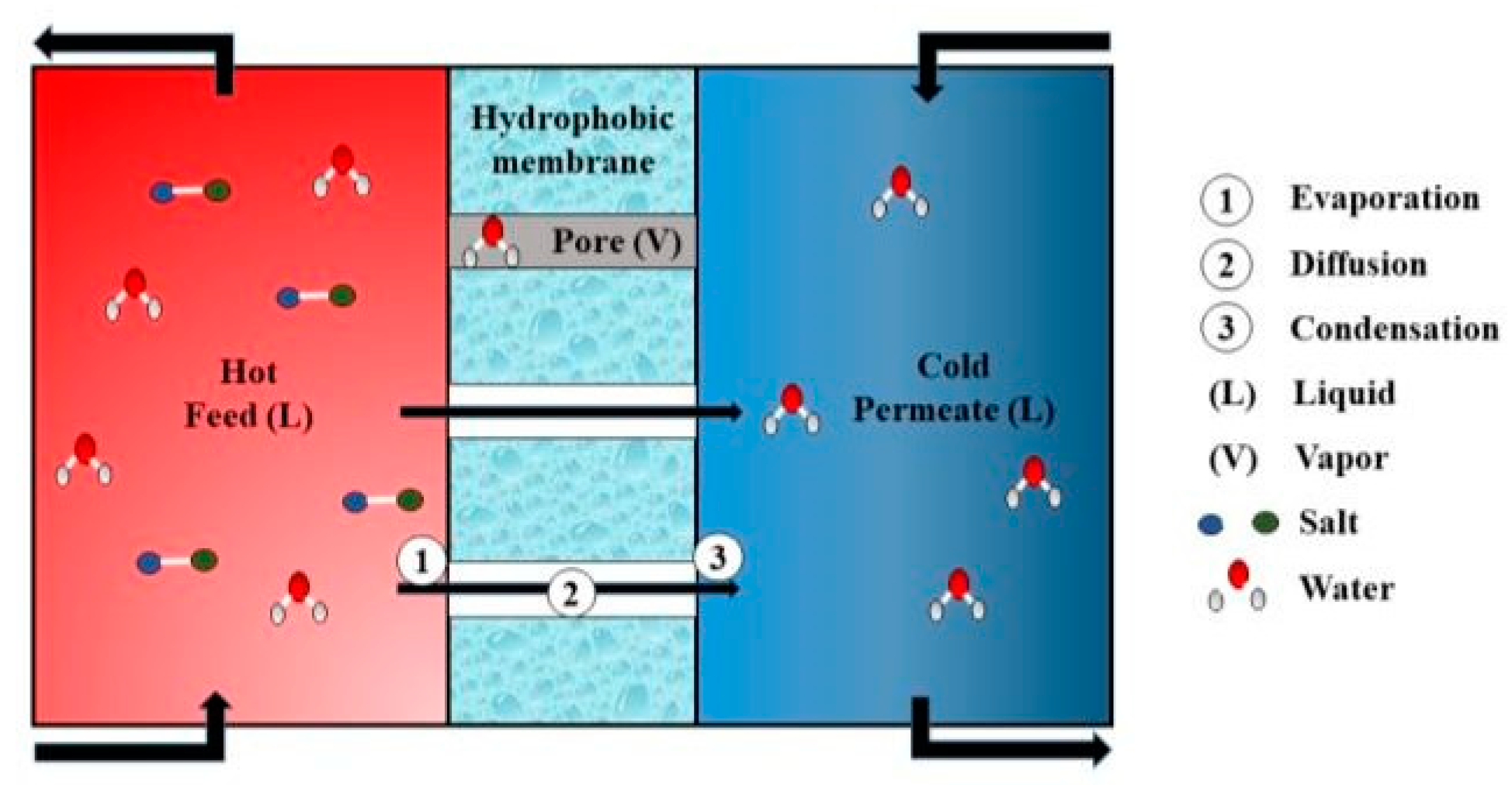
2]. To overcome the prevailing water scarcity, water desalination techniques are needed. Thermal and membrane-based desalination processes are in use. Membrane distillation (MD) is a technique that uses a hydrophobic membrane, as shown in
Figure 1, allowing only water vapors to pass through its pores and condense on the other side of the membrane—usually deionized water [
3]. The hydrophobic nature of the membrane helps develop a liquid–vapor barrier at the entrance of each pore, which acts as a driving force in the process of MD [
4].
Geopolymerization is a process in which a geopolymer material (aluminosilicate-based material, such as metakaolin) is mixed with an alkaline activator solution to produce an amorphous paste. The paste then stays inside a specific mold at 60 °C for 24 h to complete the geopolymerization reaction inside it [
5]. This process is obtaining the focus of membrane researchers who apply it to porous geopolymeric membranes due to its green synthesis nature.
In the current work, the technique of geopolymerization has been used to synthesize a porous geopolymeric membrane substrate. The substrate was then coated with methyltrichlorosilane, which was applied for desalination through direct contact membrane distillation.
2. Materials and Methods
In this study, sodium silicate and sodium hydroxide were used to prepare an alkaline activator solution. A hydrophobic agent, namely, methyltrichlorosilane (MTCS), was used to enhance the membrane hydrophobicity. Ethanol was utilized in preparing the hydrophobic solution and as a hydroxylation pre-coating solution to activate the membrane surface. Metakaolin was used as an aluminosilicate source material. Hydrogen peroxide worked as a foaming agent, while egg albumen helped in pore uniformity and prevented pore rupture.
Metakaolin and egg albumen were mixed in an alkaline solution along with hydrogen peroxide. The slurry was then poured into a mold with rectangular shaped boxes after proper mixing and was stored at room temperature for 24 h. After that, the membrane substrates were taken out of the mold and gently abraded for hydrophobic coating. Before coating, the membranes were kept dipped in a 2:1 ethanol–water solution for 2 h called hydroxylation. A 30–70 vol% solution of MTCS-ethanol was prepared, and the membranes were kept dipped inside it for a day at room temperature [
6].
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
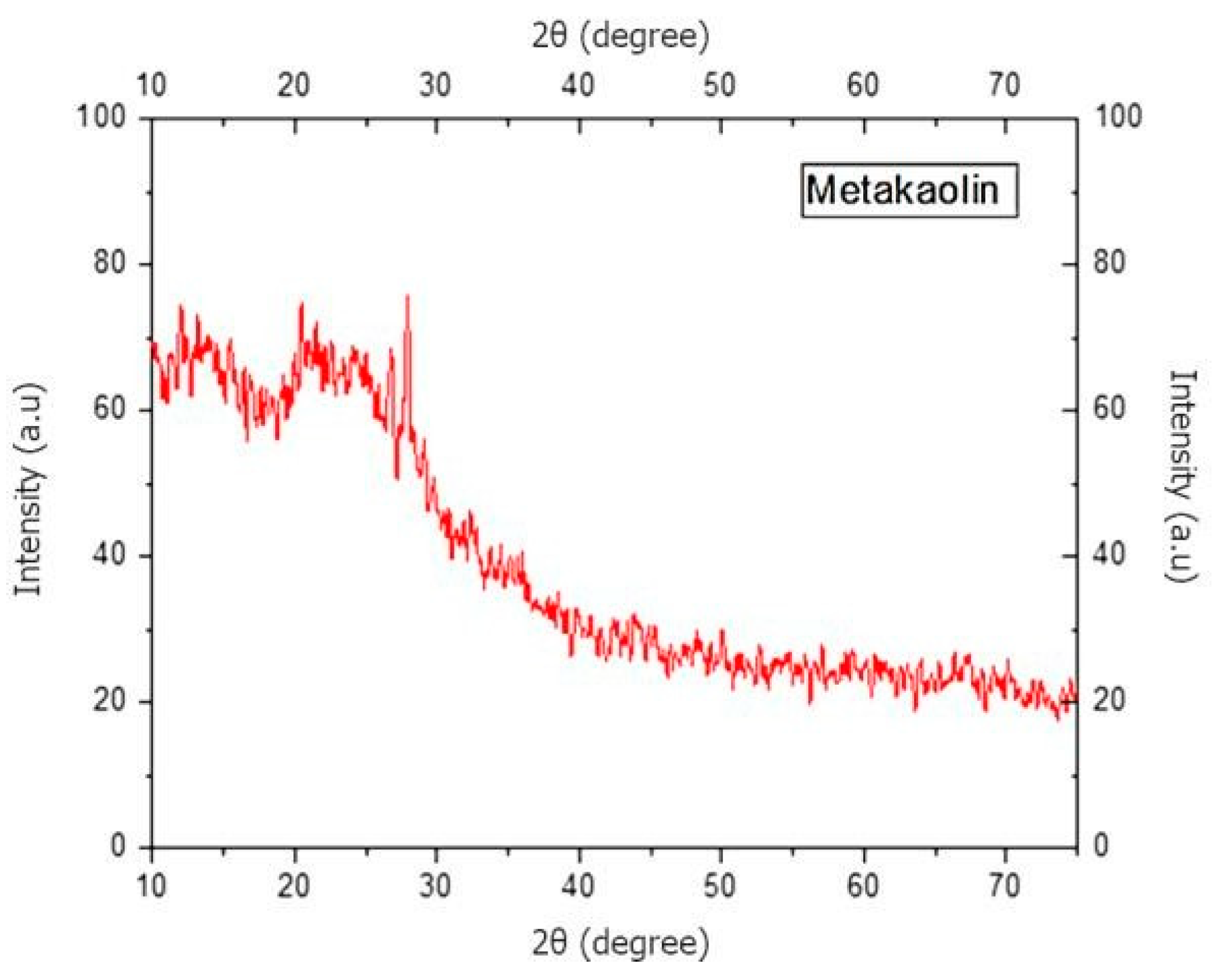
The amorphousness of metakaolin was determined to examine its suitability for the geopolymerization process using XRD, and the results are shown in
Figure 2. A crystalline material has sharp peaks in its XRD pattern, as found by Kumar et al. [
7].
Figure 2 shows that the metakaolin is in amorphous form. The two peaks between 20 and 30 degrees were attributed to the presence of quartz powder.
3.2. Contact Angle
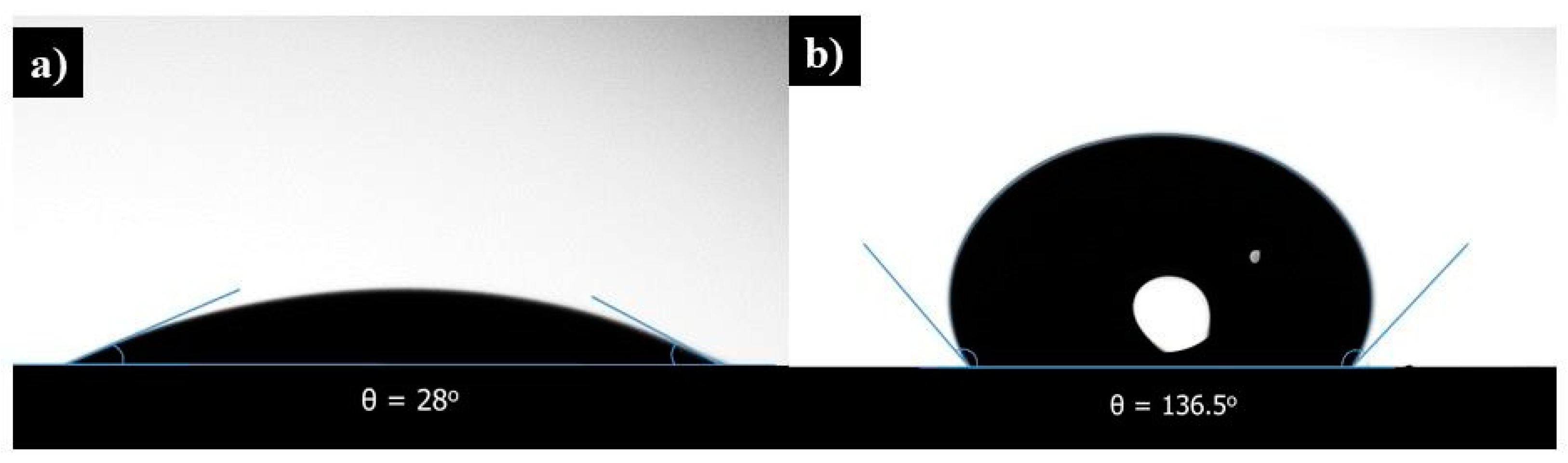
The hydrophobicity of both uncoated and MTCS-coated membranes was measured through the sessile drop method using an optical tensiometer as shown in
Figure 3. The contact angle is the measure of hydrophobicity. A surface having a contact angle less than 90° is hydrophilic, while that above 90° and less than 150° is termed hydrophobic. A super-hydrophobic surface has a contact angle greater than 150°. The uncoated membrane showed an angle of 28°, while that coated with MTCS exhibited 136.5° and was nearly super-hydrophobic. The modified membrane surface with reduced roughness helps the membrane repel liquid water and allows water vapor to pass through it [
8]. The results show that MTCS is an appropriate hydrophobic coating agent for geopolymer materials.
3.3. Performance Evaluation
Water Vapor Flux
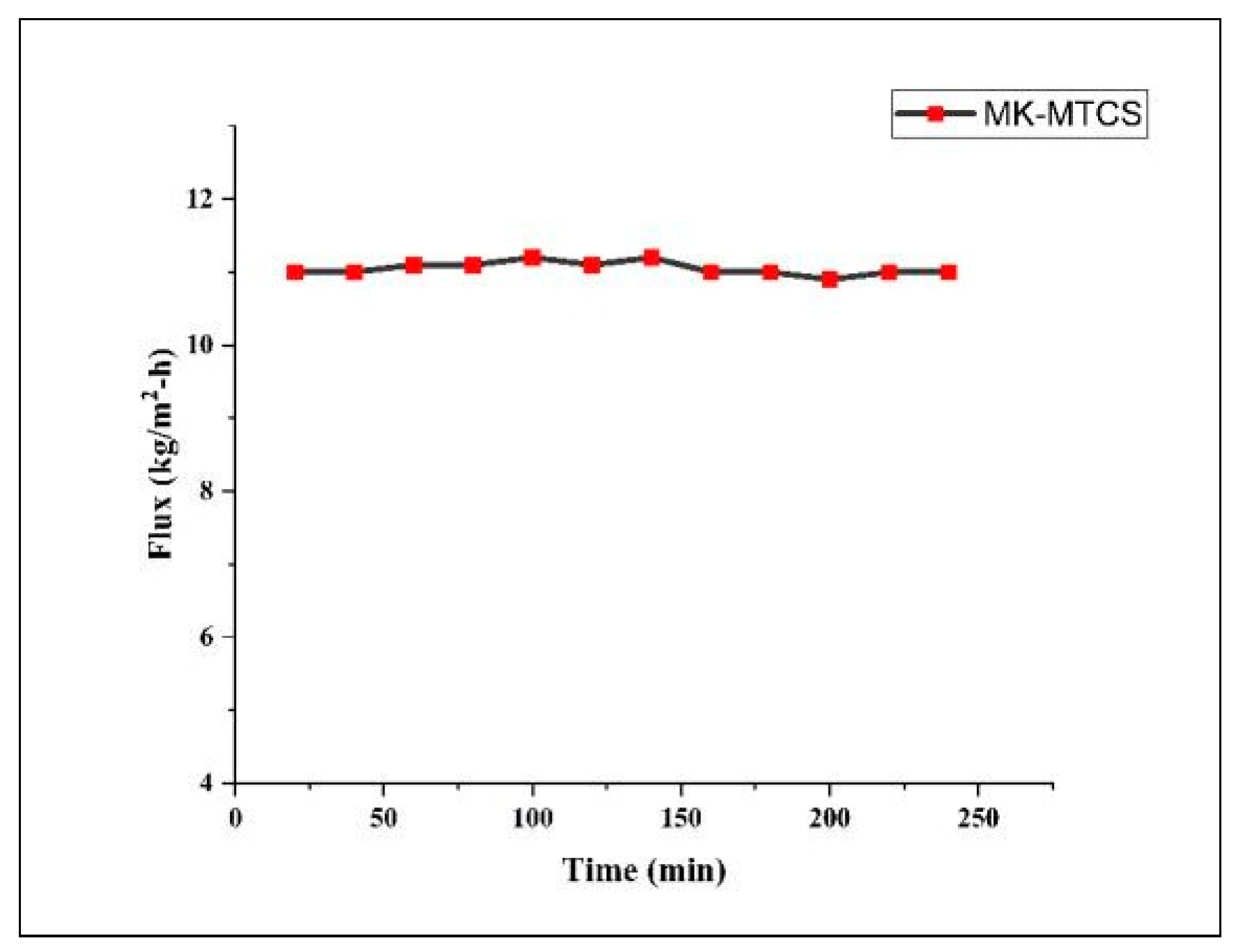
A 3 h DCMD permeation experiment was performed using an MTCS-coated membrane and results are presented in
Figure 4. A 3M NaCl solution was treated using deionized water as a draw solution. The NaCl solution was kept at 50 °C, while the temperature of the deionized water was maintained at 20 °C. The average vapor flux was recorded to be 11 kg/m
2.h. The vapor flux was found to match the literature data for ceramic and polymeric membranes [
5,
6]. The main reason behind the competitive flux is that the membrane was coated with MTCS, which has a smaller hydrophobic chain compared to other organosilane hydrophobic agents, leading to a small change in pore size and porosity [
9].
The uncoated membrane porosity was calculated to be 48%, while that of the coated membrane was 46.86%, reducing it merely by 1.14%. Furthermore, the coated membrane was found to have low pore tortuosity, i.e., 2.3, which helps the water vapor molecules to diffuse and move to the permeate side easily.
4. Conclusions
This study introduces a novel approach towards fabricating hydrophobic geopolymer membranes for water desalination for application in DCMD. Unlike ceramic membranes, they do not need high sintering temperatures. In contrast, the geopolymer membrane synthesis requires comparatively lower temperatures of less than 100 °C. The outcomes of the work are that MTCS nearly turned a hydrophilic surface into a superhydrophobic surface, making it an appropriate agent for synthesizing the hydrophobic geopolymer materials. The MTCS-coated membrane also showed a water vapor flux of 11 kg/m2.h, which is even greater than some ceramic and polymeric membranes in the literature. In a nutshell, this work concludes that the geopolymer membrane is a novel medium for water desalination and that metakaolin is a proper source material.
Author Contributions
Conceptualization, N.A. and K.K.; methodology N.A. and K.K.; software, N.A. and K.K.; validation, N.A. and K.K.; formal analysis, N.A.; investigation, N.A.; resources, N.A.; data curation, N.A.; writing—original draft preparation, N.A.; writing—review and editing, N.A.; visualization, N.A. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Higher Education Commission of Pakistan [20-16456/NRPU/R&D/HEC/2021-2020].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are confidential and will be provided upon request by the corresponding author.
Acknowledgments
The authors would like to acknowledge Saeed Gul for his unwavering support and guidance throughout this research work.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bundschuh, J.; Tomaszewska, B. (Eds.) Geothermal Water Management; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Shiklomanov, I.A. Appraisal and Assessment of World Water Resources. Water Int. 2000, 25, 11–32. [Google Scholar] [CrossRef]
- Ameen, N.A.M.; Ibrahim, S.S.; Alsalhy, Q.F.; Figoli, A. Highly Saline Water Desalination Using Direct Contact Membrane Distillation (DCMD): Experimental and Simulation Study. Water 2020, 12, 1575. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, L.; Zhang, J.-W.; Xu, X.; Chen, C.-S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Silanization of tubular ceramic membranes for application in membrane distillation. J. Membr. Sci. 2020, 601, 117911. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, Q.; Xiao, C. Preparation and vacuum membrane distillation performance of a silane coupling agent-modified polypropylene hollow fiber membrane. Desalination 2019, 468, 114060. [Google Scholar] [CrossRef]
- Dashairya, L.; Barik, D.D.; Saha, P. Methyltrichlorosilane functionalized silica nanoparticles-treated superhydrophobic cotton for oil–water separation. J. Coatings Technol. Res. 2019, 16, 1021–1032. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).